Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review
Abstract
:1. Introduction
2. Synthetization of Carbonaceous Materials
2.1. Activated Carbons (ACs)
2.1.1. Physical Activation
- (a)
- The omission of volatile matter and the degradation of tar;
- (b)
- The establishment of new micropores;
- (c)
- Additional expansion of the pores present.
2.1.2. Chemical Activation
2.1.3. Physiochemical Activation
2.1.4. Microwave-Assisted Activation
2.2. Carbon Nanotubes
2.2.1. Electrolysis
2.2.2. Chemical Vapor Deposition (CVD)
2.2.3. Mechano-Thermal Methods
2.2.4. Laser Ablation
2.2.5. Flame Synthesis
2.2.6. Arc Discharge
2.3. Graphene
2.3.1. Mechanical Exfoliation
2.3.2. Chemical Reduction of Graphite
2.3.3. Chemical Exfoliation
2.3.4. Pyrolysis
2.3.5. Chemical Vapor Deposition (CVD)
2.3.6. Epitaxial Growth
2.3.7. Plasma Synthesis
3. Water Treatment
3.1. Activated Carbons in Water Treatment
3.1.1. Dye Removal
3.1.2. Heavy Metal Removal
3.1.3. Removal of Pesticides
3.1.4. Phenolic Removal
| Applications of ACs in Phenolic Removal from Water | ||||||
|---|---|---|---|---|---|---|
| Adsorbent | Activator | Adsorbate | SBET (m2/g) | pH | Qm (mg/g) | Ref. |
| Acacia mangium ACs | H3PO4 | Phenol | 1767 | 3 | 53.8 | [193] |
| Soybean-curd-residue ACs | KOH | Phenol | 253.14 | 2 | - | [194] |
| Antibiotic-mycelial-residue ACs | - | Phenol | 1369.76 | - | - | [195] |
| Kenaf-rapeseed ACs | CO2 | Phenol | 1112 | 10 | 84.1 | [196] |
| Date-pit ACs | CO2 | Phenol | - | - | 161.8 | [69] |
| Avocado-kernel ACs | CO2 | Phenol | 206 | 6 | 90 | [196] |
| Açaí-seed ACs | Ar/CO2 | Phenol | 496 | 3.5–8 | 133 | [197] |
| Baobab-wood ACs | H3PO4 | Phenol | 1682 | 3 | 240 | [198] |
| Date-pit ACs | ZnCl2 | Phenol | - | 7 | 169.5 | [69] |
| Coconut-husk ACs | KOH | 2,4,6-trichlorophenol | - | 2 | 716.10 | [196] |
| Loosestrife ACs | H3PO4 | 2,4,6-trichlorophenol | 1255.75 | - | 367.65 | [199] |
| Date-pit ACs | H3PO4 | o-Nitrophenol | - | - | 142.9 | [69] |
| Date-pit ACs | KOH | p-Cresol | - | - | 322.5 | [69] |
| Avocado-seed ACs | H2SO4 | Resorcinol, 3-aminophenol | 1432 | 7 | Resorcinol: 407 3-aminophenol: 454 | [192] |
| Durian-peel ACs | H2SO4 | Bisphenol A | - | - | 4.2 | [192] |
| Argan-nutshell ACs | H3PO4 | Bisphenol A | 1372 | - | 1250 | [191] |
| Elimination of Pesticides by ACs | ||||||
| Activated Carbon Sources | Activator | Adsorbate | SBET (m2/g) | pH | Qm (mg/g) | Ref. |
| Coconut charcoal | Methanol | Monocrotophos | 79.4 | 7 | 103.9 | [200] |
| Navel orange (Citrus sinensis L.) | H3PO4 | Chlorpyrifos | 94.26 | 6.8 | - | [201] |
| Dates (Phoenix dactylifera L.) | H3PO4 | Chlorpyrifos | 111.75 | 6.8 | - | [201] |
| Pomegranates (Punicagranatum L.) | H3PO4 | Chlorpyrifos | 183.89 | 6.8 | - | [201] |
| Bananas (Musa sp.) | H3PO4 | Chlorpyrifos | 289.86 | 6.8 | - | [201] |
| Date stones | Steam | Aldrin | - | - | 373.23 | [187] |
| Date stones | Steam | Dieldrin | - | - | 295.30 | [187] |
| Date stones | Steam | Endrin | - | - | 228.05 | [187] |
| Date seeds | CO2/KOH | Bentazon | 1322 | 5.5 | 86.26 | [188] |
| Date seeds | CO2/KOH | Carbofuran | - | - | 135.14 | [186] |
| Date seeds | CO2/KOH | Bentazon | - | - | 78.13 | [186] |
| Date seeds | CO2/KOH | Dichlorophenoxyacetic acid | - | 3.6 | 238.10 | [69] |
| Olive kernels | Steam | Bromopropylate | 600 | - | Up to 100% removal | [189] |
| Soya stalks | Steam | Bromopropylate | 570 | - | Up to 100% removal | [189] |
| Rapeseed stalks | Steam | Bromopropylate | 490 | - | Up to 100% removal | [189] |
3.1.5. Pharmaceutical Component Removal
3.2. Carbon Nanotubes
3.2.1. Dye Removal
3.2.2. Heavy Metal Removal
3.2.3. Pathogen Removal
3.2.4. Removal of Other Contaminants
3.3. Graphene-Based Materials
3.3.1. Heavy Metal Removal
3.3.2. Organic Pollutants
3.3.3. Pharmaceutical Compound Removal
3.3.4. Other Pollutants
4. Energy Storage
4.1. Activated Carbons in Energy Storage
4.2. Carbon Nanotubes for Energy Storage
4.3. Graphene for Energy Storage
5. Carbon Dioxide (CO2) Capture
5.1. Activated Carbon for CO2 Capture
5.2. Carbon Nanotubes for CO2 Capture
5.3. Graphene
6. Recommendations for Future Directions
- i.
- Carbonaceous materials have so many allotropes with different applications. These allotrope materials can be used for sensitive applications, such as fuel cells, medicine, cosmetics, catalysts, etc. Similarly, other carbonaceous materials (fullerene, carbon onions, peapods, nanohoms, etc.) can also be investigated.
- ii.
- The applications of activated carbons are concentrated mostly in water treatment, whereas the applications of ACs in energy storage and CO2 capture are relatively few and can be further improved. Applications of activated carbons for CO2 adsorption from soil can amend soil quality by reducing greenhouse gases.
- iii.
- Improving the surface properties of carbon nanotubes for precise organic matter adsorption may be a major research project in the near future to further improve water treatment. The degree of oxidation of the surface of carbonaceous adsorbents should be considered since the actual surface areas of CNTs are about 1000 m2/g (the theoretical maximum is 1315 m2/g).
- iv.
- Surface hydrophobicity and fast agglomeration in aqueous solutions are two major drawbacks of graphene, limiting its adsorption potential in practical applications. Therefore, the performance of graphene can be improved by functionalizing it to overcome these shortcomings. Moreover, surfactants can be used to change the surfaces of hydrophobic materials.
- v.
- The reduction of graphene oxide to produce graphene is a promising field for low-cost graphene production on a large scale in the future. Furthermore, the less costly graphene manufacturing technology has made little progress. As a result, new graphene synthesis techniques will increase the range of potential applications and enable the development of new green synthesis methods to regenerate sustainable resources.
- vi.
- Carbonaceous materials are currently being employed as catalysts in fuel cells. The improved performance of these catalysts in comparison to an industry standard may be developed by overcoming the weaknesses of the present catalytic technology and boosting lifespan to fulfill the durability criterion.
- vii.
- Comparisons of these carbonaceous materials can be evaluated in terms of characteristics, advantages, and disadvantages for a specific application.
- viii.
- Additional modifications to activated carbons, graphene, and carbon nanotubes may be made to improve properties while lowering costs and improving efficiency, and environmentally friendly paths will be built to open new trails based on the attributes, allowing for more research into highly replicable real-time applications.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and Graphene Oxide: Biofunctionalization and Applications in Biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Azad, A.K.; Bakar, M.S.A.; Karim, M.R.; Sharifpur, M.; Taweekun, J. Evaluation of Thermochemical Characteristics and Pyrolysis of Fish Processing Waste for Renewable Energy Feedstock. Sustainability 2022, 14, 1203. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Amin, M.R.; Taweekun, J.; Azad, A.K. Progress in Nanomaterials Fabrication and Their Prospects in Artificial Intelligence towards Solid Oxide Fuel Cells: A Review. Int. J. Hydrogen Energy, 2022, in press. [CrossRef]
- Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability 2023, 15, 1643. [Google Scholar] [CrossRef]
- Syazaidah, I.; Abu Bakar, M.S.; Reza, M.S.; Azad, A.K. Ex-Situ Catalytic Pyrolysis of Chicken Litter for Bio-Oil Production: Experiment and Characterization. J. Environ. Manag. 2021, 297, 113407. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of Food Waste, Fish Waste and Food Processing Waste for China’s Aquaculture Industry: Needs and Challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Lin, X.; Zhang, X.; Wu, D.; Matyjaszewski, K. Emerging Functional Porous Polymeric and Carbonaceous Materials for Environmental Treatment and Energy Storage. Adv. Funct. Mater. 2020, 30, 1907006. [Google Scholar] [CrossRef]
- Gupta, T. Carbon the Black, the Gray and the Transparent; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319664057. [Google Scholar]
- Mangler, C.; Meyer, J.C. Using Electron Beams to Investigate Carbonaceous Materials. C. R. Phys. 2014, 15, 241–257. [Google Scholar] [CrossRef]
- Sheoran, K.; Thakur, V.K.; Siwal, S.S. Synthesis and Overview of Carbon-Based Materials for High Performance Energy Storage Application: A Review. Mater. Today Proc. 2022, 56, 9–17. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated Carbon, Carbon Nanotubes and Graphene: Materials and Composites for Advanced Water Purification. C 2017, 3, 18. [Google Scholar] [CrossRef]
- Nazal, M.K.; Nazal, M.K. An Overview of Carbon-Based Materials for the Removal of Pharmaceutical Active Compounds. In Carbon-Based Material for Environmental Protection and Remediation; IntechOpen: London, UK, 2020; pp. 1–19. ISBN 978-1-78984-587-7. [Google Scholar]
- Dhandapani, E.; Thangarasu, S.; Ramesh, S.; Ramesh, K.; Vasudevan, R.; Duraisamy, N. Recent Development and Prospective of Carbonaceous Material, Conducting Polymer and Their Composite Electrode Materials for Supercapacitor—A Review. J. Energy Storage 2022, 52, 104937. [Google Scholar] [CrossRef]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of Activated Carbon from Biomass and Its’ Applications in Water and Gas Purification, a Review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Marco-Lozar, J.P.; Kunowsky, M.; Suárez-García, F.; Linares-Solano, A. Sorbent Design for CO2 Capture under Different Flue Gas Conditions. Carbon 2014, 72, 125–134. [Google Scholar] [CrossRef]
- Hussain, A.; Mehdi, S.M.; Abbas, N.; Hussain, M.; Naqvi, R.A. Synthesis of Graphene from Solid Carbon Sources: A Focused Review. Mater. Chem. Phys. 2020, 248, 122924. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-Based Nano Composites and Their Applications. A Review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.H. A Review of the Electrical and Mechanical Properties of Carbon Nanofiller-Reinforced Polymer Composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- ullah Rather, S. Preparation, Characterization and Hydrogen Storage Studies of Carbon Nanotubes and Their Composites: A Review. Int. J. Hydrogen Energy 2020, 45, 4653–4672. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of Dye Molecules from Aqueous Solution by Carbon Nanotubes and Carbon Nanotube Functional Groups: Critical Review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon Nanotubes in Li-Ion Batteries: A Review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P.; Singh, G.; Khandai, M.; Sarangi, R.R.; Kar, M.K. Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges. C 2022, 9, 3. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Amin, R.; Kumar, P.R.; Belharouak, I.; Amin, R.; Kumar, P.R.; Belharouak, I. Carbon Nanotubes: Applications to Energy Storage Devices. In Carbon Nanotubes—Redefining the World of Electronics; IntechOpen: London, UK, 2020; pp. 1–23. ISBN 978-1-83881-185-3. [Google Scholar]
- Halada, Š.; Zlatník, J.; Mazúr, P.; Charvát, J.; Slouka, Z. Fast Screening of Carbon-Based Nanostructured Materials as Potential Electrode Materials for Vanadium Redox Flow Battery. Electrochim. Acta 2022, 430, 141043. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z. Benefits of Using Carbon Nanotubes in Fuel Cells: A Review. Int. J. Energy Res. 2017, 41, 92–102. [Google Scholar] [CrossRef]
- Shukrullah, S.; Mohamed, N.M.; Shaharun, M.S.; Ullah, S.; Naz, M.Y. Effective CO2 Adsorption on Pristine and Chemically Functionalized MWCNTs. In Proceedings of the 4th International Conference on Fundamental and Applied Sciences (ICFAS2016), Kuala Lumpur, Malaysia, 15–17 August 2016; Volume 1787, p. 50025. [Google Scholar]
- Hashim, H.; Shukor Salleh, M.; Zaidi Omar, M. Homogenous Dispersion and Interfacial Bonding of Carbon Nanotube Reinforced with Aluminum Matrix Composite: A Review. Rev. Adv. Mater. Sci. 2019, 58, 295–303. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A. Electrochemical Carbon Based Nanosensors: A Promising Tool in Pharmaceutical and Biomedical Analysis. J. Pharm. Biomed. Anal. 2018, 147, 439–457. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Y.; Lu, X.; Xie, J. Fullerenes for Rechargeable Battery Applications: Recent Developments and Future Perspectives. J. Energy Chem. 2021, 55, 70–79. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable Activated Carbons from Dead Ginkgo Leaves for Supercapacitor Electrode Active Materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of Energy Storage in Renewable Energy Systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A Review of Carbon Materials for Supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Giri, A.; Bharti, V.K.; Kalia, S.; Arora, A.; Balaje, S.S.; Chaurasia, O.P. A Review on Water Quality and Dairy Cattle Health: A Special Emphasis on High-Altitude Region. Appl. Water Sci. 2020, 10, 79. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of Plant Based Biosorbents in the Removal of Heavy Metals: A Review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Tahmid, M.; Shoronika, A.Z.; Fariha, A.; Roy, H.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. A Comprehensive Review on the Sustainable Treatment of Textile Wastewater: Zero Liquid Discharge and Resource Recovery Perspectives. Sustainability 2022, 14, 15398. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.B.M.K.; Afroze, S.; Muhammad, S.; Bakar, A.; Taweekun, J.; Azad, A.K. Analysis on Preparation, Application, and Recycling of Activated Carbon to Aid in COVID-19 Protection. Int. J. Integr. Eng. 2020, 12, 233–244. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Hashim, R.; Hafiz, M.R.; Ghazali, A.; Sulaiman, O.; Hiziroglu, S. Characterization and Adsorption Kinetic Study of Surfactant Treated Oil Palm (Elaeis guineensis) Empty Fruit Bunches. Desalin. Water Treat. 2016, 57, 9474–9487. [Google Scholar] [CrossRef]
- Thekkudan, V.N.; Vaidyanathan, V.K.; Ponnusamy, S.K.; Charles, C.; Sundar, S.; Vishnu, D.; Anbalagan, S.; Vaithyanathan, V.K.; Subramanian, S. Review on Nanoadsorbents: A Solution for Heavy Metal Removal from Wastewater. IET Nanobiotechnol. 2017, 11, 213–224. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol. 2012, 2, 154–158. [Google Scholar] [CrossRef]
- Water Pollution. Available online: http://www.informaction.org/index.php?menu=menua.txt&main=waterpol_gen.txt&s=Water+sewage (accessed on 20 September 2022).
- UNenvironment Greenhouse Gases Are Depriving Our Oceans of Oxygen. Available online: https://www.unenvironment.org/news-and-stories/story/greenhouse-gases-are-depriving-our-oceans-oxygen (accessed on 10 December 2022).
- Chiang, Y.C.; Juang, R.S. Surface Modifications of Carbonaceous Materials for Carbon Dioxide Adsorption: A Review. J. Taiwan Inst. Chem. Eng. 2017, 71, 214–234. [Google Scholar] [CrossRef]
- CO2 Emissions by Year—Worldometer. Available online: https://www.worldometers.info/co2-emissions/co2-emissions-by-year/ (accessed on 22 September 2020).
