Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant
Abstract
:1. Introduction
2. Materials and Methods
Nanolubricants Preparation
3. Experimental Details
3.1. Thermal Transport Evaluations
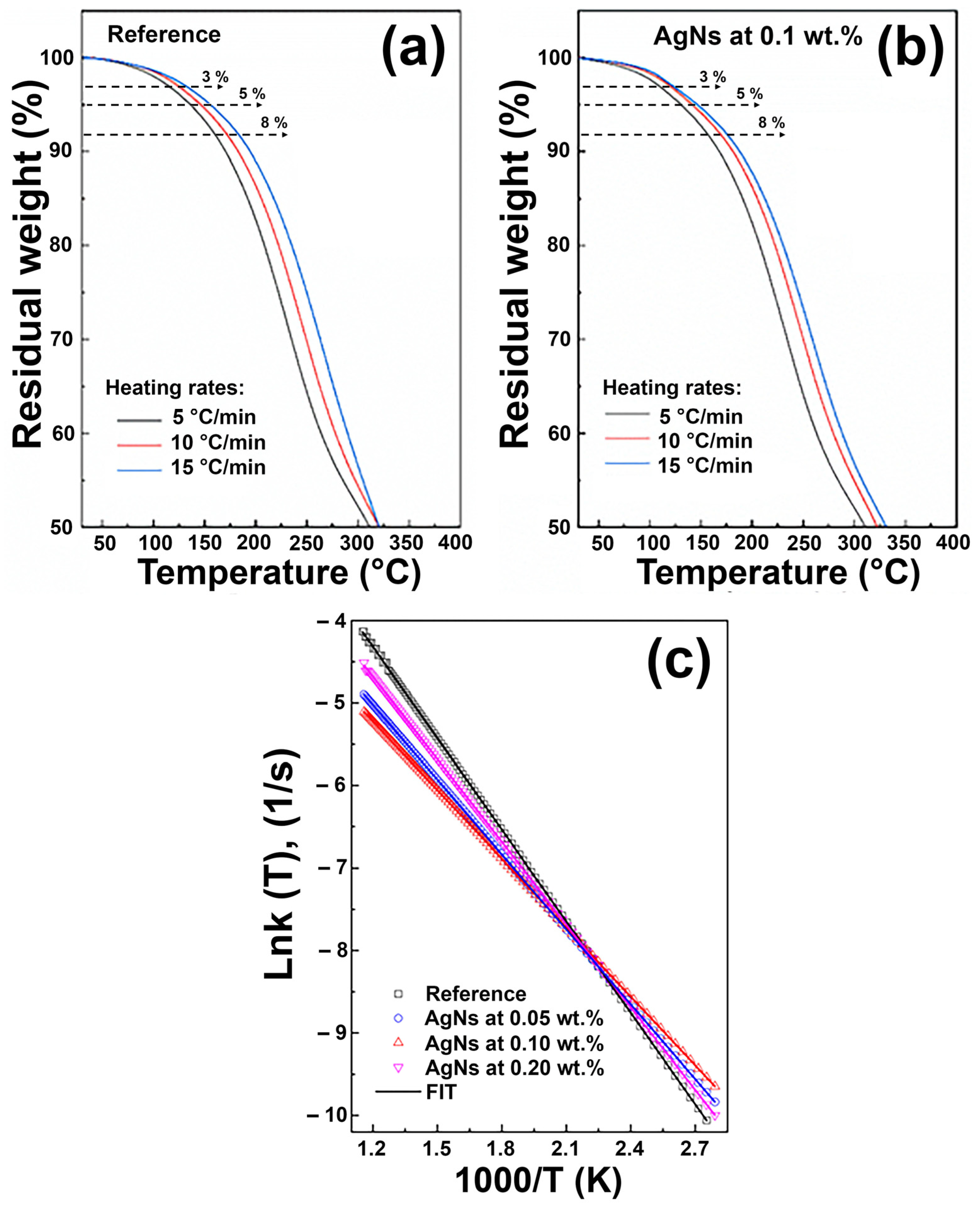
3.2. Thermogravimetric Analysis (TGA)
4. Results and Discussion
4.1. Thermal Transport Evaluations
4.2. Thermogravimetric Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diabb-Zavala, J.M.; Martínez-Romero, O.; Elías-Zúñiga, A.; Leija-Gutiérrez, H.M.; Estrada-De La Vega, A.; Taha-Tijerina, J. Study of Friction and Wear Effects in Aluminum Parts Manufactured via Single Point Incremental Forming Process Using Petroleum and Vegetable Oil-Based Lubricants. Materials 2021, 14, 3973. [Google Scholar] [CrossRef] [PubMed]
- Krolczyk, G.M.; Maruda, R.W.; Krolczyk, J.B.; Wojciechowski, S.; Mia, M.; Nieslony, P. Ecological trends in machining as a key factor in sustainable production—A review. J. Clean. Prod. 2019, 218, 601–615. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, R.A.; Fadare, D.A.; Ikumapayi, O.M.; Adediran, A.A.; Aliyu, S.J.; Akinlabi, S.A. Advances in the Application of Vegetable-Oil-Based Cutting Fluids to Sustainable Machining Operations: A Review. Lubricants 2022, 10, 69. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil—Biodegradable and potential base stoke for industrial lubricants. Ind. Crops Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A. A review on properties, challenges and commercial aspects of eco-friendly biolubricants productions. Chemosphere 2022, 309, 136622. [Google Scholar] [CrossRef] [PubMed]
- Uppar, R.; Dinesha, P.; Kumar, S. A critical review on vegetable oil-based bio-lubricants: Preparation, characterization, and challenges. Environ. Dev. Sustain. 2022, 1–36. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.J.; Martínez, J.M.; Euresti, D.; Arquieta-Guillén, P.Y. Carbon Nanotori Reinforced Lubricants in Plastic Deformation Processes. Lubricants 2022, 10, 74. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Aviña, K.; Diabb, J.M. Tribological and thermal transport performance of SiO2-based natural lubricants. Lubricants 2019, 7, 71. [Google Scholar] [CrossRef]
