Post-Harvest Eucalyptus Residue Removal Reduces Soil Aggregation and Biological Activities in Central-West Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Management History
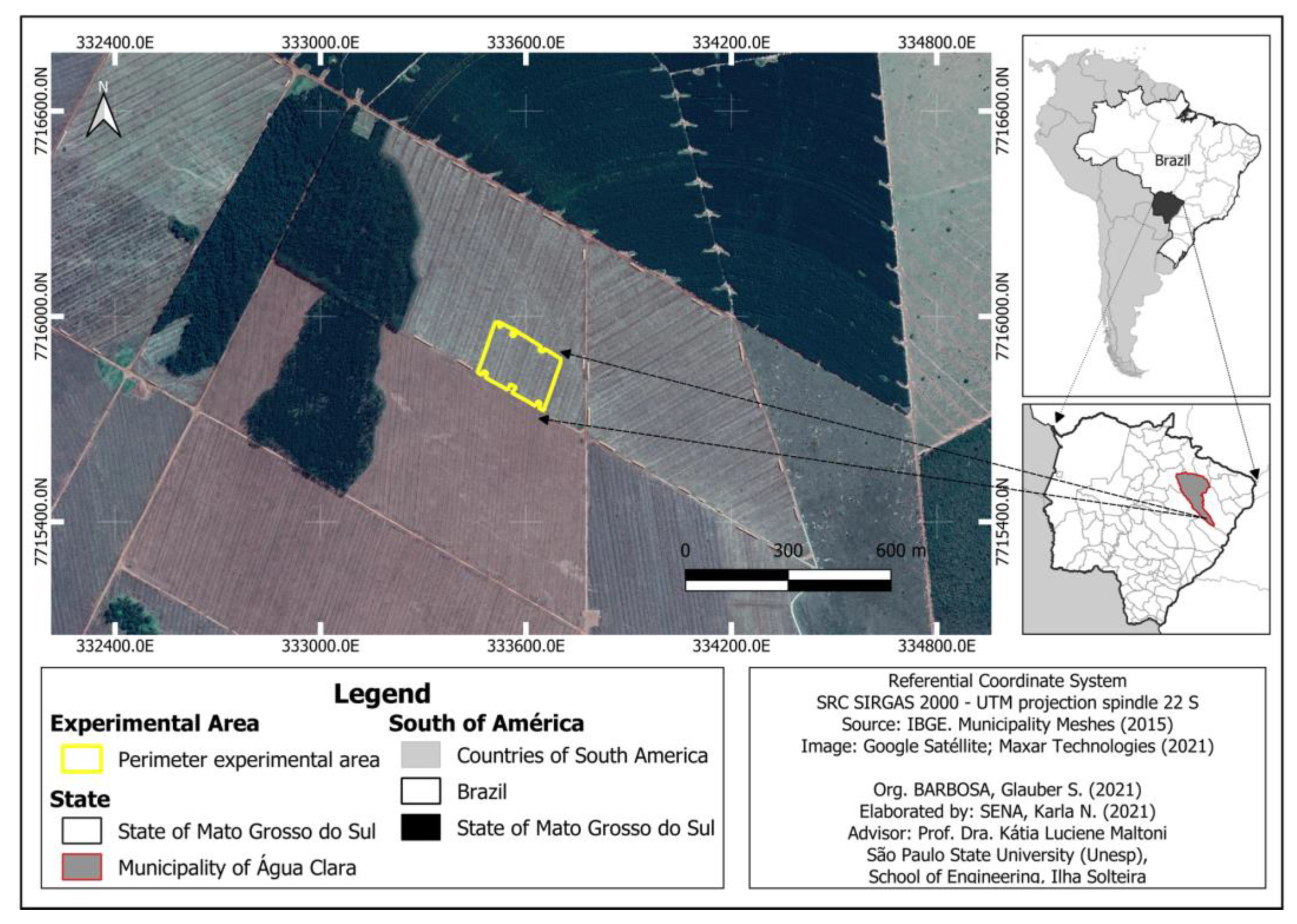
2.2. Treatments and Experimental Design
2.3. Sampling and Aggregates from Dry Sieve Preparation
2.4. Sand Correction of Aggregates
2.5. Soil Aggregates Respiration Measurement and Microbial Biomass Carbon (MBC)
2.6. Enzyme Activity
2.7. Total Carbon and Nitrogen
2.8. Active Carbon
2.9. Statistical Analyzes
3. Results
4. Discussion
4.1. Availability and C:N Ratio of Residues Affect C Cycle-Related Indicators
4.2. Stability and C Accumulation in Soil Aggregates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Gonçalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; de Barros Ferraz, S.F.; de Paula Lima, W.; Brancalion, P.H.S.; Hubner, A. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Laclau, J.P.; Ranger, J.; de Moraes Gonçalves, J.L.; Maquère, V.; Krusche, A.V.; M’bou, A.T.; Nouvellon, Y.; Saint-André, L.; Bouillet, J.P.; de Cassia Piccolo, M. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; de Moraes Gonçalves, J.L.; Brandani, C.B.; de Vicente Ferraz, A.; Franci, A.F.; Marques, E.R.G.; Arthur Junior, J.C.; Hubner, A. Forest residue removal decreases soil quality and affects wood productivity even with high rates of fertilizer application. For. Ecol. Manag. 2018, 430, 188–195. [Google Scholar] [CrossRef]
- Ferreira, G.W.D.; Soares, E.M.B.; Oliveira, F.C.C.; Silva, I.R.; Dungait, J.A.J.; Souza, I.F.; Vergutz, L. Nutrient release from decomposing Eucalyptus harvest residues following simulated management practices in multiple sites in Brazil. For. Ecol. Manag. 2016, 370, 1–11. [Google Scholar] [CrossRef]
- Vinhal-Freitas, I.C.; Corrêa, G.F.; Wendling, B.; Bobuľská, L.; Ferreira, A.S. Soil textural class plays a major role in evaluating the effects of land use on soil quality indicators. Ecol. Indic. 2017, 74, 182–190. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Du, H.; Zeng, F.; Peng, W.; Wang, K.; Zhang, H.; Liu, L.; Song, T. Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China. Forests 2015, 6, 1763–1778. [Google Scholar] [CrossRef]
- Malinovski, J.R.; Camargo, C.M.S.; Malinovski, R.A.; Malinovski, R.A.; Castro, G.P. Sistemas. In Colheita Florestal, 3rd ed.; Machado, C.C., Ed.; UFV: Viçosa, Brasil, 2014; Volume 3, pp. 178–205. [Google Scholar]
- De Souza, I.F.; de Barros, N.F.; da Silva, I.R.; Renier, R.F.; de Ávila Silva, L.; de Novais, R.F. Decomposition of eucalypt harvest residues as affected by management practices, climate and soil properties across southeastern Brazil. For. Ecol. Manag. 2016, 374, 186–194. [Google Scholar] [CrossRef]
- Barros, I.B.; Cavalcante, V.S.; Moulin, A.S.; da Silva, I.R.; de Barros, N.F.; Vergütz, L.; Valadares, S.V. Integrating Forest residue and mineral fertilization: Effects on nutrient acquisition, nutrient use efficiency and growth of eucalypt plants. For. Ecol. Manag. 2021, 496, 119461. [Google Scholar] [CrossRef]