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous Materials as Adsorbents for CO2 Capture: Synthesis and Modification. Carbon Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.B.M.K.; Ahmed, A.S.; Afroze, S.; Bakar, M.S.A.; Islam, S.N.; Azad, A.K. COVID-19 Prevention: Role of Activated Carbon. J. Eng. Technol. Sci. 2021, 53, 210404. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Q.; Jiao, T.; Zhang, Z.; Wang, S.; Sun, Q.; Gao, F. Rationally Designed Porous Polystyrene Encapsulated Zirconium Phosphate Nanocomposite for Highly Efficient Fluoride Uptake in Waters. Sci. Rep. 2013, 3, 2551. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Liu, Y.; Liang, J.; Tang, J. Renewable Phenols Production by Catalytic Microwave Pyrolysis of Douglas Fir Sawdust Pellets with Activated Carbon Catalysts. Bioresour. Technol. 2013, 142, 546–552. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Adsorption of Cephalexin onto Activated Carbons from Albizia Lebbeck Seed Pods by Microwave-Induced KOH and K2CO3 Activations. Chem. Eng. J. 2012, 211–212, 200–207. [Google Scholar] [CrossRef]
- Chayid, M.A.; Ahmed, M.J. Amoxicillin Adsorption on Microwave Prepared Activated Carbon from Arundo Donax Linn: Isotherms, Kinetics, and Thermodynamics Studies. J. Environ. Chem. Eng. 2015, 3, 1592–1601. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from Microwave Pyrolysis of Biomass: A Review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Gayathiri, M.; Pulingam, T.; Lee, K.T.; Sudesh, K. Activated Carbon from Biomass Waste Precursors: Factors Affecting Production and Adsorption Mechanism. Chemosphere 2022, 294, 133764. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Afroze, S.; Azad, A.K.; Sukri, R.S.; Shams, S.; Taweekun, J.; Saghir, M.; Phusunti, N.; Bakar, M.S.A. Thermochemical Characterization of Invasive Axonopus Compressus Grass as a Renewable Energy Source. In Proceedings of the 5th International Conference of Chemical Engineering and Industrial Biotechnology (ICCEIB 2020), Kuala Lumpur, Malaysia, 9–11 August 2020; pp. 1–6. [Google Scholar]
- Reza, M.S.; Islam, S.N.; Afroze, S.; Abu Bakar, M.S.; Sukri, R.S.; Rahman, S.; Azad, A.K.; Bakar, M.S.A.; Sukri, R.S.; Rahman, S.; et al. Evaluation of the Bioenergy Potential of Invasive Pennisetum Purpureum through Pyrolysis and Thermogravimetric Analysis. Energy Ecol. Environ. 2020, 5, 118–133. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.A.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar Characterization of Invasive Pennisetum Purpureum Grass: Effect of Pyrolysis Temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Taweekun, J.; Ja’afar, F.; Bakar, M.S.A.; Azad, A.K.; Roy, H.; et al. Ex Situ Catalytic Pyrolysis of Invasive Pennisetum Purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability 2023, 15, 7628. [Google Scholar] [CrossRef]
- Nguyen, M.-V.; Lee, B.-K. A Novel Removal of CO2 Using Nitrogen Doped Biochar Beads as a Green Adsorbent. Process Saf. Environ. Prot. 2016, 104, 490–498. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Silvestre-Albero, J. Nanoporous Materials for Gas Storage; Springer: Singapore, 2019; ISBN 9789811335037. [Google Scholar]
- Wang, C.; Chen, P.; Li, Z. Progress in Preparation and Application of Organic Waste Based Activated Carbon. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 042004. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar Pyrolytically Produced from Municipal Solid Wastes for Aqueous As(V) Removal: Adsorption Property and Its Improvement with KOH Activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Burhenne, L.; Aicher, T. Benzene Removal over a Fixed Bed of Wood Char: The Effect of Pyrolysis Temperature and Activation with CO2 on the Char Reactivity. Fuel Process. Technol. 2014, 127, 140–148. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Gil, M.V.; Volperts, A.; Centeno, T.A. Production of Nanoporous Carbons from Wood Processing Wastes and Their Use in Supercapacitors and CO2 Capture. Biomass Bioenergy 2012, 46, 145–154. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of Activated Carbons from Date (Phoenix dactylifera L.) Palm Stones and Application for Wastewater Treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of Carbonaceous Adsorbents from Lignocellulosic Biomass and Their Use in Removal of Contaminants from Aqueous Solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of Agricultural Based Activated Carbons by Microwave and Conventional Activations for Basic Dye Adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Q.; Liu, J.; Yan, H.; Wei, Y. Pilot-Scale Study of Sludge Pretreatment by Microwave and Sludge Reduction Based on Lysis–cryptic Growth. Bioresour. Technol. 2015, 190, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Vinu, R. Peroxide-Assisted Microwave Activation of Pyrolysis Char for Adsorption of Dyes from Wastewater. Bioresour. Technol. 2016, 216, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Izhar, T.; Nayak, J.; Mumtaz, N. Comparative Study between Pre-Engineered RCC Structure and Usual RCC Structure. Int. J. Sci. Res. Dev. 2017, 5, 1714–1717. [Google Scholar]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave Assisted Preparation of Activated Carbon from Biomass: A Review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Abraham, J.; Thomas, S.; Kalarikkal, N. Handbook of Carbon Nanotubes; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-91346-5. [Google Scholar]
- Sawant, S.V.; Patwardhan, A.W.; Joshi, J.B.; Dasgupta, K. Boron Doped Carbon Nanotubes: Synthesis, Characterization and Emerging Applications—A Review. Chem. Eng. J. 2022, 427, 131616. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Production of Carbon Nanotubes on Bio-Char at Low Temperature via Microwave-Assisted CVD Using Ni Catalyst. Diam. Relat. Mater. 2019, 91, 98–106. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon Nanotube- and Graphene-Based Nanomaterials and Applications in High-Voltage Supercapacitor: A Review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Singh, N.P.; Gupta, V.K.; Singh, A.P. Graphene and Carbon Nanotube Reinforced Epoxy Nanocomposites: A Review. Polymer 2019, 180, 121724. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Janas, D. From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes. Sustainability 2020, 12, 4115. [Google Scholar] [CrossRef]
- Szabó, A.; Perri, C.; Csató, A.; Giordano, G.; Vuono, D.; Nagy, J.B. Synthesis Methods of Carbon Nanotubes and Related Materials. Materials 2010, 3, 3092–3140. [Google Scholar] [CrossRef]
- Mahto, R.K.; Kumar, S.; Mahto, R.K.; Kumar, S. Synthesis and Characterization of Low Dimensional Structure of Carbon Nanotubes. Int. J. Sci. Res. Arch. 2022, 7, 571–582. [Google Scholar] [CrossRef]
- Ren, Z.; Lan, Y.; Wang, Y. Aligned Carbon Nanotubes: Physics, Concepts, Fabrication and Devices; Springer: Berlin, Germany, 2013; Volume 5. [Google Scholar] [CrossRef]
- Rafique, M.M.A.; Iqbal, J. Production of Carbon Nanotubes by Different Routes-A Review. J. Encapsulation Adsorpt. Sci. 2011, 1, 29–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent Advanced Thermal Interfacial Materials: A Review of Conducting Mechanisms and Parameters of Carbon Materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Torres, T. Carbon Nanotubes and Related Structures. Synthesis, Characterization, Functionalization, and Applications. Edited by Dirk M. Guldi and Nazario Martín. Angew. Chem. Int. Ed. 2011, 50, 1473–1474. [Google Scholar] [CrossRef]
- Mirabootalebi, S.O.; Akbari, G.H. Methods for Synthesis of Carbon Nanotubes-Review. Int. J. Bio-Inorg. Hybr. Nanomater. 2017, 6, 49–57. [Google Scholar]
- Choudhury, S.; Paul, S.; Goswami, S.; Deb, K. Methods for Nanoparticle Synthesis and Drug Delivery. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 21–44. ISBN 9780323884501. [Google Scholar]
- Abdullayeva, S.H. Characterization of High Quality Carbon Nanotubes Synthesized via Aerosol-CVD. J. Adv. Phys. 2015, 11, 3229–3240. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; He, M.; Li, X.; Chen, H. Synthesis of High-Quality Carbon Nanotube Arrays without the Assistance of Water. J. Nanomater. 2012, 2012, 542582. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, J.; Zhang, J.; Gu, L.; Yang, Z.; Li, X.; Yu, H.; Zhu, X.; Yang, R.; Shi, D.; et al. Oxygen-Assisted Chemical Vapor Deposition Growth of Large Single-Crystal and High-Quality Monolayer MoS2. J. Am. Chem. Soc. 2015, 137, 15632–15635. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Manafi, S.; Rahimipour, M.R.; Mobasherpour, I.; Soltanmoradi, A. The Synthesis of Peculiar Structure of Springlike Multiwall Carbon Nanofibers/nanotubes via Mechanothermal Method. J. Nanomater. 2012, 2012, 803546. [Google Scholar] [CrossRef]
- Kudiyarov, V.N.; Elman, R.R.; Kurdyumov, N.E. The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes. Metals 2021, 11, 1409. [Google Scholar] [CrossRef]
- Herrera-Ramirez, J.M.; Perez-Bustamante, R.; Aguilar-Elguezabal, A. An Overview of the Synthesis, Characterization, and Applications of Carbon Nanotubes. In Carbon-Based Nanofillers and Their Rubber Nanocomposites: Carbon Nano-Objects; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–75. [Google Scholar] [CrossRef]
- Dutta, S.D.; Lim, K.T.; Patel, D.K. Carbon Nanotube-Based Nanohybrids for Agricultural and Biological Applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–535. ISBN 9780128213544. [Google Scholar]
- Zhao, G.; Liu, H.Y.; Du, X.; Zhou, H.; Pan, Z.; Mai, Y.W.; Jia, Y.Y.; Yan, W. Flame Synthesis of Carbon Nanotubes on Glass Fibre Fabrics and Their Enhancement in Electrical and Thermal Properties of Glass Fibre/epoxy Composites. Compos. Part B Eng. 2020, 198, 108249. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Ji, D.; Liu, Y.; Li, L.; Yuan, D.; Zhang, Z.; Ren, J.; Lefler, M.; Wang, B.; et al. One-Pot Synthesis of Nanostructured Carbon Materials from Carbon Dioxide via Electrolysis in Molten Carbonate Salts. Carbon 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc Discharge Synthesis of Carbon Nanotubes: Comprehensive Review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Bhagabati, P.; Rahaman, M.; Bhandari, S.; Roy, I.; Dey, A.; Gupta, P.; Ansari, M.A.; Dutta, A.; Chattopadhyay, D. Synthesis/Preparation of Carbon Materials. In Carbon-Containing Polymer Composites; Springer: Singapore, 2019; pp. 1–64. [Google Scholar]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Jariwala, D.; Srivastava, A.; Ajayan, P.M. Graphene Synthesis and Band Gap Opening. J. Nanosci. Nanotechnol. 2011, 11, 6621–6641. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K. (Eds.) Graphene: Synthesis, Properties, and Phenomena; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 9783527651122. [Google Scholar]
- Lim, J.Y.; Mubarak, N.M.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M. Inamuddin Recent Trends in the Synthesis of Graphene and Graphene Oxide Based Nanomaterials for Removal of Heavy Metals—A Review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Santhiran, A.; Iyngaran, P.; Abiman, P.; Kuganathan, N.; Bellucci, S. Graphene Synthesis and Its Recent Advances in Applications—A Review. C 2021, 7, 76. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-Reduction of Graphite- and Graphene Oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Gnanaseelan, M.; Samanta, S.; Pionteck, J.; Jehnichen, D.; Simon, F.; Pötschke, P.; Voit, B. Vanadium Salt Assisted Solvothermal Reduction of Graphene Oxide and the Thermoelectric Characterisation of the Reduced Graphene Oxide in Bulk and as Composite. Mater. Chem. Phys. 2019, 229, 319–329. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.D.P.; Romero, A.; Garrido, J.; Sanchez-Silva, L.; Valverde, J.L. Influence of Different Improved Hummers Method Modifications on the Characteristics of Graphite Oxide in Order to Make a More Easily Scalable Method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; ullah Khan, A. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. Advances in Synthesis of Graphene Derivatives Using Industrial Wastes Precursors; Prospects and Challenges. J. Mater. Res. Technol. 2020, 9, 15924–15951. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A Comprehensive Review on Selected Graphene Synthesis Methods: From Electrochemical Exfoliation through Rapid Thermal Annealing towards Biomass Pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Tetlow, H.; Posthuma de Boer, J.; Ford, I.J.; Vvedensky, D.D.; Coraux, J.; Kantorovich, L. Growth of Epitaxial Graphene: Theory and Experiment. Phys. Rep. 2014, 542, 195–295. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Chen, S. Large Area CVD Growth of Graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, L.; Ren, H.; Sun, X. CVD Synthesis of Graphene. In Thermal Transport in Carbon-Based Nanomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 19–56. ISBN 9780323473460. [Google Scholar]
- Chaste, J.; Saadani, A.; Jaffre, A.; Madouri, A.; Alvarez, J.; Pierucci, D.; Ben Aziza, Z.; Ouerghi, A. Nanostructures in Suspended Mono- and Bilayer Epitaxial Graphene. Carbon 2017, 125, 162–167. [Google Scholar] [CrossRef]
- Fogarassy, Z.; Rümmeli, M.H.; Gorantla, S.; Bachmatiuk, A.; Dobrik, G.; Kamarás, K.; Biró, L.P.; Havancsák, K.; Lábár, J.L. Dominantly Epitaxial Growth of Graphene on Ni (1 1 1) Substrate. Appl. Surf. Sci. 2014, 314, 490–499. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Chai, Z.; Hu, W. Low-Temperature Plasma Synthesis of Carbon Nanotubes and Graphene Based Materials and Their Fuel Cell Applications. Chem. Soc. Rev. 2013, 42, 8821–8834. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Li, X.; Yang, J.; Du, C.; Shen, W.; Yan, J. Upcycling Waste Lard Oil into Vertical Graphene Sheets by Inductively Coupled Plasma Assisted Chemical Vapor Deposition. Nanomaterials 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, A.; Abro, R.; Kandhro, A.; Khaskheli, A.R.; Jalbani, N.; Gishkori, K.A.; Mahar, A.M.; Qaisar, S.; Panhwar, A.; Abro, R.; et al. Global Water Mapping, Requirements, and Concerns over Water Quality Shortages. In Water Quality—New Perspectives [Working Title]; IntechOpen: Rijeka, Croatia, 2022; ISBN 978-1-83969-010-5. [Google Scholar]
- Bottero, M.; Abastante, F.; Wambui Mumbi, A.; Watanabe, T. Cost Estimations of Water Pollution for the Adoption of Suitable Water Treatment Technology. Sustainability 2022, 14, 649. [Google Scholar] [CrossRef]
- Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 28 April 2023).