- Wu, H.; Kamali, H.; Huo, M.; Lin, F.; Huang, S.; Huang, H. Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants 2020, 8, 96. [Google Scholar] [CrossRef]
- Morshed, A.; Wu, H.; Jiang, Z. A Comprehensive Review of Water-Based Nanolubricants. Lubricants 2021, 9, 89. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Taha-Tijerina, J.J. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef]
- Suresh, K.; Selvakumar, P.; Kumaresan, G.; Vijayakumar, M.; Ravikumar, M.; Jenita, N.R. A critical review on the effect of morphology, stability, and thermophysical properties of graphene nanoparticles in nanolubricants and nanofluids. J. Therm. Anal. Calorim. 2022, 148, 451–472. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Scherer, B.; Good, S.; Wohld, J.; Vafaei, S. Nanofluid Heat Transfer: Enhancement of the Heat Transfer Coefficient inside Microchannels. Nanomaterials 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Abro, K.A.; Souayeh, B.; Yasmin, H.; Giwa, S.O.; Noor, S. Reproduction of Nanofluid Synthesis, Thermal Properties and Experiments in Engineering: A Research Paradigm Shift. Energies 2023, 16, 1145. [Google Scholar]
- Ito, H.; Tomaru, M.; Suzuki, T. Physical and chemical aspects of grease deterioration in sealed ball bearings. Lubr. Eng. 1988, 44, 872–879. [Google Scholar]
- Mustafa, W.A.A.; Dassenoy, F.; Sarno, M.; Senatore, A. A review on potentials and challenges of nanolubricants as promising lubricants for electric vehicles. Lubr. Sci. 2022, 34, 1–29. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H. Improving the heat transfer capability and thermal stability of vehicle engine oils using Al2O3/TiO2 nanomaterials. Powder Technol. 2020, 363, 48–58. [Google Scholar] [CrossRef]