- Momolli, D.R.; Schumacher, M.V. O manejo dos resíduos florestais afeta a produção de biomassa e a sustentabilidade de povoamentos de Eucalyptus sp. Rev. Bras. Gestão Ambient. Sustentabilidade 2019, 6, 251–261. [Google Scholar] [CrossRef]
- Tisdall, J.; Oades, J. Organic-matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, X.; Zhu, A.; Yang, W.; Zhang, J.; Ding, S.; Mu, L.; Shao, L. Linking macroaggregation to soil microbial community and organic carbon accumulation under different tillage and residue managements. Soil Tillage Res. 2018, 178, 99–107. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Nicolodi, M.; Gianello, C. Understanding Soil as an Open System and Fertility as an Emergent Property of the Soil System. Sustain. Agric. Res. 2014, 4, 94. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Erickson, T.E.; Dixon, K.W.; Merritt, D.J. Soil quality indicators to assess functionality of restored soils in degraded semiarid ecosystems. Rest. Ecol. 2016, 24, S43–S52. [Google Scholar] [CrossRef]
- Insam, H.; Haselwandter, K. Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 1989, 79, 174–178. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Cesarz, S.; Leite, L.F.C.; Borges, C.D.; Tsai, S.M.; Eisenhauer, N. Soil microbial properties and temporal stability in degraded and restored lands of Northeast Brazil. Soil Biol. Biochem. 2013, 66, 175–181. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, 3–9. [Google Scholar] [CrossRef]
- Vicente, L.C.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Marciano, C.R. Organic carbon within soil aggregates under forestry systems and pasture in a southeast region of Brazil. Catena 2019, 182, 104139. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Zhang, Y. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration. Geoderma 2019, 337, 444–452. [Google Scholar] [CrossRef]
- Li, N.; Yao, S.H.; Qiao, Y.F.; Zou, W.X.; You, M.Y.; Han, X.Z.; Zhang, B. Separation of soil microbial community structure by aggregate size to a large extent under agricultural practices during early pedogenesis of a Mollisol. Appl. Soil Ecol. 2015, 88, 9–20. [Google Scholar] [CrossRef]
- Zhang, H.J.; Ding, W.X.; Yu, H.Y.; He, X.H. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: Result of 20 years compost and inorganic fertilizers repeated application experiment. Biol. Fertil. Soils 2015, 51, 137–150. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports, 106; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2015. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Mapa de Biomas do Brasil; Primeira Aproximação. Escala 1:5 000.000. Projeção Policônica. Meridiano de Referência: 54 W. Gr; Paralelo de Referência: 0, 2014. Available online: https://geoftp.ibge.gov.br/informacoes_ambientais/estudos_ambientais/biomas/mapas/biomas_5000mil.pdf (accessed on 9 March 2023).