- Pyrzynska, K. Removal of Cadmium from Wastewaters with Low-Cost Adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Alcamo, J.; Henrichs, T.; Rösch, T. World Water in 2025: Global Modeling and Scenario Analysis for the World Commission on Water for the 21st Century; Center for Environmental Systems Research University of Kassel: Kassel, Germany, 2017. [Google Scholar]
- Thamilselvi, V.; Radha, K.V. Silver Nanoparticle Loaded Corncob Adsorbent for Effluent Treatment. J. Environ. Chem. Eng. 2017, 5, 1843–1854. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lee, S.S.; Seo, D.-C.; Tsang, D.C.W.; Ok, Y.S. Steam Activation of Biochars Facilitates Kinetics and pH-Resilience of Sulfamethazine Sorption. J. Soils Sediments 2016, 16, 889–895. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated Carbon Derived from Carbon Residue from Biomass Gasification and Its Application for Dye Adsorption: Kinetics, Isotherms and Thermodynamic Studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Boateng, L.K.; Flora, J.R.V.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive Adsorption of Selected Non-Steroidal Anti-Inflammatory Drugs on Activated Biochars: Experimental and Molecular Modeling Study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Ang, T.N.; Young, B.R.; Burrell, R.; Taylor, M.; Aroua, M.K.; Baroutian, S. Oxidative Hydrothermal Surface Modification of Activated Carbon for Sevoflurane Removal. Chemosphere 2021, 264, 128535. [Google Scholar] [CrossRef] [PubMed]
- Kristiana, I.; Joll, C.; Heitz, A. Powdered Activated Carbon Coupled with Enhanced Coagulation for Natural Organic Matter Removal and Disinfection by-Product Control: Application in a Western Australian Water Treatment Plant. Chemosphere 2011, 83, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Loganathan, P.; Listowski, A.; Kandasamy, J.; Khourshed, C.; Vigneswaran, S. Simultaneous Removal of Natural Organic Matter and Micro-Organic Pollutants from Reverse Osmosis Concentrate Using Granular Activated Carbon. Water Res. 2019, 155, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Phonlam, T.; Weerasuk, B.; Sataman, P.; Duangmanee, T.; Thongphanit, S.; Nilgumhang, K.; Anantachaisilp, S.; Chutimasakul, T.; Kwamman, T.; Chobpattana, V. Ammonia Modification of Activated Carbon Derived from Biomass via Gamma Irradiation vs. Hydrothermal Method for Methylene Blue Removal. S. Afr. J. Chem. Eng. 2023, 43, 67–78. [Google Scholar] [CrossRef]
- Li, J.; Lv, F.; Yang, R.; Zhang, L.; Tao, W.; Liu, G.; Gao, H.; Guan, Y. N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application. Polymers 2022, 14, 3889. [Google Scholar] [CrossRef] [PubMed]
- Iwar, R.T.; Ogedengbe, K.; Katibi, K.K.; Oshido, L.E. Meso-Microporous Activated Carbon Derived from Raffia Palm Shells: Optimization of Synthesis Conditions Using Response Surface Methodology. Heliyon 2021, 7, e07301. [Google Scholar] [CrossRef]
- Rattanapan, S.; Srikram, J.; Kongsune, P. Adsorption of Methyl Orange on Coffee Grounds Activated Carbon. Energy Procedia 2017, 138, 949–954. [Google Scholar] [CrossRef]
- Nuithitikul, K.; Srikhun, S.; Hirunpraditkoon, S. Kinetics and Equilibrium Adsorption of Basic Green 4 Dye on Activated Carbon Derived from Durian Peel: Effects of Pyrolysis and Post-Treatment Conditions. J. Taiwan Inst. Chem. Eng. 2010, 41, 591–598. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Porkodi, K.; Varadarajan, P.R.; Subburaam, C.V. Adsorption of Dissolved Reactive Red Dye from Aqueous Phase onto Activated Carbon Prepared from Agricultural Waste. Bioresour. Technol. 2006, 97, 1618–1625. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Equilibrium, Kinetics and Mechanism of Malachite Green Adsorption on Activated Carbon Prepared from Bamboo by K2CO3 Activation and Subsequent Gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Loh, M.M.; Aziz, J.A. Preparation and Characterization of Activated Carbon from Oil Palm Wood and Its Evaluation on Methylene Blue Adsorption. Dye Pigment 2007, 75, 263–272. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Martins, A.C.; Moraes, J.C.G.; Garcia, E.E.; Gauze, G.F.; Costa, W.F.; Almeida, V.C. Kinetic and Equilibrium Studies: Adsorption of Food Dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on Activated Carbon from Flamboyant Pods. Chem. Eng. J. 2012, 181–182, 243–250. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Flores, E.D.; Genuino, D.A.D.; Futalan, C.M.; Wan, M.W. Adsorption of Eriochrome Black T (EBT) Dye Using Activated Carbon Prepared from Waste Rice Hulls-Optimization, Isotherm and Kinetic Studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 646–653. [Google Scholar] [CrossRef]
- Thinakaran, N.; Panneerselvam, P.; Baskaralingam, P.; Elango, D.; Sivanesan, S. Equilibrium and Kinetic Studies on the Removal of Acid Red 114 from Aqueous Solutions Using Activated Carbons Prepared from Seed Shells. J. Hazard. Mater. 2008, 158, 142–150. [Google Scholar] [CrossRef]
- Uma; Banerjee, S.; Sharma, Y.C. Equilibrium and Kinetic Studies for Removal of Malachite Green from Aqueous Solution by a Low Cost Activated Carbon. J. Ind. Eng. Chem. 2013, 19, 1099–1105. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium Isotherms and Kinetics Modeling of Methylene Blue Adsorption on Agricultural Wastes-Based Activated Carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Kaouah, F.; Boumaza, S.; Berrama, T.; Trari, M.; Bendjama, Z. Preparation and Characterization of Activated Carbon from Wild Olive Cores (Oleaster) by H3PO4 for the Removal of Basic Red 46. J. Clean. Prod. 2013, 54, 296–306. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and Characterization of Activated Carbon from Pineapple Waste Biomass for Dye Removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Kumar, M.; Tamilarasan, R. Modeling Studies for the Removal of Methylene Blue from Aqueous Solution Using Acacia Fumosa Seed Shell Activated Carbon. J. Environ. Chem. Eng. 2013, 1, 1108–1116. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Using of Activated Carbon Produced from Spent Tea Leaves for the Removal of Malachite Green from Aqueous Solution. Ecol. Eng. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Angin, D. Utilization of Activated Carbon Produced from Fruit Juice Industry Solid Waste for the Adsorption of Yellow 18 from Aqueous Solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ng, D.H.L.; Song, P.; Kong, C.; Song, Y. Synthesis of SnO2-Activated Carbon Fiber Hybrid Catalyst for the Removal of Methyl Violet from Water. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 194, 1–8. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A. Adsorptive Removal of an Acid Dye by Lignocellulosic Waste Biomass Activated Carbon: Equilibrium and Kinetic Studies. Chemosphere 2011, 82, 1367–1372. [Google Scholar] [CrossRef]
- Hajati, S.; Ghaedi, M.; Yaghoubi, S. Local, Cheep and Nontoxic Activated Carbon as Efficient Adsorbent for the Simultaneous Removal of Cadmium Ions and Malachite Green: Optimization by Surface Response Methodology. J. Ind. Eng. Chem. 2015, 21, 760–767. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.-J.; Gaspard, S. Adsorption Studies of Methylene Blue and Phenol onto Vetiver Roots Activated Carbon Prepared by Chemical Activation. J. Hazard. Mater. 2009, 165, 1029–1039. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of Malachite Green from Aqueous Solution by Activated Carbon Prepared from the Epicarp of Ricinus Communis by Adsorption. J. Hazard. Mater. 2010, 179, 178–186. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Sillanpää, M.; Khan, M.A.; Mubarak, N.M.; Tan, Y.H. Rapid Adsorptive Removal of Chromium from Wastewater Using Walnut—Derived Biosorbents. Sci. Rep. 2023, 13, 6859. [Google Scholar] [CrossRef]
- Bumajdad, A.; Hasila, P. Surface Modification of Date Palm Activated Carbonaceous Materials for Heavy Metal Removal and CO2 Adsorption. Arab. J. Chem. 2023, 16, 104403. [Google Scholar] [CrossRef]
- Jimenez-Paz, J.; Lozada-Castro, J.J.; Lester, E.; Williams, O.; Stevens, L.; Barraza-Burgos, J. Solutions to Hazardous Wastes Issues in the Leather Industry: Adsorption of Chromium Iii and vi from Leather Industry Wastewaters Using Activated Carbons Produced from Leather Industry Solid Wastes. J. Environ. Chem. Eng. 2023, 11, 109715. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Elhadidy, H. Adsorption of Arsenic by Activated Carbon, Calcium Alginate and Their Composite Beads. Int. J. Biol. Macromol. 2014, 68, 125–130. [Google Scholar] [CrossRef] [PubMed]
- St. Vassileva, P.; Detcheva, A. Adsorption of Some Transition Metal Ions [Cu(II), Fe(III), Cr(III) and Au(III)] onto Lignite-Based Activated Carbons Modified by Oxidation. Adsorpt. Sci. Technol. 2010, 28, 229–242. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of Copper (II) and Lead (II) Ions from Aqueous Solutions by Adsorption on Activated Carbon from a New Precursor Hazelnut Husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Giraldo-Gutiérrez, L.; Moreno-Piraján, J.C. Pb(II) and Cr(VI) Adsorption from Aqueous Solution on Activated Carbons Obtained from Sugar Cane Husk and Sawdust. J. Anal. Appl. Pyrolysis 2008, 81, 278–284. [Google Scholar] [CrossRef]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive Adsorption of Lead and Zinc from Aqueous Solution on Activated Carbon Prepared from Van Apple Pulp: Study in Single- and Multi-Solute Systems. Chem. Eng. J. 2012, 200–202, 224–236. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Lin, H.; Chen, J. Fabrication and Characterization of Mesoporous Activated Carbon from Lemna Minor Using One-Step H3PO4 Activation for Pb(II) Removal. Appl. Surf. Sci. 2014, 317, 422–431. [Google Scholar] [CrossRef]
- Boudrahem, F.; Aissani-Benissad, F.; Aït-Amar, H. Batch Sorption Dynamics and Equilibrium for the Removal of Lead Ions from Aqueous Phase Using Activated Carbon Developed from Coffee Residue Activated with Zinc Chloride. J. Environ. Manag. 2009, 90, 3031–3039. [Google Scholar] [CrossRef]
- Sugashini, S.; Begum, K.M.M.S. Preparation of Activated Carbon from Carbonized Rice Husk by Ozone Activation for Cr(VI) Removal. New Carbon Mater. 2015, 30, 252–261. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, İ.; Tümsek, F.; Karabacakoğlu, B. Adsorption of chromium(VI) from Aqueous Solution by Activated Carbon Derived from Olive Bagasse and Applicability of Different Adsorption Models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of Hexavalent Chromium from Aqueous Solution by Activated Carbon Prepared from Longan Seed: Kinetics, Equilibrium and Thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- El Nemr, A.; Khaled, A.; Abdelwahab, O.; El-Sikaily, A. Treatment of Wastewater Containing Toxic Chromium Using New Activated Carbon Developed from Date Palm Seed. J. Hazard. Mater. 2008, 152, 263–275. [Google Scholar] [CrossRef] [PubMed]
- AL-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent Chromium Removal from Aqueous Medium by Activated Carbon Prepared from Peanut Shell: Adsorption Kinetics, Equilibrium and Thermodynamic Studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Zabihi, M.; Haghighi Asl, A.; Ahmadpour, A. Studies on Adsorption of Mercury from Aqueous Solution on Activated Carbons Prepared from Walnut Shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, S.; Sohrabi, R.; Javadian, H.; Ghasemi, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Rapid Removal of Hg (II) from Aqueous Solution by Rice Straw Activated Carbon Prepared by Microwave-Assisted H2SO4 Activation: Kinetic, Isotherm and Thermodynamic Studies. J. Mol. Liq. 2016, 215, 144–153. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Vennilamani, N.; Pattabhi, S. Mercury (II) Adsorption by Activated Carbon Made from Sago Waste. Carbon 2004, 42, 745–752. [Google Scholar] [CrossRef]
- Asasian, N.; Kaghazchi, T.; Soleimani, M. Elimination of Mercury by Adsorption onto Activated Carbon Prepared from the Biomass Material. J. Ind. Eng. Chem. 2012, 18, 283–289. [Google Scholar] [CrossRef]
- Saman, N.; Abdul Aziz, A.; Johari, K.; Song, S.T.; Mat, H. Adsorptive Efficacy Analysis of Lignocellulosic Waste Carbonaceous Adsorbents toward Different Mercury Species. Process Saf. Environ. Prot. 2015, 96, 33–42. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Foul, A.A. Cadmium Removal from Aqueous Solution Using Microwaved Olive Stone Activated Carbon. J. Environ. Chem. Eng. 2013, 1, 589–599. [Google Scholar] [CrossRef]
- Fouladi Tajar, A.; Kaghazchi, T.; Soleimani, M. Adsorption of Cadmium from Aqueous Solutions on Sulfurized Activated Carbon Prepared from Nut Shells. J. Hazard. Mater. 2009, 165, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Obregón-Valencia, D.; del Rosario Sun-Kou, M. Comparative Cadmium Adsorption Study on Activated Carbon Prepared from Aguaje (Mauritia flexuosa) and Olive Fruit Stones (Olea europaea L.). J. Environ. Chem. Eng. 2014, 2, 2280–2288. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022; ISBN 978-1-80355-580-5. [Google Scholar]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A Comprehensive Review on Human Health Effects of Chromium: Insights on Induced Toxicity. Environ. Sci. Pollut. Res. Int. 2022, 29, 70686–70705. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.M.; Hussein, F.H. Batch Adsorber Design for Different Solution Volume/Adsorbate Mass Ratios of Bentazon, Carbofuran and 2,4-D Adsorption on to Date Seeds Activated Carbon. J. Environ. Anal. Chem. 2014, 2, 2. [Google Scholar] [CrossRef]