- Smook, L.A.; Sathwik, S.C.; Lugt, P.M. Evaluating the oxidation properties of lubricants via non-isothermal thermogravimetric analysis: Estimating induction times and oxidation stability. Tribol. Int. 2022, 171, 107569. [Google Scholar] [CrossRef]
- Dos Santos-Politi, J.R.; De Matos, P.R.R.; Sales, M.J.A. Comparative study of the oxidative and thermal stability of vegetable oils to be used as lubricant bases. J. Therm. Anal. Calorim. 2013, 111, 1437–1442. [Google Scholar] [CrossRef]
- Khan, U.; Zaib, A.; Ishak, A. Impact of Thermal and Activation Energies on Glauert Wall Jet (WJ) Heat and Mass Transfer Flows Induced by ZnO-SAE50 Nano Lubricants with Chemical Reaction: The Case of Brinkman-Extended Darcy Model. Lubricants 2023, 11, 22. [Google Scholar] [CrossRef]
- Lugt, P.M. Grease Aging. In Grease Lubrication in Rolling Bearings; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 171–190. [Google Scholar]
- Fasehullah, M.; Wang, F.; Jamil, S.; Bhutta, M.S. Influence of Emerging Semiconductive Nanoparticles on AC Dielectric Strength of Synthetic Ester Midel-7131 Insulating Oil. Materials 2022, 15, 4689. [Google Scholar] [CrossRef] [PubMed]
- Wan-Nik, W.B.; Ani, F.N.; Masjuki, H.H. Thermal stability evaluation of palm oil as energy transport media. Energy Convers. Manag. 2005, 46, 2198–2215. [Google Scholar] [CrossRef]
- López, T.D.-F.; González, A.F.; Del Reguero, Á.; Matos, M.; Díaz-García, M.E.; Badía-Laíño, R. Engineered silica nanoparticles as additives in lubricant oils. Sci. Technol. Adv. Mater. 2015, 16, 055005. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.K.; Khalid, M.; Javeed, A.; Rashmi, W.; Gupta, T.C.S.M.; Chan, A. Heat transfer and tribological performance of graphene nanolubricant in an internal combustion engine. Tribol. Int. 2016, 103, 504–515. [Google Scholar] [CrossRef]
- Ghaednia, H.; Hossain, M.S.; Jackson, R.L. Tribological Performance of Silver Nanoparticle-Enhanced Polyethylene Glycol Lubricants. Tribol. Trans. 2015, 59, 585–592. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio. Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef]