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Tian, J.; Pausch, J.; Yu, G.R.; Blagodatskaya, E.; Gao, Y.; Kuzyakov, Y. Aggregate size and their disruption affect C-14-labeled glucose mineralization and priming effect. Appl. Soil Ecol. 2015, 90, 1–10. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kec value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. The metabolic quotient of CO2 (qCO2) as a specific activity parameter to assess the effects of environmental condition, such as pH, on the microbial of forest soil. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis: Part 2. Microbiological and Biochemical Properties, 1st ed.; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America Book Series; Soil Science Society of America: Madison, WI, USA, 1994; Volume 3, pp. 775–833. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Benckiser, G.; Schnell, S. Biodiversity in Agricultural Production Systems; CRC Press—Taylor and Fancis Group: Boca Raton, FL, USA, 2006; p. 429. [Google Scholar]
- Weil, R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. AJAA 2003, 18, 3–17. [Google Scholar]
- Moebius-Clune, B.N.; Moebius-Clune, D.J.; Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Ristow, A.J.; van Es, H.M.; Thies, J.E.; Shayler, H.A.; McBride, M.B.; et al. Comprehensive Assessment of Soil Health. In The Cornell Framework, 3rd ed.; Cornell University: Geneva, Switzerland, 2017; pp. 53–54. ISBN 0-967-6507-6-3. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 March 2021).
- Van Bavel, C.H.M. Mean weight-diameter of soil aggregates as a statistical index of aggregation. Soil Sci. Soc. Am. J. 1950, 14, 20–23. [Google Scholar] [CrossRef]
- Feketeová, Z.; Hrabovský, A.; Šimkovic, I. Microbial Features Indicating the Recovery of Soil Ecosystem Strongly Affected by Mining and Ore Processing. Int. J. Environ. Res. Public. Health 2021, 18, 3240. [Google Scholar] [CrossRef] [PubMed]
- Okolo, C.C.; Gebresamuel, G.; Zenebe, A.; Haile, M.; Eze, P.N. Accumulation of organic carbon in various soil aggregate sizes under different land use systems in a semi-arid environment. Agric. Ecosyst. Environ. 2020, 297, 106924. [Google Scholar] [CrossRef]
- Sena, K.N.; Maltoni, K.L.; Troleis, M.J.B.; Faria, G.A. Forest harvest management systems and residual phytomass affecting physical properties of a sandy soil. Rev. Bras. Cienc. Solo 2021, 45, e0200190. [Google Scholar] [CrossRef]
- Du, X.; Jian, J.; Du, C.; Stewart, R.D. Conservation management decreases surface runoff and soil erosion. Int. Soil Water Conserv. Res. 2022, 10, 188–196. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal Fungi Influence Soil Structure. In Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds, D.D., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 3–18. [Google Scholar]
- Mohanty, M.; Sinha, N.K.; Sammi Reddy, K.; Chaudhary, R.S.; Subba Rao, A.; Dalal, R.C.; Menzies, N.W. How Important is the Quality of Organic Amendments in Relation to Mineral N Availability in Soils? Agric. Res. 2013, 2, 99–110. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, X.; Yang, W.; Zhu, A.; Ding, S. Short-term decomposition, turnover and retention of residue-derived carbon are influenced by the fertility level in a sandy loam soil. Geoderma 2019, 349, 68–78. [Google Scholar] [CrossRef]