- El Bakouri, H.; Usero, J.; Morillo, J.; Rojas, R.; Ouassini, A. Drin Pesticides Removal from Aqueous Solutions Using Acid-Treated Date Stones. Bioresour. Technol. 2009, 100, 2676–2684. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Bentazon and Carbofuran Adsorption onto Date Seed Activated Carbon: Kinetics and Equilibrium. Chem. Eng. J. 2011, 173, 361–368. [Google Scholar] [CrossRef]
- Ioannidou, O.A.; Zabaniotou, A.A.; Stavropoulos, G.G.; Islam, M.A.; Albanis, T.A. Preparation of Activated Carbons from Agricultural Residues for Pesticide Adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Theydan, S.K. Equilibrium Isotherms, Kinetics and Thermodynamics Studies of Phenolic Compounds Adsorption on Palm-Tree Fruit Stones. Ecotoxicol. Environ. Saf. 2012, 84, 39–45. [Google Scholar] [CrossRef]
- Zbair, M.; Ainassaari, K.; Drif, A.; Ojala, S.; Bottlinger, M.; Pirilä, M.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Toward New Benchmark Adsorbents: Preparation and Characterization of Activated Carbon from Argan Nut Shell for Bisphenol A Removal. Environ. Sci. Pollut. Res. 2018, 25, 1869–1882. [Google Scholar] [CrossRef]
- Min, H.S.; Abbas, M.; Kanthasamy, R.; Abdul Aziz, H.; Tay, C.C. Activated Carbon: Prepared from Various Precursors; Ideal International E—Publication Pvt. Ltd.: Indore, India, 2017; ISBN 9789386675071. [Google Scholar]
- Alam, M.G.; Danish, M.; Alanazi, A.M.; Ahmad, T.; Khalil, H.P.S.A. Response Surface Methodology Approach of Phenol Removal Study Using High-Quality Activated Carbon Derived from H3PO4 Activation of Acacia Mangium Wood. Diam. Relat. Mater. 2023, 132, 109632. [Google Scholar] [CrossRef]
- Dat, N.D.; Huynh, Q.S.; Tran, K.A.T.; Nguyen, M.L. Performance of Heterogeneous Fenton Catalyst from Solid Wastes for Removal of Emerging Contaminant in Water: A Potential Approach to Circular Economy. Results Eng. 2023, 18, 101086. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Yang, J.; Liu, P.; Li, X.; Wu, Y.; Wu, S. Adsorption of Phenol from Aqueous Solution on Activated Carbons Prepared from Antibiotic Mycelial Residues and Traditional Biomass. Fuel Process. Technol. 2023, 242, 107663. [Google Scholar] [CrossRef]
- González-García, P. Activated Carbon from Lignocellulosics Precursors: A Review of the Synthesis Methods, Characterization Techniques and Applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Kecira, Z.; Benturki, A.; Daoud, M.; Benturki, O. Effect of Chemical Activation on the Surface Properties of Apricot Stones Based Activated Carbons and Its Adsorptive Properties Toward Aniline. In Proceedings of the Third International Symposium on Materials and Sustainable Development; Springer: New York, NY, USA, 2018; pp. 228–240. [Google Scholar]
- Tchikuala, E.F.; Mourão, P.A.M.; Nabais, J.M.V. Removal of Phenol by Adsorption on Activated Carbon from Aqueous Solution. In Proceedings of the WASTES 2017 International Conference: Solutions, Treatments and Opportunities, Porto, Portugal, 25–26 September 2017; Faculty of Engineering of the University of Porto: Porto, Portugal, 2017; pp. 1–3. [Google Scholar]
- Fan, J.; Zhang, J.; Zhang, C.; Ren, L.; Shi, Q. Adsorption of 2,4,6-Trichlorophenol from Aqueous Solution onto Activated Carbon Derived from Loosestrife. Desalination 2011, 267, 139–146. [Google Scholar] [CrossRef]
- Kodali, J.; Talasila, S.; Arunraj, B.; Nagarathnam, R. Activated Coconut Charcoal as a Super Adsorbent for the Removal of Organophosphorous Pesticide Monocrotophos from Water. Case Stud. Chem. Environ. Eng. 2021, 3, 100099. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Abdel Rahim, E.A.; Fouzy, A.S.M. Preparation and Characterization of Activated Carbon from Agricultural Wastes and Their Ability to Remove Chlorpyrifos from Water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.C.; Aggarwal, D.; Goyal, M.; Kaistha, B.C. Influence of Carbon-Oxygen Surface Groups on the Adsorption of Phenol by Activated Carbons. Indian J. Chem. Technol. 2002, 9, 290–296. [Google Scholar]
- Ma, R.; Xue, Y.; Ma, Q.; Chen, Y.; Yuan, S.; Fan, J. Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials 2022, 12, 4045. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of Non-Steroidal Anti-Inflammatory Drugs from Aqueous Solution Using Activated Carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Boudrahem, N.; Delpeux-Ouldriane, S.; Khenniche, L.; Boudrahem, F.; Aissani-Benissad, F.; Gineys, M. Single and Mixture Adsorption of Clofibric Acid, Tetracycline and Paracetamol onto Activated Carbon Developed from Cotton Cloth Residue. Process Saf. Environ. Prot. 2017, 111, 544–559. [Google Scholar] [CrossRef]
- Pereira, D.; Gil, M.V.; Esteves, V.I.; Silva, N.J.O.; Otero, M.; Calisto, V. Ex-Situ Magnetic Activated Carbon for the Adsorption of Three Pharmaceuticals with Distinct Physicochemical Properties from Real Wastewater. J. Hazard. Mater. 2023, 443, 130258. [Google Scholar] [CrossRef] [PubMed]
- Al-sareji, O.J.; Meiczinger, M.; Somogyi, V.; Al-Juboori, R.A.; Grmasha, R.A.; Stenger-Kovács, C.; Jakab, M.; Hashim, K.S. Removal of Emerging Pollutants from Water Using Enzyme-Immobilized Activated Carbon from Coconut Shell. J. Environ. Chem. Eng. 2023, 11, 109803. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Moreno-Andrés, J.; Rey, A.; Corada-Fernández, C.; Mikola, A.; Manzano, M.A.; Levchuk, I. Post-Treatment of Real Municipal Wastewater Effluents by Means of Granular Activated Carbon (GAC) Based Catalytic Processes: A Focus on Abatement of Pharmaceutically Active Compounds. Water Res. 2021, 192, 116833. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent Advances in Applications of Activated Carbon from Biowaste for Wastewater Treatment: A Short Review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The Use of Activated Carbon for the Removal of Pharmaceuticals from Aqueous Solutions: A Review. Rev. Environ. Sci. Biotechnol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Oliveira, J.A.B.P.; Santos, S.M.; Gil, M.V.; Otero, M.; Calisto, V.; Esteves, V.I. Production of Highly Efficient Activated Carbons from Industrial Wastes for the Removal of Pharmaceuticals from Water-A Full Factorial Design. J. Hazard. Mater. 2019, 370, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Wang, P. Rational Design of Nanomaterials for Water Treatment. Nanoscale 2015, 7, 17167–17194. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef]
- Ohnishi, M.; Shiga, T.; Shiomi, J. Effects of Defects on Thermoelectric Properties of Carbon Nanotubes. Phys. Rev. B 2017, 95, 155405. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wu, W.; Lu, G.; Xu, F.; Tong, Y.; Liu, W.; Du, J. Enhanced Adsorption of Malachite Green onto Carbon Nanotube/polyaniline Composites. J. Appl. Polym. Sci. 2013, 127, 2475–2482. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Ghazisaeidi, R.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption Behavior of Methylene Blue Dye on Nanocomposite Multi-Walled Carbon Nanotube Functionalized Thiol (MWCNT-SH) as New Adsorbent. J. Mol. Liq. 2016, 216, 830–835. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Vaghetti, J.C.P.; Simon, N.M.; Calvete, T.; Veses, R.C. Applications of Brazilian Pine-Fruit Shell in Natural and Carbonized Forms as Adsorbents to Removal of Methylene Blue from Aqueous Solutions-Kinetic and Equilibrium Study. J. Hazard. Mater. 2009, 164, 1213–1222. [Google Scholar] [CrossRef]
- Moradi, O. Adsorption Behavior of Basic Red 46 by Single-Walled Carbon Nanotubes Surfaces. Fuller. Nanotub. Carbon Nanostruct. 2013, 21, 286–301. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Sadik, Z.; Mahdi, D.K.; Alshrefi, S.M.; Al-Sammarraie, A.M.; Alamgir, F.M.; Singh, P.M.; Aljeboree, A.M. Preparation, Structure and Adsorption Properties of Synthesized Multiwall Carbon Nanotubes for Highly Effective Removal of Maxilon Blue Dye. Korean J. Chem. Eng. 2015, 32, 2456–2462. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for Remediation of Heavy Metals: Current Status and Their Future Prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, Characterization, Adsorption Kinetics and Thermodynamics of Novel Magnetic Chitosan Enwrapping Nanosized γ-Fe2O3 and Multi-Walled Carbon Nanotubes with Enhanced Adsorption Properties for Methyl Orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef]
- Machado, F.M.; Bergmann, C.P.; Lima, E.C.; Adebayo, M.A.; Fagan, S.B. Adsorption of a Textile Dye from Aqueous Solutions by Carbon Nanotubes. Mater. Res. 2014, 17, 153–160. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Kord Mostafapour, F.; Rahdar, S.; Mahvi, A.H. Equilibrium and Thermodynamics Studies for Decolorization of Reactive Black 5 (RB5) by Adsorption onto MWCNTs. Desalin. Water Treat. 2015, 54, 2241–2251. [Google Scholar] [CrossRef]
- Machado, F.M.; Bergmann, C.P.; Fernandes, T.H.M.; Lima, E.C.; Royer, B.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Red M-2BE Dye from Water Solutions by Multi-Walled Carbon Nanotubes and Activated Carbon. J. Hazard. Mater. 2011, 192, 1122–1131. [Google Scholar] [CrossRef]
- Wu, C.H. Adsorption of Reactive Dye onto Carbon Nanotubes: Equilibrium, Kinetics and Thermodynamics. J. Hazard. Mater. 2007, 144, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Prola, L.D.T.; Machado, F.M.; Bergmann, C.P.; de Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebayo, M.A.; Dias, S.L.P.; Calvete, T. Adsorption of Direct Blue 53 Dye from Aqueous Solutions by Multi-Walled Carbon Nanotubes and Activated Carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Geyikçi, F. Adsorption of Acid Blue 161 (AB 161) Dye from Water by Multi-Walled Carbon Nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2013, 21, 579–593. [Google Scholar] [CrossRef]
- Exley, J.M.; Hunter, T.N.; Pugh, T.; Tillotson, M.R. Influence of Flake Size and Electrolyte Conditions on Graphene Oxide Adsorption of Ionic Dyes. Powder Technol. 2023, 421, 118387. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated Carbon Derived from Waste Orange and Lemon Peels for the Adsorption of Methyl Orange and Methylene Blue Dyes from Wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Ahmad, N.; Suryani Arsyad, F.; Royani, I.; Lesbani, A. Charcoal Activated as Template Mg/Al Layered Double Hydroxide for Selective Adsorption of Direct Yellow on Anionic Dyes. Results Chem. 2023, 5, 100766. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.H.; Yang, H.; Chen, X.H.; Yang, Q.; Yu, L.Y.; Jiang, J.H.; Chen, X.Q. Aqueous Adsorption and Removal of Organic Contaminants by Carbon Nanotubes. Sci. Total Environ. 2014, 482–483, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Khani, H.; Moradi, O. Influence of Surface Oxidation on the Morphological and Crystallographic Structure of Multi-Walled Carbon Nanotubes via Different Oxidants. J. Nanostruct. Chem. 2013, 3, 73. [Google Scholar] [CrossRef]
- Usman Farid, M.; Luan, H.Y.; Wang, Y.; Huang, H.; An, A.K.; Jalil Khan, R. Increased Adsorption of Aqueous Zinc Species by Ar/O2 Plasma-Treated Carbon Nanotubes Immobilized in Hollow-Fiber Ultrafiltration Membrane. Chem. Eng. J. 2017, 325, 239–248. [Google Scholar] [CrossRef]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of Acid Modification on Adsorption of Hexavalent Chromium (Cr(VI)) from Aqueous Solution by Activated Carbon and Carbon Nanotubes. Desalin. Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Ihsanullah; Al Amer, A.M.; Laoui, T.; Abbas, A.; Al-Aqeeli, N.; Patel, F.; Khraisheh, M.; Atieh, M.A.; Hilal, N. Fabrication and Antifouling Behaviour of a Carbon Nanotube Membrane. Mater. Des. 2016, 89, 549–558. [Google Scholar] [CrossRef]
- Pyrzynska, K. Recent Applications of Carbon Nanotubes for Separation and Enrichment of Lead Ions. Separations 2023, 10, 152. [Google Scholar] [CrossRef]
- Šolic, M.; Maletic, S.; Isakovski, M.K.; Nikic, J.; Watson, M.; Kónya, Z.; Trickovic, J. Comparing the Adsorption Performance of Multiwalled Carbon Nanotubes Oxidized by Varying Degrees for Removal of Low Levels of Copper, Nickel and Chromium(VI) from Aqueous Solutions. Water 2020, 12, 723. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy Metal Removal from Aqueous Solution by Advanced Carbon Nanotubes: Critical Review of Adsorption Applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and Opportunities in Functional Carbon Nanotubes for Membrane-Based Water Treatment and Desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of Surfactants on Pb(II) Adsorption from Aqueous Solutions Using Oxidized Multiwall Carbon Nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Mitra, S. Removal of Trace Arsenic to Meet Drinking Water Standards Using Iron Oxide Coated Multiwall Carbon Nanotubes. J. Chem. Eng. Data 2011, 56, 2077–2083. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Pashameah, R.A.; Ahmed, H.A.; Alrefaee, S.H.; Alamro, F.S.; Faqih, H.H.; Mwafy, E.A.; Mostafa, A.M. Preparation of NiO/MWCNTs Nanocomposite for the Removal of Cadmium Ions. J. Mater. Res. Technol. 2022, 19, 1961–1971. [Google Scholar] [CrossRef]
- Dobrzyńska, J.; Mróz, A.; Olchowski, R.; Zięba, E.; Dobrowolski, R. Modified Multi-Walled Carbon Nanotubes as Effective Pt(IV) Ions Adsorbent with Respect to Analytical Application. Appl. Surf. Sci. 2022, 602, 154388. [Google Scholar] [CrossRef]