- Du, F.; Li, C.; Li, D.; Sa, X.; Yu, Y.; Li, C. Research Progress Regarding the Use of Metal and Metal Oxide Nanoparticles as Lubricant Additives. Lubricants 2022, 10, 196. [Google Scholar] [CrossRef]
- Chinnachamy, R.; Durairaj, V.; Saravanamuthu, M.; Rajagopal, V. Evaluation of the effect of silver nanoparticles on the tribological and thermophysical properties of bio-lubricants. Proc. Inst. Mech. Eng. E J. Process. Mech. Eng. 2022, 237, 410–417. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Shaji, S.; Kanakkillam, S.S.; Mendivil-Palma, M.I.; Aviña, K. Tribological and Thermal Transport of Ag-Vegetable Nanofluids Prepared by Laser Ablation. Appl. Sci. 2020, 10, 1779. [Google Scholar] [CrossRef]
- Akgul, Y.; Simsir, H. Anti-wear behaviour of silver nanoparticles on Al-Si alloy. Surf. Topogr. Metrol. Prop. 2021, 9, 025031. [Google Scholar] [CrossRef]
- Duan, L.; Li, J.; Duan, H. Nanomaterials for lubricating oil application: A review. Friction 2022, 11, 647–684. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, N.K. Effect of silver nanoparticle volume fraction on thermal conductivity, specific heat and viscosity of ethylene glycol base silver nanofluid: A molecular dynamics investigation. J. Mol. Liq. 2023, 378, 121635. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Behi, M.; Gan, Y.; Shen, L. Particle-shape-, temperature-, and concentration-dependent thermal conductivity and viscosity of nanofluids. Phys. Rev. E 2019, 99, 043109. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 31246. [Google Scholar] [CrossRef]
- Kumara, C.; Luo, H.; Leonard, D.N.; Meyer, H.M.; Qu, J. Organic-Modified Silver Nanoparticles as Lubricant Additives. ACS Appl. Mater. Interfaces 2017, 9, 37227–37237. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, W.; Liang, W.; Yan, T.; Li, T.; Zhang, L. Inorganic nanomaterial lubricant additives for base fluids, to improve tribological performance: Recent developments. Friction 2022, 10, 645–676. [Google Scholar] [CrossRef]