- Boni, T.S.; Pereira, E.I.P.; Santos, A.A.; Cassiolato, A.M.R.; Maltoni, K.L. Biomass residues improve soil chemical and biological properties reestablishing native species in an exposed subsoil in Brazilian Cerrado. PLoS ONE 2022, 17, e0270215. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Kong, A.Y.Y.; Six, J.; Bryant, D.C.; Denison, R.F.; van Kessel, C. The relationship between carbon input, aggregation, and soil organic carbon stabilization in sustainable cropping systems. Soil Sci. Soc. Am. J. 2005, 69, 1078–1085. [Google Scholar] [CrossRef]

| Properties | RMS | AgDi | ||
|---|---|---|---|---|
| MI | SM | LM | ||
| 0–250 | 250–2000 | 2000–4000 | ||
| TN (mg N g−1 soil) | CTL | 0.08 bA | 0.01 cA | 0.55 aB |
| TL | 0.03 bA | 0.01 bA | 0.66 aA | |
| BL | 0.08 bA | 0.01 cA | 0.47 aC | |
| TOC (mg C g−1 soil) * | CTL | 1.35 bA | 0.27 bA | 10.99 aB |
| TL | 0.55 bA | 0.14 bA | 13.67 aA | |
| BL | 1.24 bA | 0.17 bA | 8.54 aC | |
| TOC (mg C g−1 aggregate) * | CTL | 7.42 bA | 6.44 bA | 14.59 aA |
| TL | 6.16 bA | 5.29 bA | 15.70 aA | |
| BL | 7.04 bA | 5.08 bA | 13.98 aA | |
| BSR (μg C-CO2 g−1 soil day −1) | CTL | 4.51 bB | 0.74 cA | 15.33 aB |
| TL | 2.32 bC | 0.48 cA | 19.35 aA | |
| BL | 5.99 bA | 0.54 cA | 15.50 aB | |
| MBC (μg C g −1 soil) | CTL | 256.45 bA | 32.81 cA | 475.59 aA |
| TL | 53.80 bB | 46.19 bA | 500.60 aA | |
| BL | 234.56 bA | 55.82 cA | 393.30 aB | |
| qCO2 (μg1 CO2-C soil day−1 of μg−1 MBC) | CTL | 0.019 bB | 0.023 abA | 0.032 aA |
| TL | 0.094 aA | 0.010 cB | 0.040 bA | |
| BL | 0.028 bB | 0.010 cB | 0.042 aA | |
| qMic (%) | CTL | 19.97 aA | 13.05 bC | 4.08 cA |
| TL | 14.05 bB | 26.77 aB | 3.56 cA | |
| BL | 18.91 bA | 32.99 aA | 4.25 cA | |
| FDA (mg fluorescein g−1 soil h−1) | CTL | 6.41 bA | 1.34 cA | 31.43 aA |
| TL | 2.93 bA | 0.70 bA | 33.98 aA | |
| BL | 6.86 bA | 0.83 cA | 26.77 aB | |
| β-Glu (mg PNP g−1 soil h−1) | CTL | 5.16 bA | 1.75 cA | 31.68 aA |
| TL | 2.12 bB | 0.96 bA | 28.79 aB | |
| BL | 5.09 bA | 1.09 cA | 24.98 aC | |
| Active Carbon (mg C g−1 soil) | CTL | 0.299 bB | 0.522 cA | 1.072 aB |
| TL | 0.148 bC | 0.364 cC | 1.239 aA | |
| BL | 0.361 bA | 0.394 cB | 1.022 aC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, K.N.; Boni, T.S.; Maltoni, K.L.; Cassiolato, A.M.R.; Pujol Pereira, E.I. Post-Harvest Eucalyptus Residue Removal Reduces Soil Aggregation and Biological Activities in Central-West Brazil. Sustainability 2023, 15, 8790. https://doi.org/10.3390/su15118790
Sena KN, Boni TS, Maltoni KL, Cassiolato AMR, Pujol Pereira EI. Post-Harvest Eucalyptus Residue Removal Reduces Soil Aggregation and Biological Activities in Central-West Brazil. Sustainability. 2023; 15(11):8790. https://doi.org/10.3390/su15118790
Chicago/Turabian StyleSena, Karla Nascimento, Thaís Soto Boni, Kátia Luciene Maltoni, Ana Maria Rodrigues Cassiolato, and Engil Isadora Pujol Pereira. 2023. "Post-Harvest Eucalyptus Residue Removal Reduces Soil Aggregation and Biological Activities in Central-West Brazil" Sustainability 15, no. 11: 8790. https://doi.org/10.3390/su15118790
APA StyleSena, K. N., Boni, T. S., Maltoni, K. L., Cassiolato, A. M. R., & Pujol Pereira, E. I. (2023). Post-Harvest Eucalyptus Residue Removal Reduces Soil Aggregation and Biological Activities in Central-West Brazil. Sustainability, 15(11), 8790. https://doi.org/10.3390/su15118790