- Moghaddam, H.K.; Pakizeh, M. Experimental Study on Mercury Ions Removal from Aqueous Solution by MnO2/CNTs Nanocomposite Adsorbent. J. Ind. Eng. Chem. 2015, 21, 221–229. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, L.; Bai, X.; Shao, Y.; Zhao, G.; Qu, Y.; Wang, C.; Li, Y. Facile Synthesis of Multiwall Carbon Nanotubes/iron Oxides for Removal of Tetrabromobisphenol A and Pb(ii). J. Mater. Chem. 2012, 22, 15853–15862. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Jaiswal, M.; Verma, N. Composite Nanofloral Clusters of Carbon Nanotubes and Activated Alumina: An Efficient Sorbent for Heavy Metal Removal. Chem. Eng. J. 2014, 235, 1–9. [Google Scholar] [CrossRef]
- Moradi, O. The Removal of Ions by Functionalized Carbon Nanotube: Equilibrium, Isotherms and Thermodynamic Studies. Chem. Biochem. Eng. Q. 2011, 25, 229–240. [Google Scholar]
- Dichiara, A.B.; Webber, M.R.; Gorman, W.R.; Rogers, R.E. Removal of Copper Ions from Aqueous Solutions via Adsorption on Carbon Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 15674–15680. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chiu, H.; Liu, C. Removal of zinc(II) from Aqueous Solution by Purified Carbon Nanotubes: Kinetics and Equilibrium Studies. Ind. Eng. Chem. Res. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of Divalent Heavy Metal Ions from Water Using Carbon Nanotube Sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pütün, A.E.; Özbay, N.; Önal, E.P.; Pütün, E. Fixed-Bed Pyrolysis of Cotton Stalk for Liquid and Solid Products. Fuel Process. Technol. 2005, 86, 1207–1219. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon Nanotube Membranes for Water Purification: A Bright Future in Water Desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon Nanotubes—The Promising Adsorbent in Wastewater Treatment. J. Phys. Conf. Ser. 2007, 61, 698–702. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Daniel, S.; Khalid, M.; Tan, J. Comparative Study of Functionalize and Non- Functionalized Carbon Nanotube for Removal of Copper from Polluted Water. Int. J. Chem. Environ. Eng. 2012, 3, 6–9. [Google Scholar]
- Rao, G.P.; Lu, C.; Su, F. Sorption of Divalent Metal Ions from Aqueous Solution by Carbon Nanotubes: A Review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Kang, M.S.; Kwon, M.; Jang, H.J.; Jeong, S.J.; Han, D.W.; Kim, K.S. Biosafety of Inorganic Nanomaterials for Theranostic Applications. Emergent Mater. 2022, 5, 1995–2029. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.E.; Elgabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of Carbon Nanotubes in Removal of Heavy Metals from Wastewater: A Review of the CNTs’ Potential and Current Challenges. Appl. Phys. A Mater. Sci. Process. 2020, 126, 38. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and Anti-Adhesive Properties of Carbon Nanotube-Based Surfaces for Medical Applications: A Systematic Review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef] [PubMed]
- Indarto, A.; Ikhsan, N.A.; Wibowo, I. Applications of Carbon Nanotubes for Controlling Waterborne Pathogens. In Waterborne Pathogens: Detection and Treatment; Butterworth-Heinemann: Oxford, UK, 2020; pp. 433–461. ISBN 9780128187838. [Google Scholar]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced Adsorption of Phenols from Liquids by Aluminum Oxide/carbon Nanotubes: Comprehensive Study from Synthesis to Surface Properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Rananga, L.E.; Magadzu, T. Interaction of Silver Doped Carbon Nanotubes-Cyclodextrin Nanocomposites with Escherichia Coli Bacteria during Water Purification. Water Sci. Technol. Water Supply 2014, 14, 367–375. [Google Scholar] [CrossRef]
- Sheng, L.; Huang, S.; Sui, M.; Zhang, L.; She, L.; Chen, Y. Deposition of Copper Nanoparticles on Multiwalled Carbon Nanotubes Modified with Poly (Acrylic Acid) and Their Antimicrobial Application in Water Treatment. Front. Environ. Sci. Eng. 2015, 9, 625–633. [Google Scholar] [CrossRef]
- Liu, C.; Xie, X.; Zhao, W.; Liu, N.; Maraccini, P.A.; Sassoubre, L.M.; Boehm, A.B.; Cui, Y. Conducting Nanosponge Electroporation for Affordable and High-Efficiency Disinfection of Bacteria and Viruses in Water. Nano Lett. 2013, 13, 4288–4293. [Google Scholar] [CrossRef]
- Sui, M.; Zhang, L.; Sheng, L.; Huang, S.; She, L. Synthesis of ZnO Coated Multi-Walled Carbon Nanotubes and Their Antibacterial Activities. Sci. Total Environ. 2013, 452–453, 148–154. [Google Scholar] [CrossRef]
- Lilly, M.; Dong, X.; McCoy, E.; Yang, L. Inactivation of Bacillus Anthracis Spores by Single-Walled Carbon Nanotubes Coupled with Oxidizing Antimicrobial Chemicals. Environ. Sci. Technol. 2012, 46, 13417–13424. [Google Scholar] [CrossRef] [PubMed]
- Zalipour, Z.; Lashanizadegan, A.; Sadeghfar, F.; Ghaedi, M.; Asfaram, A.; Sadegh, F. Electrochemical Synthesis of CNTs–Zn: ZnO@SDS/PEG@Ni2P Nanocomposite and Its Application for Ultrasound-Assisted Removal of Methylene Blue and Investigation of Its Antibacterial Property. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100721. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Luo, R.; Guo, Q.; Xu, F.; Yang, F.; Zhang, M.; Jia, L.; Yuan, S. Design of Tandem CuO/CNTs Composites for Enhanced Tetracycline Degradation and Antibacterial Activity. Sep. Purif. Technol. 2023, 306, 122548. [Google Scholar] [CrossRef]
- Liu, J.R.; Wang, X.Y.; Saberi, A.; Heydari, Z. The Effect of Co-Encapsulated GNPs-CNTs Nanofillers on Mechanical Properties, Degradation and Antibacterial Behavior of Mg-Based Composite. J. Mech. Behav. Biomed. Mater. 2023, 138, 105601. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Abd Hamid, S.B.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional Carbon Nanotubes in Water Treatment: The Present, Past and Future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of Natural Organic Matter (NOM) and Its Constituents from Water by Adsorption—A Review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shahid, S.; Shen, J.; Emanuelsson, E.A.C.; Patterson, D.A. A Wide Range and High Resolution One-Filtration Molecular Weight Cut-off Method for Aqueous Based Nanofiltration and Ultrafiltration Membranes. J. Memb. Sci. 2017, 525, 304–311. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Goodwin, D.; Marsh, K.; Fairbrother, D.H.; Schwab, K.J.; Jacangelo, J.G.; Huang, H. Modification of Low Pressure Membranes with Carbon Nanotube Layers for Fouling Control. Water Res. 2012, 46, 5645–5654. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Huang, H.; Cho, H.H. Carbon Nanotube Composite Membranes for Microfiltration of Pharmaceuticals and Personal Care Products: Capabilities and Potential Mechanisms. J. Memb. Sci. 2015, 479, 165–174. [Google Scholar] [CrossRef]
- Engel, M.; Chefetz, B. Adsorption and Desorption of Dissolved Organic Matter by Carbon Nanotubes: Effects of Solution Chemistry. Environ. Pollut. 2016, 213, 90–98. [Google Scholar] [CrossRef]
- Yang, X.; Lee, J.; Yuan, L.; Chae, S.R.; Peterson, V.K.; Minett, A.I.; Yin, Y.; Harris, A.T. Removal of Natural Organic Matter in Water Using Functionalised Carbon Nanotube Buckypaper. Carbon 2013, 59, 160–166. [Google Scholar] [CrossRef]
- Yin, S.; López, J.F.; Solís, J.J.C.; Wong, M.S.; Villagrán, D. Enhanced Adsorption of PFOA with Nano MgAl2O4@CNTs: Influence of pH and Dosage, and Environmental Conditions. J. Hazard. Mater. Adv. 2023, 9, 100252. [Google Scholar] [CrossRef]
- Nagorzanski, M.; Qian, J.; Martinez, A.; Cwiertny, D.M. Electrospun Nanofiber Mats as Sorbents for Polar Emerging Organic Contaminants: Demonstrating Tailorable Material Performance for Uptake of Neonicotinoid Insecticides from Water. J. Hazard. Mater. Adv. 2023, 9, 100219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Rastgar, M.; Wang, C.; Ke, S.; He, L.; Chen, X.; Song, Y.; He, C.; Wang, J.; Sadrzadeh, M. Robust PANI-Entangled CNTs Electro-Responsive Membranes for Enhanced In-Situ Generation of H2O2 and Effective Separation of Charged Contaminants. Sep. Purif. Technol. 2022, 303, 122274. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Zhu, J.; Ye, N.; Zhang, X.; Huang, H. Multi-Walled Carbon Nanotubes with Selected Properties for Dynamic Filtration of Pharmaceuticals and Personal Care Products. Water Res. 2016, 92, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, M.; Li, Z.; Li, D.; Pan, Z. Removal of Humic Acid from Aqueous Solution by Magnetic Multi-Walled Carbon Nanotubes Decorated with Calcium. J. Mol. Liq. 2017, 230, 520–528. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and Properties of Nanocomposite Polysulfone/multi-Walled Carbon Nanotubes Membranes for Desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Shao, D.; Sheng, G.; Chen, C.; Wang, X.; Nagatsu, M. Removal of Polychlorinated Biphenyls from Aqueous Solutions Using β-Cyclodextrin Grafted Multiwalled Carbon Nanotubes. Chemosphere 2010, 79, 679–685. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wei, X. Influence of Wastewater Precoagulation on Adsorptive Filtration of Pharmaceutical and Personal Care Products by Carbon Nanotube Membranes. Chem. Eng. J. 2018, 333, 66–75. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, N.; Liu, S.B. Graphene and Graphene-Based Nanocomposites Used for Antibiotics Removal in Water Treatment: A Review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of Divalent Metal Ions from Aqueous Solutions Using Graphene Oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.H.; Kang, F. Adsorption of lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Yang, J.W. Self-Assembled Flower-like TiO2 on Exfoliated Graphite Oxide for Heavy Metal Removal. J. Ind. Eng. Chem. 2012, 18, 1178–1185. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide-MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef] [PubMed]
- Al-Mhyawi, S.R.; Bader, D.M.D.; Bajaber, M.A.; El Dayem, S.M.A.; Ragab, A.H.; Abd El-Rahem, K.A.; Gado, M.A.; Atia, B.M.; Cheira, M.F. Zirconium Oxide with Graphene Oxide Anchoring for Improved Heavy Metal Ions Adsorption: Isotherm and Kinetic Study. J. Mater. Res. Technol. 2023, 22, 3058–3074. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, C.; Zhao, G.; Yang, X.; Li, J.; Wang, X. Removal of Cu(II) and Fulvic Acid by Graphene Oxide Nanosheets Decorated with Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 4991–5000. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, Y.G.; Zeng, G.M.; You, S.H.; Wang, H.; Hu, X.; Guo, Y.M.; Tan, X.F.; Guo, F.Y. Effects of Background Electrolytes and Ionic Strength on Enrichment of Cd(II) Ions with Magnetic Graphene Oxide-Supported Sulfanilic Acid. J. Colloid Interface Sci. 2014, 435, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.; Ijaz, I.; Zain, H.; Mehmood, U.; Mudassir Iqbal, M.; Gilani, E.; Nazir, A. Introduction of CdO Nanoparticles into Graphene and Graphene Oxide Nanosheets for Increasing Adsorption Capacity of Cr from Wastewater Collected from Petroleum Refinery. Arab. J. Chem. 2023, 16, 104445. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Xu, W.; Guo, X.; Cui, L.; Gao, L.; Wei, Q.; Du, B. Adsorption of Pb(II) and Hg(II) from Aqueous Solution Using Magnetic CoFe2O4-Reduced Graphene Oxide. J. Mol. Liq. 2014, 191, 177–182. [Google Scholar] [CrossRef]
- Nandi, D.; Basu, T.; Debnath, S.; Ghosh, A.K.; De, A.; Ghosh, U.C. Mechanistic Insight for the Sorption of Cd(II) and Cu(II) from Aqueous Solution on Magnetic Mn-Doped Fe(III) Oxide Nanoparticle Implanted Graphene. J. Chem. Eng. Data 2013, 58, 2809–2818. [Google Scholar] [CrossRef]
- Dan, S.; Bagheri, H.; Shahidizadeh, A.; Hashemipour, H. Performance of Graphene Oxide/SiO2 Nanocomposite-Based: Antibacterial Activity, Dye and Heavy Metal Removal. Arab. J. Chem. 2023, 16, 104450. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, Y.G.; Wang, H.; Chen, A.W.; Zeng, G.M.; Liu, S.M.; Guo, Y.M.; Hu, X.; Li, T.T.; Wang, Y.Q.; et al. Removal of Cu(II) Ions from Aqueous Solution Using Sulfonated Magnetic Graphene Oxide Composite. Sep. Purif. Technol. 2013, 108, 189–195. [Google Scholar] [CrossRef]
- Hur, J.; Shin, J.; Yoo, J.; Seo, Y.S. Competitive Adsorption of Metals onto Magnetic Graphene Oxide: Comparison with Other Carbonaceous Adsorbents. Sci. World J. 2015, 2015, 836287. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Song, H.; Zhang, L.; Wan, X.; Tang, Y.; Lv, Y. SiO2/graphene Composite for Highly Selective Adsorption of Pb(II) Ion. J. Colloid Interface Sci. 2012, 369, 381–387. [Google Scholar] [CrossRef]
- Thu, V.T.; Trieu, M.H.; An, N.H.T.; Dat, N.T.; Linh, N.D.; Manh, N.B. Mussel—Inspired Biosorbent Combined with Graphene Oxide for Removal of Organic Pollutants from Aqueous Solutions. Ecotoxicol. Environ. Saf. 2023, 255, 114793. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wang, S.; Huang, M.; Zhang, J.; Wang, P. Removal of Water-Soluble Lignin Model Pollutants with Graphene Oxide Loaded Ironic Sulfide as an Efficient Adsorbent and Heterogeneous Fenton Catalyst. Arab. J. Chem. 2022, 15, 104338. [Google Scholar] [CrossRef]
- Khaliha, S.; Bianchi, A.; Kovtun, A.; Tunioli, F.; Boschi, A.; Zambianchi, M.; Paci, D.; Bocchi, L.; Valsecchi, S.; Polesello, S.; et al. Graphene Oxide Nanosheets for Drinking Water Purification by Tandem Adsorption and Microfiltration. Sep. Purif. Technol. 2022, 300, 121826. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, C.; Zhao, J.; Ma, L.; Sun, S.; Zhao, C. Polyethersulfone Enwrapped Graphene Oxide Porous Particles for Water Treatment. Chem. Eng. J. 2013, 215–216, 72–81. [Google Scholar] [CrossRef]