- Sunil, J.; Pooja, M.D.; Ginil, R.; Alex, S.N.; Pravin, A.A. Thermal conductivity and dynamic viscosity of aqueous-silver nanoparticle dispersion. Mater. Today Proc. 2021, 37, 122–124. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Experimental Investigation on the Thermal Conductivity and Viscosity of Silver-Deionized Water. Nanofluids 2010, 23, 317–332. [Google Scholar] [CrossRef]
- Paul, G.; Sarkar, S.; Pal, T.; Das, P.K.; Manna, I. Concentration and size dependence of nano-silver dispersed water based nanofluids. J. Colloid. Interface Sci. 2012, 371, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.D.; Doganay, S.; Turgut, A. Thermal characteristics and performance of Ag-water nanofluid: Application to natural circulation loops. Energy Convers. Manag. 2017, 135, 9–20. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Aberoumand, H.; Javaherdeh, K. A Complete Experimental Investigation on The Rheological Behavior of Silver Oil Based Nanofluid. Heat Transf. Res. 2017, 46, 294–304. [Google Scholar] [CrossRef]
- Khamliche, T.; Khamlich, S.; Doyle, T.B.; Makinde, D.; Maaza, M. Thermal conductivity enhancement of nano-silver particles dispersed ethylene glycol based nanofluids. Mater. Res. Express 2018, 5, 035020. [Google Scholar] [CrossRef]
- Cárdenas-Contreras, E.M.; Oliveira, G.A.; Bandarra-Filho, E.P. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Shafi, W.K.; Charoo, M.S. An overall review on the tribological, thermal and rheological properties of nanolubricants. Tribol. Mater. Surf. Interfaces 2020, 15, 20–54. [Google Scholar] [CrossRef]
- Karthikeyan, K.M.B.; Vijayanand, J.; Arun, K.; Rao, V.S. Thermophysical and wear properties of eco-friendly nano lubricants. Mater. Today Proc. 2021, 39, 285–291. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Mohammed, D.B.; Abdulhadi, A.M.; Uktamov, K.F.; Alsultany, F.H.; Izzat, S.E. Nanofluids: Properties and applications. J. Sol-Gel Sci. Technol. 2022, 104, 1–35. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Aviña, K.; Martínez, J.M.; Arquieta-Guillén, P.Y.; González-Escobedo, M. Carbon Nanotori Structures for Thermal Transport Applications on Lubricants. Nanomaterials 2021, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