- Xie, G.; Xi, P.; Liu, H.; Chen, F.; Huang, L.; Shi, Y.; Hou, F.; Zeng, Z.; Shao, C.; Wang, J. A Facile Chemical Method to Produce Superparamagnetic Graphene Oxide-Fe3O4 Hybrid Composite and Its Application in the Removal of Dyes from Aqueous Solution. J. Mater. Chem. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhong, X.; Liu, Y.; Zhu, G.; Xu, X.; Chen, K. One-Pot Solvothermal Preparation of Magnetic Reduced Graphene Oxide-Ferrite Hybrids for Organic Dye Removal. Carbon 2012, 50, 2337–2346. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. A Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Dye Removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, Characterization, and Adsorption Properties of Magnetic Fe3O4@graphene Nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Jahan, N.; Roy, H.; Reaz, A.H.; Arshi, S.; Rahman, E.; Firoz, S.H.; Islam, M.S. A Comparative Study on Sorption Behavior of Graphene Oxide and Reduced Graphene Oxide towards Methylene Blue. Case Stud. Chem. Environ. Eng. 2022, 6, 100239. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of Methylene Blue from Aqueous Solution by Graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of Graphene Based Materials for Adsorption of Pharmaceutical Traces from Water and Wastewater-a Review. Desalin. Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Ghaedi, M.; Zeinali, N.; Ghaedi, A.M.; Teimuori, M.; Tashkhourian, J. Artificial Neural Network-Genetic Algorithm Based Optimization for the Adsorption of Methylene Blue and Brilliant Green from Aqueous Solution by Graphite Oxide Nanoparticle. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 125, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fang, Q.; Chen, B. Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection. Environ. Sci. Technol. 2015, 49, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide-Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Bakhtiari, M.; Oveisi, M.; Mahmoodi, B.; Hayati, B. Green Synthesis of Eco-Friendly Magnetic Metal-Organic Framework Nanocomposites (AlFum -Graphene Oxide) and Pollutants (Dye and Pharmaceuticals) Removal Capacity from Water. Mater. Chem. Phys. 2023, 302, 127720. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Bandyopadhyay, D.; Powell, C.D.; Arnusch, C.J. Electrochemical Degradation of Emerging Pollutants via Laser-Induced Graphene Electrodes. Chem. Eng. J. Adv. 2021, 8, 100195. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and Removal of Tetracycline Antibiotics from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, S.; Li, J. Fast and Highly Efficient Tetracyclines Removal from Environmental Waters by Graphene Oxide Functionalized Magnetic Particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption Properties of Tetracycline onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. PLoS ONE 2013, 8, e79254. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of Sulfamethoxazole and Ciprofloxacin from Aqueous Solutions by Graphene Oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Bi, D. Enhanced Adsorptive Removal of Selected Pharmaceutical Antibiotics from Aqueous Solution by Activated Graphene. Environ. Sci. Pollut. Res. 2015, 22, 4715–4724. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-Enhanced Removal of Ciprofloxacin from Water by Porous Graphene Hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Li, Y.; Du, Q.; Sun, J.; Wang, Y.; Wang, X.; Xia, Y.; Wang, Z.; Xia, L. Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. Materials 2013, 6, 2026–2042. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Sun, Y.; Wu, J.; Wu, B.; Creamer, A.E.; Gao, B. Graphene Oxide as Filter Media to Remove Levofloxacin and Lead from Aqueous Solution. Chemosphere 2016, 150, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhiyan, S.S.; Muthu Prabhu, S.; Kim, Y.; Park, C.M. Lanthanum-Substituted Bimetallic Magnetic Materials Assembled Carboxylate-Rich Graphene Oxide Nanohybrids as Highly Efficient Adsorbent for Perfluorooctanoic Acid Adsorption from Aqueous Solutions. Appl. Surf. Sci. 2020, 509, 144716. [Google Scholar] [CrossRef]
- Jones, S.; Pramanik, A.; Kanchanapally, R.; Viraka Nellore, B.P.; Begum, S.; Sweet, C.; Ray, P.C. Multifunctional Three-Dimensional Chitosan/Gold Nanoparticle/Graphene Oxide Architecture for Separation, Label-Free SERS Identification of Pharmaceutical Contaminants, and Effective Killing of Superbugs. ACS Sustain. Chem. Eng. 2017, 5, 7175–7187. [Google Scholar] [CrossRef]
- Tian, T.; Shi, X.; Cheng, L.; Luo, Y.; Dong, Z.; Gong, H.; Xu, L.; Zhong, Z.; Peng, R.; Liu, Z. Graphene-Based Nanocomposite as an Effective, Multifunctional, and Recyclable Antibacterial Agent. ACS Appl. Mater. Interfaces 2014, 6, 8542–8548. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-Based Sustainable Nanomaterials for Water Treatment: State-of-Art and Future Perspectives. Chemosphere 2020, 263, 128005. [Google Scholar] [CrossRef]
- Fera, M.; Macchiaroli, R.; Iannone, R.; Miranda, S.; Riemma, S. Economic Evaluation Model for the Energy Demand Response. Energy 2016, 112, 457–468. [Google Scholar] [CrossRef]
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen Production from Water Splitting Through Photocatalytic Activity of Carbon-Based Materials, A Review. Chem. Eng. Technol. 2023, 46, 420–434. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; PS, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A Sustainable Fuel for Future of the Transport Sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of Energy Storage Systems in Energy Transition from Fossil Fuels to Renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Chen, H.; Li, Y.; Chen, S. Globalization, Green Economy and Environmental Challenges: State of the Art Review for Practical Implications. Front. Environ. Sci. 2022, 10, 199. [Google Scholar] [CrossRef]
- Radenahmad, N.; Reza, S.; Saifullah, M.; Bakar, A.; Shams, S.; Tesfai, A.; Taweekun, J.; Khalilpoor, N.; Azad, A.K.; Issakhov, A. Evaluation of the Bioenergy Potential of Temer Musa: An Invasive Tree from the African Desert. Int. J. Chem. Eng. 2021, 2021, 6693071. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Lu, M.; Tao, P.-Y.; Zhang, Y.-J.; Gong, X.-T.; Yang, Z.; Zhang, G.-Q.; Li, H.-L. Hierarchically Porous and Heteroatom Doped Carbon Derived from Tobacco Rods for Supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Azcárate, C.; Mallor, F.; Mateo, P. Tactical and Operational Management of Wind Energy Systems with Storage Using a Probabilistic Forecast of the Energy Resource. Renew. Energy 2017, 102, 445–456. [Google Scholar] [CrossRef]
- Holze, R.F.; Béguin, E. Frąckowiak (Eds): Supercapacitors—Materials, Systems, and Applications. J. Solid State Electrochem. 2015, 19, 1253. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for Energy Conversion and Storage. Chem. Soc. Rev. 2013, 42, 3127. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-Based Composite Materials for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. From Soybean Residue to Advanced Supercapacitors. Sci. Rep. 2015, 5, 16618. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Ayinla, R.T.; Dennis, J.O.O.; Zaid, H.M.M.; Sanusi, Y.K.K.; Usman, F.; Adebayo, L.L.L. A Review of Technical Advances of Recent Palm Bio-Waste Conversion to Activated Carbon for Energy Storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Liang, J. Performance of Electric Double Layer Capacitors Using Active Carbons Prepared from Petroleum Coke by KOH and Vapor Re-Etching. J. Mater. Sci. Technol. 2003, 19, 265–269. [Google Scholar]
- Zhang, C.; Long, D.; Xing, B.; Qiao, W.; Zhang, R.; Zhan, L.; Liang, X.; Ling, L. The Superior Electrochemical Performance of Oxygen-Rich Activated Carbons Prepared from Bituminous Coal. Electrochem. Commun. 2008, 10, 1809–1811. [Google Scholar] [CrossRef]
- Hasegawa, G.; Aoki, M.; Kanamori, K.; Nakanishi, K.; Hanada, T.; Tadanaga, K. Monolithic Electrode for Electric Double-Layer Capacitors Based on Macro/meso/microporous S-Containing Activated Carbon with High Surface Area. J. Mater. Chem. 2011, 21, 2060–2063. [Google Scholar] [CrossRef]
- Fang, B.; Wei, Y.Z.; Maruyama, K.; Kumagai, M. High Capacity Supercapacitors Based on Modified Activated Carbon Aerogel. J. Appl. Electrochem. 2005, 35, 229–233. [Google Scholar] [CrossRef]
- Toupin, M.; Bélanger, D.; Hill, I.R.; Quinn, D. Performance of Experimental Carbon Blacks in Aqueous Supercapacitors. J. Power Sources 2005, 140, 203–210. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, C.; Dai, Y.; Xie, J. Synthesis of Mesoporous Carbon as Electrode Material for SC. J. Cent. South Univ. 2005, 12, 647–652. [Google Scholar] [CrossRef]
- Jin, J.; Tanaka, S.; Egashira, Y.; Nishiyama, N. KOH Activation of Ordered Mesoporous Carbons Prepared by a Soft-Templating Method and Their Enhanced Electrochemical Properties. Carbon 2010, 48, 1985–1989. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Chen, X.; Shi, C. Preparation and Performance of Straw Based Activated Carbon for Supercapacitor in Non-Aqueous Electrolytes. Microporous Mesoporous Mater. 2010, 131, 303–309. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Q.; Xia, K.; Hu, J. Enhanced Electrical Capacitance of Porous Carbons by Nitrogen Enrichment and Control of the Pore Structure. Microporous Mesoporous Mater. 2009, 118, 28–34. [Google Scholar] [CrossRef]
- Yun, Y.S.; Cho, S.Y.; Shim, J.; Kim, B.H.; Chang, S.J.; Baek, S.J.; Huh, Y.S.; Tak, Y.; Park, Y.W.; Park, S.; et al. Microporous Carbon Nanoplates from Regenerated Silk Proteins for Supercapacitors. Adv. Mater. 2013, 25, 1993–1998. [Google Scholar] [CrossRef]
- Jiang, Q.; Qu, M.Z.; Zhou, G.M.; Zhang, B.L.; Yu, Z.L. A Study of Activated Carbon Nanotubes as Electrochemical Super Capacitors Electrode Materials. Mater. Lett. 2002, 57, 988–991. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. Nanoporous Carbon Electrode from Waste Coffee Beans for High Performance Supercapacitors. Electrochem. Commun. 2008, 10, 1594–1597. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From Coconut Shell to Porous Graphene-like Nanosheets for High-Power Supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Asfaw, H.D.; Kucernak, A.; Greenhalgh, E.S.; Shaffer, M.S.P. Electrochemical Performance of Supercapacitor Electrodes Based on Carbon Aerogel-Reinforced Spread Tow Carbon Fiber Fabrics. Compos. Sci. Technol. 2023, 238, 110042. [Google Scholar] [CrossRef]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Effective Conversion of Waste Banana Bract into Porous Carbon Electrode for Supercapacitor Energy Storage Applications. Results Surf. Interfaces 2023, 10, 100096. [Google Scholar] [CrossRef]
- Tu, J.; Qiao, Z.; Wang, Y.; Li, G.; Zhang, X.; Li, G.; Ruan, D. American Ginseng Biowaste-Derived Activated Carbon for High-Performance Supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 16–24. [Google Scholar] [CrossRef]
- Shrestha, D. Activated Carbon and Its Hybrid Composites with Manganese (IV) Oxide as Effectual Electrode Materials for High Performance Supercapacitor. Arab. J. Chem. 2022, 15, 103946. [Google Scholar] [CrossRef]
- Taberna, P.-L.; Gaspard, S. Chapter 9 Nanoporous Carbons for High Energy Density Supercapacitors. In Biomass for Sustainable Applications: Pollution Remediation and Energy; The Royal Society of Chemistry: London, UK, 2014; pp. 366–399. ISBN 978-1-84973-600-8. [Google Scholar]
- Gupta, R.K.; Dubey, M.; Kharel, P.; Gu, Z.; Fan, Q.H. Biochar Activated by Oxygen Plasma for Supercapacitors. J. Power Sources 2015, 274, 1300–1305. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Xu, H.; Xu, Q.; Jiang, L.; Shi, Y.; Zhang, Q. Scandium-Doped PrBaCo2-XScXO6-δ Oxides as Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 12035–12042. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly Ordered Macroporous Woody Biochar with Ultra-High Carbon Content as Supercapacitor Electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, Y.; Wang, X.; Agung Susantyoko, R.; Zhang, Q. Large Scale Low Cost Fabrication of Diameter Controllable Silicon Nanowire Arrays. Nanotechnology 2014, 25, 255302. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical Properties of High-Power Supercapacitors Using Single-Walled Carbon Nanotube Electrodes. Adv. Funct. Mater. 2001, 11, 387–392. [Google Scholar] [CrossRef]
- Islam, M.R.; Pias, S.M.N.S.; Alam, R.B.; Khondaker, S.I. Enhanced Electrochemical Performance of Solution-Processed Single-Wall Carbon Nanotube Reinforced Polyvinyl Alcohol Nanocomposite Synthesized via Solution-Cast Method. Nano Express 2020, 1, 30013. [Google Scholar] [CrossRef]
- Picó, F.; Rojo, J.M.; Sanjuán, M.L.; Ansón, A.; Benito, A.M.; Callejas, M.A.; Maser, W.K.; Martínez, M.T. Single-Walled Carbon Nanotubes as Electrodes in Supercapacitors. J. Electrochem. Soc. 2004, 151, A831. [Google Scholar] [CrossRef]
- Frackowiak, E.; Jurewicz, K.; Delpeux, S.; Béguin, F. Nanotubular Materials for Supercapacitors. J. Power Sources 2001, 97–98, 822–825. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Béguin, F. Optimisation of an Asymmetric Manganese Oxide/activated Carbon Capacitor Working at 2 V in Aqueous Medium. J. Power Sources 2006, 153, 183–190. [Google Scholar] [CrossRef]
- Lal, M.S.; Badam, R.; Matsumi, N.; Ramaprabhu, S. Hydrothermal Synthesis of Single-Walled Carbon nanotubes/TiO2 for Quasi-Solid-State Composite-Type Symmetric Hybrid Supercapacitors. J. Energy Storage 2021, 40, 102794. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, L.; Lin, G.; Liu, X. SWCNTs/phthalocyanine Polymer Composite Derived Nitrogen Self-Doped Graphene-like Carbon for High-Performance Supercapacitors Electrodes. Mater. Chem. Phys. 2022, 277, 125433. [Google Scholar] [CrossRef]
- Ma, S.B.; Nam, K.W.; Yoon, W.S.; Yang, X.Q.; Ahn, K.Y.; Oh, K.H.; Kim, K.B. Electrochemical Properties of Manganese Oxide Coated onto Carbon Nanotubes for Energy-Storage Applications. J. Power Sources 2008, 178, 483–489. [Google Scholar] [CrossRef]
- Wang, G.X.; Ahn, J.H.; Yao, J.; Lindsay, M.; Liu, H.K.; Dou, S.X. Preparation and Characterization of Carbon Nanotubes for Energy Storage. J. Power Sources 2003, 119–121, 16–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, L.; Jing, P.; Hou, X.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y.; Zhai, C. In Situ Construction of Hierarchical polyaniline/SnS2@carbon Nanotubes on Carbon Fibers for High-Performance Supercapacitors. J. Colloid Interface Sci. 2021, 588, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Hao, Y.; Chen, C. Facile Synthesis of N-Doped NiCo2S4/CNTs with Coordinated Effects as Cathode Materials for High-Performance Supercapacitors. Ionics 2021, 27, 3567–3578. [Google Scholar] [CrossRef]
- Akbar, A.R.; Wu, J.; Tahir, M.; Hu, H.; Yu, C.; Qadir, M.B.; Mateen, F.; Xiong, C.; Yang, Q. Synthesis of the Novel Binary Composite of Self-Suspended Polyaniline (S-PANI) and Functionalized Multi-Walled Carbon Nanotubes for High-Performance Supercapacitors. Ionics 2021, 27, 1743–1755. [Google Scholar] [CrossRef]
- Solgi, M.; Cheraghali, R.; Aghazadeh, M. Self-Assembled Co(OH)2/functionalized MWNTs/porous Graphene Ternary Binder-Free Hybrid for Supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 151–167. [Google Scholar] [CrossRef]
- Ma, S.B.; Nam, K.W.; Yoon, W.S.; Bak, S.M.; Yang, X.Q.; Cho, B.W.; Kim, K.B. Nano-Sized Lithium Manganese Oxide Dispersed on Carbon Nanotubes for Energy Storage Applications. Electrochem. Commun. 2009, 11, 1575–1578. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Y.; Zhou, M.; Chen, G.; Deng, S.; Smirnov, S.; Luo, H.; Zou, G. Porous TiO2 Conformal Coating on Carbon Nanotubes as Energy Storage Materials. Electrochim. Acta 2015, 169, 73–81. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-Anchored Carbon Nanotubes for Enhanced Electrochemical Energy Storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational Design of Ti3C2/carbon nanotubes/MnCo2S4 Electrodes for Symmetric Supercapacitors with High Energy Storage. Appl. Surf. Sci. 2022, 581, 152432. [Google Scholar] [CrossRef]
- Shi, K.; Ren, M.; Zhitomirsky, I. Activated Carbon-Coated Carbon Nanotubes for Energy Storage in Supercapacitors and Capacitive Water Purification. ACS Sustain. Chem. Eng. 2014, 2, 1289–1298. [Google Scholar] [CrossRef]
- Decruyenaere, J.G.; Holt, J.S. Seasonality of Clonal Propagation in Giant Reed. Weed Sci. 2006, 49, 760–767. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Kitajima, K.; Joseph Wright, S.; Westbrook, J.W. Leaf Cellulose Density as the Key Determinant of Inter-and Intra-Specific Variation in Leaf Fracture Toughness in a Species-Rich Tropical Forest. Interface Focus 2016, 6, 20150100. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Obata, K.; Okuhama, Y.; Matsuda, Y.; Ishikawa, M. Activated Carbon/DNA Composite Electrodes for Electric Double Layer Capacitors with Neutral Aqueous Electrolytes. Electrochemistry 2007, 75, 592–594. [Google Scholar] [CrossRef]
- Dai, L.; Patil, A.; Gong, X.; Guo, Z.; Liu, L.; Liu, Y.; Zhu, D. Aligned Nanotubes. ChemPhysChem 2003, 4, 1150–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Lou, Z.; Chen, S.; Chen, D.; Wang, Z.M.; Jiang, K.; Shen, G. All rGO-on-PVDF-Nanofibers Based Self-Powered Electronic Skins. Nano Energy 2017, 35, 121–127. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An Ultra-Sensitive and Rapid Response Speed Graphene Pressure Sensors for Electronic Skin and Health Monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for Batteries, Supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Qin, Y.; Li, J.; Jin, X.; Jiao, S.; Chen, Y.; Cai, W.; Cao, R. Anthraquinone-Functionalized Graphene Framework for Supercapacitors and Lithium Batteries. Ceram. Int. 2020, 46, 15379–15384. [Google Scholar] [CrossRef]
- He, X.; Tang, Z.; Gao, L.L.; Wang, F.; Zhao, J.; Miao, Z.; Wu, X.; Zhou, J.; Su, Y.; Zhuo, S. Facile and Controllable Synthesis N-Doping Porous Graphene for High-Performance Supercapacitor. J. Electroanal. Chem. 2020, 871, 114311. [Google Scholar] [CrossRef]
- Kang, D.N.; Li, J.; Xu, Y.H.; Huang, W.X. Synthesis of MnO2 Nanoparticle Decorated Graphene-Based Porous Composite Electrodes for High-Performance Supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 6091–6108. [Google Scholar] [CrossRef]
- Khawaja, M.K.; Khanfar, M.F.; Oghlenian, T.; Alnahar, W. Fabrication and Electrochemical Characterization of Graphene-Oxide Supercapacitor Electrodes with Activated Carbon Current Collectors on Graphite Substrates. Comput. Electr. Eng. 2020, 85, 106678. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Chen, J.; Yuan, Z.; Liu, C.; Zhang, X.; Xu, M.; Wei, L.; Chen, Y. A Graphene-Covalent Organic Framework Hybrid for High-Performance Supercapacitors. Energy Storage Mater. 2020, 32, 448–457. [Google Scholar] [CrossRef]
- Wang, H.; Tran, D.; Moussa, M.; Stanley, N.; Tung, T.T.; Yu, L.; Yap, P.L.; Ding, F.; Qian, J.; Losic, D. Improved Preparation of MoS2/graphene Composites and Their Inks for Supercapacitors Applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114700. [Google Scholar] [CrossRef]
- Hamra, A.A.B.; Lim, H.N.; Huang, N.M.; Gowthaman, N.S.K.; Nakajima, H.; Rahman, M.M. Microwave Exfoliated Graphene-Based Materials for Flexible Solid-State Supercapacitor. J. Mol. Struct. 2020, 1220, 128710. [Google Scholar] [CrossRef]
- Mondal, A.K.; Wang, B.; Su, D.; Wang, Y.; Chen, S.; Zhang, X.; Wang, G. Graphene/MnO2 Hybrid Nanosheets as High Performance Electrode Materials for Supercapacitors. Mater. Chem. Phys. 2014, 143, 740–746. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, M.; Wang, Y.; Yan, Z.; Jing, J.; Xie, J. In Situ Fabrication of Nickel Based Oxide on Nitrogen-Doped Graphene for High Electrochemical Performance Supercapacitors. Chem. Phys. Lett. 2017, 685, 457–464. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Zhang, H.; Chen, Y.; Cheng, Z.; Zhong, Y.; Ye, Y.; Lei, X. Synthesis of the Graphene/nickel Oxide Composite and Its Electrochemical Performance for Supercapacitors. Int. J. Hydrogen Energy 2014, 39, 16171–16178. [Google Scholar] [CrossRef]
- Zhao, P.; Li, W.; Wang, G.; Yu, B.; Li, X.; Bai, J.; Ren, Z. Facile Hydrothermal Fabrication of Nitrogen-Doped graphene/Fe2O3 Composites as High Performance Electrode Materials for Supercapacitor. J. Alloys Compd. 2014, 604, 87–93. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Lin, R.; Murali, S.; Li Zhang, L.; McDonough, J.K.; Ruoff, R.S.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Outstanding Performance of Activated Graphene Based Supercapacitors in Ionic Liquid Electrolyte from −50 to 80 °C. Nano Energy 2013, 2, 403–411. [Google Scholar] [CrossRef]
- Lai, L.; Li, R.; Su, S.; Zhang, L.; Cui, Y.; Guo, N.; Shi, W.; Zhu, X. Controllable Synthesis of Reduced Graphene Oxide/nickel Hydroxide Composites with Different Morphologies for High Performance Supercapacitors. J. Alloys Compd. 2020, 820, 153120. [Google Scholar] [CrossRef]
- Xu, T.; Yang, D.; Zhang, S.; Zhao, T.; Zhang, M.; Yu, Z.-Z. Antifreezing and Stretchable All-Gel-State Supercapacitor with Enhanced Capacitances Established by graphene/PEDOT-Polyvinyl Alcohol Hydrogel Fibers with Dual Networks. Carbon 2021, 171, 201–210. [Google Scholar] [CrossRef]
- Abdallah, L.; El-Shennawy, T. Reducing Carbon Dioxide Emissions from Electricity Sector Using Smart Electric Grid Applications. J. Eng. 2013, 2013, 845051. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of Activated Carbon from Lignocellulosic Biomass and Its Applications in Air Pollution Control—A Review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pis, J.J.; Rubiera, F.; Pevida, C. Production of Microporous Biochars by Single-Step Oxidation: Effect of Activation Conditions on CO2 Capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon Dioxide Removal through Physical Adsorption Using Carbonaceous and Non-Carbonaceous Adsorbents: A Review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Kammerer, S.; Borho, I.; Jung, J.; Schmidt, M.S. Review: CO2 Capturing Methods of the Last Two Decades. Int. J. Environ. Sci. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Tlili, N.; Grévillot, G.; Vallières, C. Carbon Dioxide Capture and Recovery by Means of TSA And/or VSA. Int. J. Greenh. Gas Control 2009, 3, 519–527. [Google Scholar] [CrossRef]
- Dillon, E.P.; Crouse, C.A.; Barron, A.R. Synthesis, Characterization, and Carbon Dioxide Adsorption of Covalently Attached Polyethyleneimine-Functionalized Single-Wall Carbon Nanotubes. ACS Nano 2008, 2, 156–164. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R.M. Activated Carbons from Biomass-Based Sources for CO2 Capture Applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef] [PubMed]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R.M. Synthesis and Characterization of Activated Carbon from Biomass Date Seeds for Carbon Dioxide Adsorption. J. Environ. Chem. Eng. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Zhao, W.; Fan, M. Heteroatom Self-Doped Activated Biocarbons from Fir Bark and Their Excellent Performance for Carbon Dioxide Adsorption. J. CO2 Util. 2018, 25, 89–98. [Google Scholar] [CrossRef]
- Wei, H.; Chen, H.; Fu, N.; Chen, J.; Lan, G.; Qian, W.; Liu, Y.; Lin, H.; Han, S. Excellent Electrochemical Properties and Large CO2 Capture of Nitrogen-Doped Activated Porous Carbon Synthesised from Waste Longan Shells. Electrochim. Acta 2017, 231, 403–411. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable Porous Carbons with a Superior Performance for CO2 Capture. Energy Environ. Sci. 2011, 4, 1765. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Wang, P.-Y.; Peng, C.; Yang, J.; Yan, X.-B. Activated Carbon Produced from Paulownia Sawdust for High-Performance CO2 Sorbents. Chin. Chem. Lett. 2014, 25, 929–932. [Google Scholar] [CrossRef]
- Hong, S.-M.; Jang, E.; Dysart, A.D.; Pol, V.G.; Lee, K.B. CO2 Capture in the Sustainable Wheat-Derived Activated Microporous Carbon Compartments. Sci. Rep. 2016, 6, 34590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, W.; Ma, Q.; Xie, L.; Zhang, X.; Guo, H. Enhancement of CO2 Capture on Biomass-Based Carbon from Black Locust by KOH Activation and Ammonia Modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising Porous Carbon Derived from Celtuce Leaves with Outstanding Supercapacitance and CO2 Capture Performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zhao, Z.; Zhang, X.; Feng, J.; Li, W. CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke. Separations 2022, 9, 174. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Comparison of Optimized Isotherm Models and Error Functions for Carbon Dioxide Adsorption on Activated Carbon. J. Chem. Eng. Data 2015, 60, 3148–3158. [Google Scholar] [CrossRef]
- Liu, C.; Xing, W.; Zhou, J.; Zhuo, S.P. N-Containing Activated Carbons for CO2 Capture. Int. J. Smart Nano Mater. 2013, 4, 55–61. [Google Scholar] [CrossRef]
- Przepiórski, J.; Skrodzewicz, M.; Morawski, A.W. High Temperature Ammonia Treatment of Activated Carbon for Enhancement of CO2 Adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on Activated Carbons Prepared by Chemical Activation with Cupric Nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Nikraz, H. BOD: COD Ratio as an Indicator for River Pollution. Int. Proc. Chem. Biol. Environ. Eng. 2015, 51, 139–142. [Google Scholar] [CrossRef]
- Dantas, T.L.; Rodrigues, A.E.; Moreira, R.F.P.M. Separation of Carbon Dioxide from Flue Gas Using Adsorption on Porous Solids. In Greenhouse Gases—Capturing, Utilization and Reduction; IntechOpen: London, UK, 2012; pp. 57–80. [Google Scholar]
- Raganati, F.; Ammendola, P.; Chirone, R. CO2 capture by Adsorption on Fine Activated Carbon in a Sound Assisted Fluidized Bed. Chem. Eng. Trans. 2015, 43, 1033–1038. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 Capture by Adsorption with Nitrogen Enriched Carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of Porous Carbons Derived from Sustainable Palm Solid Waste for Carbon Dioxide Capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, G.; Jalilov, A.S.; Tarkunde, Y.R.; Fei, H.; Tour, J.M. Biochar as a Renewable Source for High-Performance CO2 Sorbent. Carbon 2016, 107, 344–351. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Shao, J.; Chen, Y.; Liao, X.; Wang, X.; Chen, H. Generalized Two-Dimensional Correlation Infrared Spectroscopy to Reveal Mechanisms of CO2 Capture in Nitrogen Enriched Biochar. Proc. Combust. Inst. 2017, 36, 3933–3940. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Shao, J.; Chen, Y.; Feng, Y.; Wang, X.; Chen, H. Effects of Hydrofluoric Acid Pre-Deashing of Rice Husk on Physicochemical Properties and CO2 Adsorption Performance of Nitrogen-Enriched Biochar. Energy 2015, 91, 903–910. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon Based Materials: A Review of Adsorbents for Inorganic and Organic Compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Grondein, A.; Bélanger, D. Chemical Modification of Carbon Powders with Aminophenyl and Aryl-Aliphatic Amine Groups by Reduction of in Situ Generated Diazonium Cations: Applicability of the Grafted Powder towards CO2 Capture. Fuel 2011, 90, 2684–2693. [Google Scholar] [CrossRef]