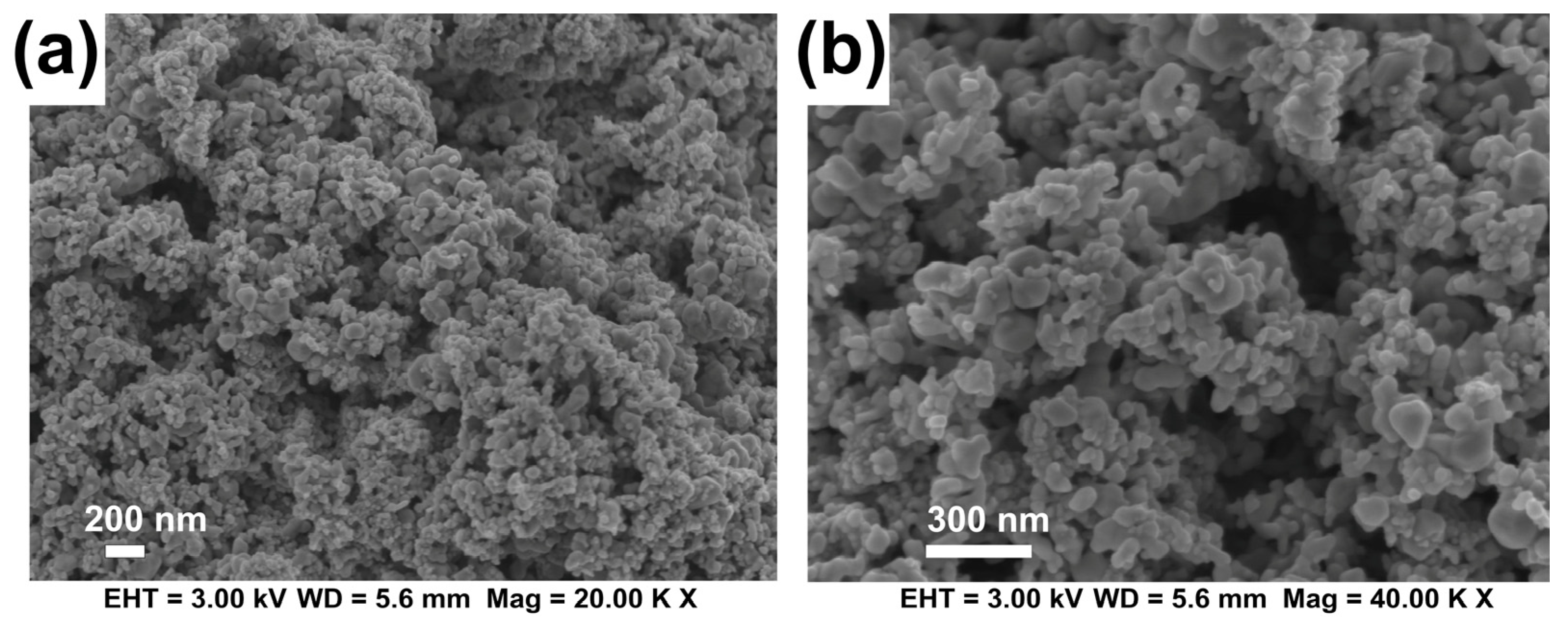
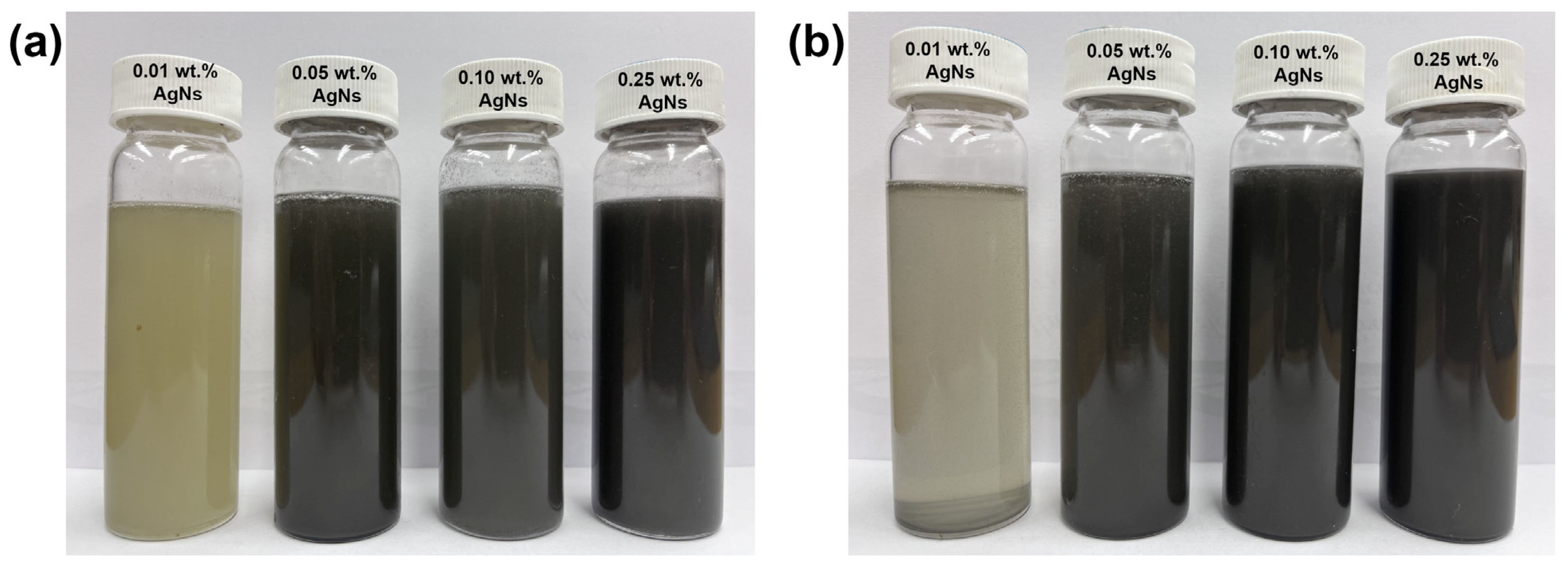



| Materials | Properties and Characteristics | |
|---|---|---|
| Base Lubricant | Density (20 °C) | Kinetic Viscosity (mm2/s) |
| Biodegradable oil | 0.915 g/cm3 | 190 @ 0 °C 32–34 @ 40 °C 7.7–8.3 @ 100 °C |
| Nanostructures | Properties | |
| Silver (AgNs) | Morphology: Spherical-like Size-diameter: 54.55 nm ± 19.37 nm Molecular weight: 107.87 g/mol | |
| SAMPLE | WEIGHT LOSS (%) | Activation Energy, Ea (KJ/mol) | ||
|---|---|---|---|---|
| First Stage (50 °C–315 °C) | Second Stage (315 °C–420 °C) | Third Stage (420 °C–510 °C) | ||
| Reference | 48.2% | 43.6% | 2.9% | 30.7 ± 2.41 |
| AgNs at 0.05 wt.% | 49.0% | 36.2% | 12.2% | 25.1 ± 3.17 |
| AgNs at 0.10 wt.% | 47.3% | 38.8% | 9.2% | 23.1 ± 3.29 |
| AgNs at 0.20 wt.% | 47.2% | 37.3% | 10.7% | 27.7 ± 2.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha-Tijerina, J.J.; Aviña, K.; Ulloa-Castillo, N.A.; Melo-Maximo, D.V. Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant. Sustainability 2023, 15, 8795. https://doi.org/10.3390/su15118795
Taha-Tijerina JJ, Aviña K, Ulloa-Castillo NA, Melo-Maximo DV. Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant. Sustainability. 2023; 15(11):8795. https://doi.org/10.3390/su15118795
Chicago/Turabian StyleTaha-Tijerina, Jose Jaime, Karla Aviña, Nicolás Antonio Ulloa-Castillo, and Dulce Viridiana Melo-Maximo. 2023. "Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant" Sustainability 15, no. 11: 8795. https://doi.org/10.3390/su15118795
APA StyleTaha-Tijerina, J. J., Aviña, K., Ulloa-Castillo, N. A., & Melo-Maximo, D. V. (2023). Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant. Sustainability, 15(11), 8795. https://doi.org/10.3390/su15118795