- Ye, Q.; Jiang, J.; Wang, C.; Liu, Y.; Pan, H.; Shi, Y. Adsorption of Low-Concentration Carbon Dioxide on Amine-Modified Carbon Nanotubes at Ambient Temperature. Energy Fuels 2012, 26, 2497–2504. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q.; Shen, M.; Shi, J.; Chen, J.; Pan, H.; Shi, Y. Carbon Dioxide Capture by Functionalized Solid Amine Sorbents with Simulated Flue Gas Conditions. Environ. Sci. Technol. 2011, 45, 5710–5716. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from Flue Gas via Multiwalled Carbon Nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Cinke, M.; Li, J.; Bauschlicher, C.W.; Ricca, A.; Meyyappan, M. CO2 Adsorption in Single-Walled Carbon Nanotubes. Chem. Phys. Lett. 2003, 376, 761–766. [Google Scholar] [CrossRef]
- Osler, K.; Twala, N.; Oluwasina, O.O.; Daramola, M.O. Synthesis and Performance Evaluation of Chitosan/Carbon Nanotube (Chitosan/MWCNT) Composite Adsorbent for Post-Combustion Carbon Dioxide Capture. Energy Procedia 2017, 114, 2330–2335. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M.; Hosseiny, A.H.; Sepehrian, M. Enhancement of CO2 Adsorption on Polyethylenimine Functionalized Multiwalled Carbon nanotubes/Cd-Nanozeolite Composites. J. Mol. Struct. 2018, 1173, 792–800. [Google Scholar] [CrossRef]
- Keller, L.; Ohs, B.; Lenhart, J.; Abduly, L.; Blanke, P.; Wessling, M. High Capacity Polyethylenimine Impregnated Microtubes Made of Carbon Nanotubes for CO2 Capture. Carbon 2018, 126, 338–345. [Google Scholar] [CrossRef]
- Ngoy, J.M.; Wagner, N.; Riboldi, L.; Bolland, O. A CO2 Capture Technology Using Multi-Walled Carbon Nanotubes with Polyaspartamide Surfactant. Energy Procedia 2014, 63, 2230–2248. [Google Scholar] [CrossRef]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified Carbon Nanotubes/tetraethylenepentamine for CO2 Capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-Functionalized Microcrystalline Cellulose–mesoporous Silica Composites for Carbon Dioxide Sorption at Elevated Temperatures. J. Mater. Chem. A 2016, 4, 4808–4819. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Li, Z.; Tang, J.; Zhao, X.S. Pebax-1657 Nanocomposite Membranes Incorporated with Nanoparticles/colloids/carbon Nanotubes for CO2/N2 and CO2/H2 Separation. J. Appl. Polym. Sci. 2013, 130, 2867–2876. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Chung, A.J.; Liao, C.H. CO2 Capture with Amine-Loaded Carbon Nanotubes via a Dual-Column Temperature/vacuum Swing Adsorption. Appl. Energy 2014, 113, 706–712. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, M.; Li, Z.; Ma, Y.; Du, A. CO2 Capture and Gas Separation on Boron Carbon Nanotubes. Chem. Phys. Lett. 2013, 575, 59–66. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Smith, S.C.; Du, A.; Zhu, Z. Electrocatalytically Switchable CO2 Capture: First Principle Computational Exploration of Carbon Nanotubes with Pyridinic Nitrogen. ChemSusChem 2014, 7, 435–441. [Google Scholar] [CrossRef]
- Lu, A.-H.; Hao, G.-P.; Zhang, X.-Q. Porous Carbons for Carbon Dioxide Capture. In Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–77. [Google Scholar]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-Combustion CO2 Capture Using Mesoporous TiO2/graphene Oxide Nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased Chitosan Hybrid Aerogels with Superior Adsorption: Role of Graphene Oxide in CO2 Capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, L.; Xu, X.; Wang, Y.; Ling, L.; Feng, X. Graphene-Based Porous Silica Sheets Impregnated with Polyethyleneimine for Superior CO2 Capture. Adv. Mater. 2013, 25, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Burress, J.; Yildirim, T. Graphene Oxide Derived Carbons (GODCs): Synthesis and Gas Adsorption Properties. Energy Environ. Sci. 2012, 5, 6453–6459. [Google Scholar] [CrossRef]
- Chandra, V.; Yu, S.U.; Kim, S.H.; Yoon, Y.S.; Kim, D.Y.; Kwon, A.H.; Meyyappan, M.; Kim, K.S. Highly Selective CO2 Capture on N-Doped Carbon Produced by Chemical Activation of Polypyrrole Functionalized Graphene Sheets. Chem. Commun. 2012, 48, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Szczęśniak, B.; Choma, J. Graphene-Containing Microporous Composites for Selective CO2 Adsorption. Microporous Mesoporous Mater. 2020, 292, 109761. [Google Scholar] [CrossRef]
- Arifutzzaman, A.; Musa, I.N.; Aroua, M.K.; Saidur, R. MXene Based Activated Carbon Novel Nano-Sandwich for Efficient CO2 Adsorption in Fixed-Bed Column. J. CO2 Util. 2023, 68, 102353. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]

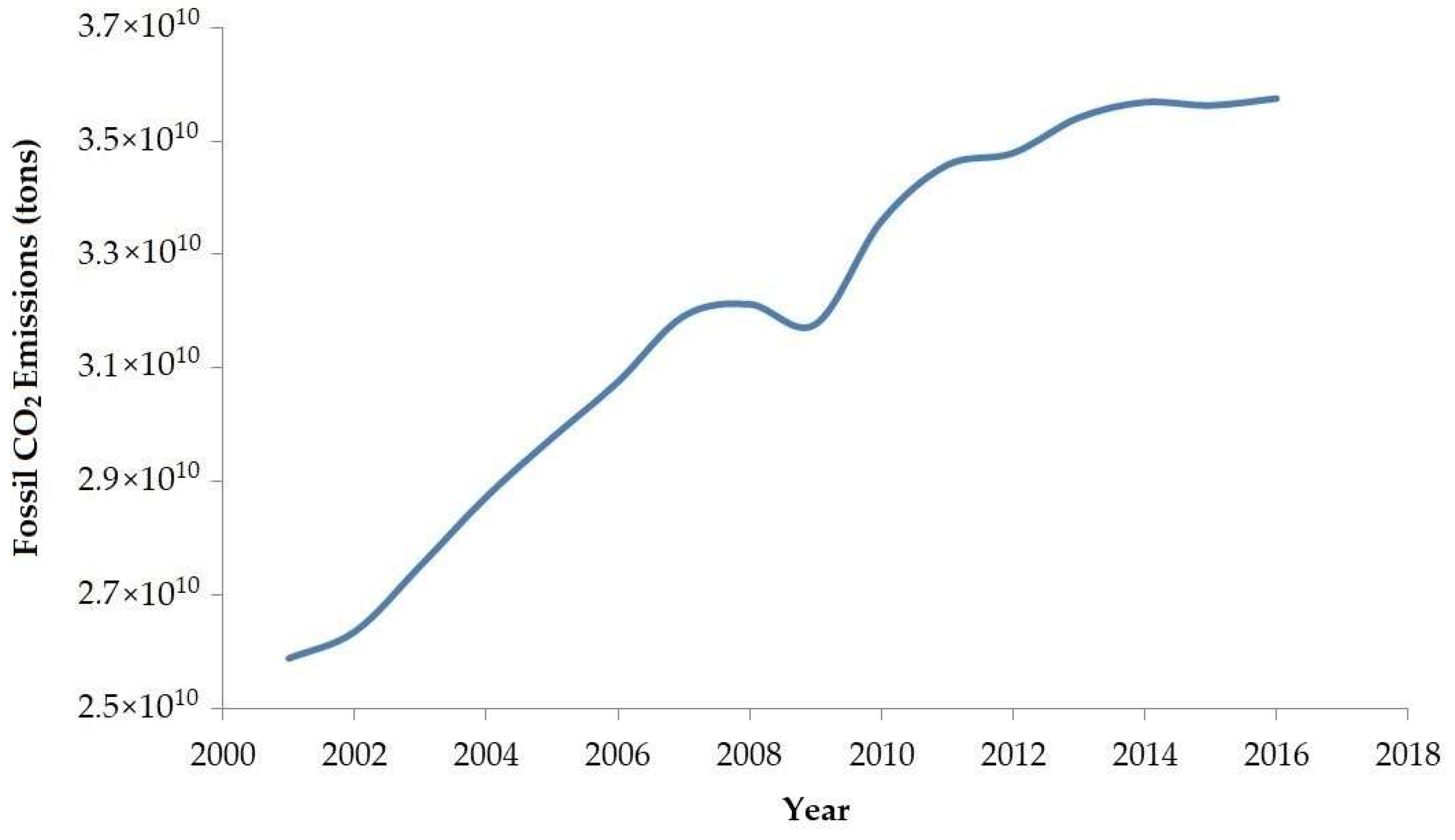
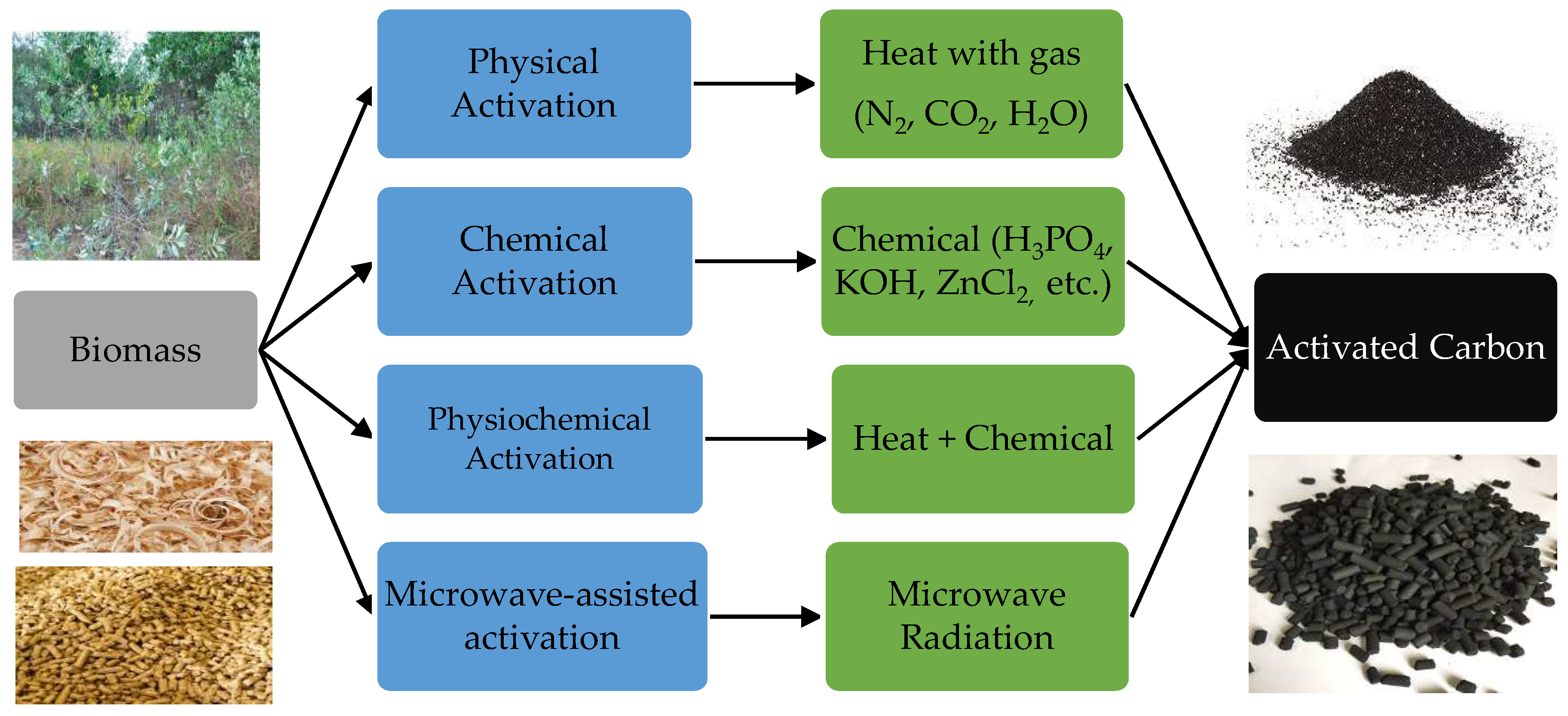
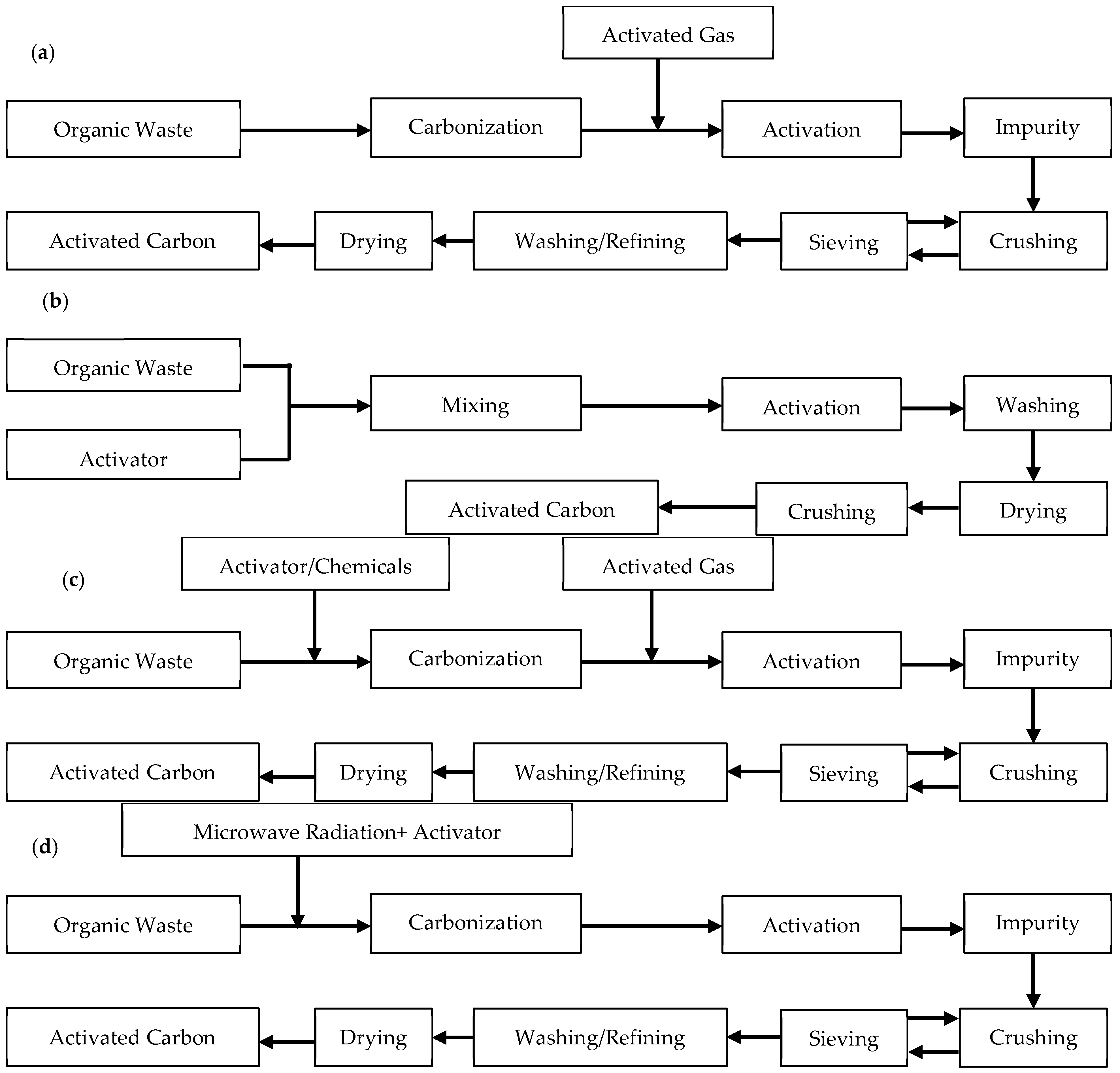


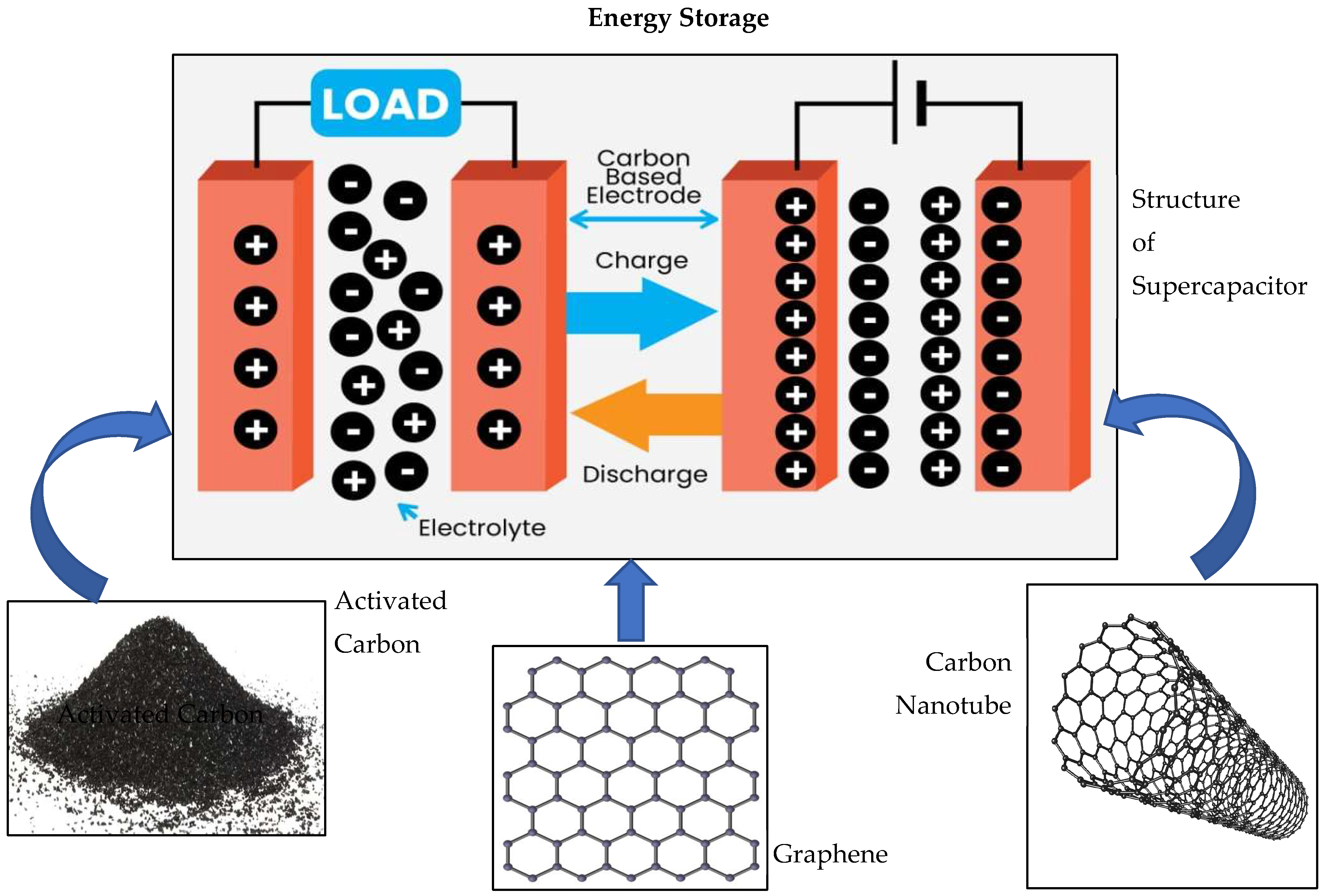
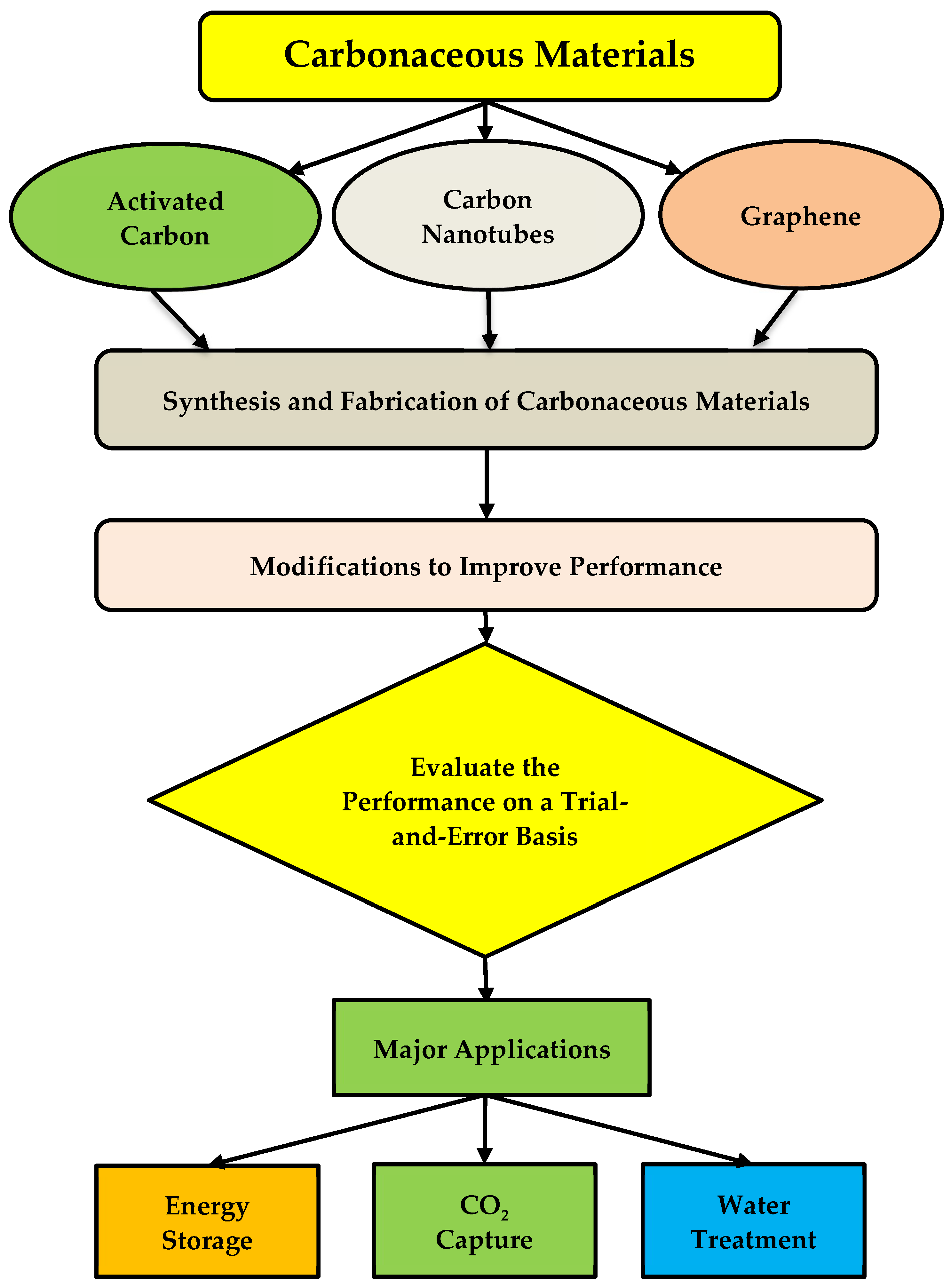
| Dye Adsorption by Activated Carbons | ||||||
|---|---|---|---|---|---|---|
| Dye | Activating Agent | Raw Material | SBET (m2/g) | pH | Adsorption Capacity (mg/g) | Ref. |
| Methylene blue | KOH | Water hyacinth | 6.6 | 587.92 | 1765.52 | [137] |
| Methylene blue | NaHCO3 | Medulla tetrapanacis | 9 | 923 | 1116.94 | [138] |
| Anionic | Phosphoric acid | Raffia palm shells | 2 | - | 1762.93 | [139] |
| Methyl orange | HNO3 | Coffee grounds | 3 | 658 | - | [140] |
| Basic green 4 | CO2 | Durian peel | - | 3 | 312.5 | [141] |
| Reactive red 120 | H3PO4 | Jute fiber | - | 6 | 200 | [142] |
| Malachite green (mg) | K2CO3/CO2 | Bamboo | 1724 | 5 | 263.58 | [143] |
| Methylene blue | CO2/steam | Oil palm wood | 1084 | - | 90.9 | [144] |
| Acid yellow 6 | NaOH | Flamboyant pods | - | 2 | 673.68 | [145] |
| Eriochrome black T | Thermal | Rice hulls | - | 2 | 0.04 | [146] |
| Acid red 114 | H2SO4 | Sesame | 229.65 | 3 | 102.4 | [147] |
| Malachite green | ZnCl2 | Coconut coir | 205.27 | - | 27.44 | [148] |
| Methylene blue | ZnCl2 | Date stones | 1045.61 | 7 | 398.19 | [149] |
| Basic red 46 | H3PO4 | Wild olive cores | 969 | 4 | 781.25 | [150] |
| Methylene blue | ZnCl2 | Pineapple waste | 914.67 | - | 288.34 | [151] |
| Methylene blue | HCl | Acacia fumosa seeds | - | 6 | 10.49 | [152] |
| Acid red 114 | H2SO4 | Pongam seeds | 324.79 | 3 | 3.33 | [147] |
| Malachite green | NaOH | Tea leaves | 134 | 4 | 256.4 | [153] |
| Yellow 18 | ZnCl2 | Sour cherry | 1704 | 2 | 75.76 | [154] |
| Methyl violet | CO2 | Kapok | 647–897 | - | 160.3 | [155] |
| Amido black 10b | H2SO4 | Palm flower | 9.57 | - | 4.04 | [156] |
| Malachite green | HNO3 | Oakwood | 68.5180 | - | 4.34 | [157] |
| Methylene blue | H3PO4 | Vetiver roots | 1004–1185 | - | 394 | [158] |
| Malachite green | H2SO4 | Ricinus communis | - | 7 | 27.78 | [159] |
| Heavy Metal Adsorption by Activated Carbons | ||||||
| Heavy Metal | Activating Agent | Raw Material | SBET (m2/g) | pH | Adsorption Capacity (mg/g) | Ref. |
| Cr (VI) | Citric acid | Waste walnut shells | 4.2 | 75.26 | 602.47 | [160] |
| Pb (II) | Cs2CO3 | Palm tree (Phoenix Dactylifera) | 7 | - | 2704 | [161] |
| Cr (VI) | KOH | Leather industry waste | 5 | 41.6 | 0.124 | [162] |
| As (V) | KOH | Apricot stones | 7.3 | 75.9 | 733.6 | [163] |
| Fe (III) | HNO3 | Bulgarian lignite | 2.7 | - | 293 | [164] |
| Pb | ZnCl2 | Hazelnut husks | 1092 | 5.7 | 13.05 | [165] |
| Pb | H3PO4 | Sugar cane bagasse | - | 4 | 8.58 | [166] |
| Pb | ZnCl2 | Van apple pulp | 1067 | 4.0 | 17.77 | [167] |
| Pb | H3PO4 | Lemna minor | 531.9 | 6 | 170.9 | [168] |
| Pb | ZnCl2 | Coffee residue | 890 | 5.8 | 63 | [169] |
| Cr | Ozone | Rice husks | 380 | 2 | 8.5 | [170] |
| Cr | Thermal | Olive bagasse | 718 | 2 | 109.89 | [171] |
| Cr | NaOH | Longan seeds | 1511.8 | 3 | 169.5 | [172] |
| Cr | H2SO4 | Date palm seeds | - | 1 | 120.48 | [173] |
| Cr | KOH | Peanut shells | 95.51 | 2 | 16.26 | [174] |
| Hg | ZnCl2 | Walnut shells | 780 | 2 | 151.5 | [175] |
| Hg | H2SO4 | Rice straw | - | 5 | 142.88 | [176] |
| Hg | H2SO4 | Sago waste | 625 | 5 | 55.6 | [177] |
| Hg | ZnCl2 | Residue of liquorice | 1492.4 | 8 | 24.78 | [178] |
| Hg | KOH | Coconut pith | 505 | - | 0.74 mmol/g | [179] |
| Cd | KOH | Olive stones | 1280.71 | 6 | 11.72 | [180] |
| Cd | HNO3 | Oak wood | 68.518 | 9 | 3.31 | [157] |
| Cd | H3PO4 | Nutshells | 1556.9 | 10 | 104.17 | [181] |
| Cd | H3PO4 | Aguaje | 1202 | 5 | 26.33 | [182] |
| Cd | H3PO4 | Olive fruit stones | 1283 | 5 | 24.83 | [182] |
| Adsorbent | Activator | Adsorbate | SBET (m2/g) | pH | Qm (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Paper-mill-sludge ACs | KOH | Carbamazepine, sulfamethoxazole, and ibuprofen | 795 | 8 | 154 ± 11, 167 ± 12 and 147 ± 8 | [206] |
| Coconut-shell ACs | HNO3, H2SO4 | 2, 2′-azino-bis 3-ethylbenzothiazole-6-sulphonic acid | 1100 | 5 | 180.5–735.2 | [207] |
| Granular activated carbons | - | Peroxymonosulfate and peroxydisulfate | 1040 | 7.13 | - | [208] |
| Cork-powder ACs | Steam | Ibuprofen | - | 2–11 | 378.1 | [204] |
| Mung-bean-husk ACs | Steam | Ibuprofen | - | 2 | 62.5 | [204] |
| Cork-powder ACs | K2CO3 | Ibuprofen | - | 2–11 | 145.2 | [204] |
| Mugwort-leaf ACs | H2SO4 | Ibuprofen | - | 2 | 16.95 | [204] |
| Waste-apricot ACs | ZnCl2 | Naproxen | - | 5.82 | 106.4 | [204] |
| Olive-waste ACs | H3PO4 | Naproxen | - | 4.1 | 39.5 | [204] |
| Olive-waste ACs | H3PO4 | Ketoprofen | - | 4.1 | 24.7 | [204] |
| Sawdust ACs | ZnCl2 | PC | - | - | 226.71 | [209] |
| Primary-paper-mill-sludge ACs | HCl | PAR | - | - | 405 | [209] |
| Lagenaria vulgaris-shell ACs | - | Ranitidine | 665 | 2–11 | 315.5 | [210] |
| Date-stone ACs | - | Levofloxacin | 817 | 2–12 | 100.4 | [210] |
| Cyclamen persicum ACs | - | Diclofenac | 799–880 | 2–12 | 22.22 | [210] |
| Paper-mill-sludge ACs | K2CO3 | SMX, CBZ, PAR | 1583 | - | SMX = 75% CBZ = 85% PAR = 84% | [211] |
| Material Type | Surface Area (m2/g) | Pollutant | Effect on Water Purification | Ref. |
|---|---|---|---|---|
| NiO/MWCNTs | 245.3 | Cadmium (Cd) (II) | - | [242] |
| CNTs-TMPDET | 120 | Platinum (Pt) (IV) | 260 mg/g | [243] |
| CNTs-MnO2 | 110.4 | Silver (Hg) (II) | 58.8 mg/g | [244] |
| CNTs/Fe3O4-NH2 | 90.7 | Lead (Pb) (II) | 75.0 mg/g | [245] |
| AA-CNTs | 203.0 | Chromium (Cr) (VI) | 264.5 mg/g | [246] |
| SWCNTs | 400.0 | Cadmium (Cd) (II) | 21.2 mg/g | [247] |
| OH-SWCNTs/RGO | - | Cupper (Cu) (II) | 256 mg/g | [248] |
| Purified SWCNTs | 423.0 | Zinc (Zn) (II) | 41.8 mg/g | [249] |
| Oxidized CNTs | - | Cobalt (Co) (II) | 85.7 mg/g | [250] |
| Adsorbate | Pollutants | Removal Efficiency | Ref. |
|---|---|---|---|
| MgAl2O4@CNTs | MgAl2O4 | 100% | [277] |
| CNTs | Polyacrylonitrile | Above 95% | [278] |
| CNTs | Polyaniline | 93.4% | [279] |
| CNT nanocomposites | PPCP | Improvement of 45% | [280] |
| CNT nanocomposites | PPCP | 95% | [274] |
| M-MWCNTs coated with Calcium | Humic acid | 90.2% | [281] |
| Functionalized CNT buckypaper | Humic acid | >93% | [276] |
| Polyelectrolyte CNTs | Bovine serum albumin (BSA) | 99.9% | [282] |
| b-cyclodextrin grafted MWCNTs (MWCNT-g-CDs) | Polychlorinated biphenyls | >96% | [283] |
| Graphene Based Nanocomposites for the Elimination of Heavy Metals | ||||
|---|---|---|---|---|
| Adsorbate | Adsorbent | SBET (m2/g) | Adsorption Capacity (mg/g) | Ref. |
| Lead (II) | GO-MnFe2O4 | 196 | 673 | [292] |
| Uranium (VI) | ZrO2/GO | 37.86 | 128 | [293] |
| Copper (II) | GO/Fe3O4 | - | 18.3 | [294] |
| Cadmium (II) | GO/Fe3O4/sulfanilic acid | - | 55.4 | [295] |
| Chromium (VI) | CdO-GO | - | 399 | [296] |
| Lead (II) | rGO/COFe2O4 | 169.9 | 299.4 | [297] |
| Cadmium (II) and cupper (II) | GO/Mn-doped Fe(III)oxide | 180 | 87.2 and 129.7 | [298] |
| Chromium (VI) | GO/SiO2 | - | 181.81 | [299] |
| Cupper (II) | GO/Fe3O4/sulfanilic acid | 92.79 | 50.7 and 56.8 | [300] |
| Copper, lead, and cadmium | GO/Fe3O4 | 49.9 | 23.1, 38.5 and 4.4 | [301] |
| Lead (II) | TiO2/GO | - | 65.6 | [291] |
| Lead (II) | SiO2–GNs | 252.5 | 113.6 | [302] |
| Organic Pollutant Adsorption Using Graphene-Based Materials | ||||
| Organic Pollutants | Adsorbent | SBET (m2/g) | Adsorption Capacity (mg/g) | Ref. |
| Methylene blue | PDOP-GO | 20 | 270 | [303] |
| Ethylene glycol | GO-Fe3S4 | 7.71 | 21.87 | [304] |
| Methylene blue | rGO | >1000 | 174 | [305] |
| Methylene blue | Polyethersulfone/GO | 24.98 | 62.50 | [306] |
| Methylene blue, Neutral Red (NR) | Fe3O4/GO hybrids | - | 167.2, 171.3 | [307] |
| Rhodamine B (RhB), Methylene blue | Reduced GO-MFe2O4 hybrids | - | 92% and 100% | [308] |
| Methylene blue, methyl violet (MV) | GO sponge | 48.409 | 397, 467 | [309] |
| Methylene blue, Congo Red (CR) | Fe3O4-GNs | 118.175 | 45.3, 33.7 | [310] |
| Methylene blue | GO | 28 | 351 | [311] |
| Methylene blue | Reduced-GO | 0.60 | 2000 | [312] |
| Methylene blue | GNs | 295.56 | 154–204 | [313] |
| Pharmaceutical Pollutants | Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|
| AlFum-CoFe2O4/GO | Tetracycline and Doxycycline | 230 | [318] |
| Ag3PO4/Fe3O4/graphene oxide | Carbamazepine | - | [319] |
| CNC-PVAm/rG | Diclofenac | 605.87 | [318] |
| Tetracycline | Graphene oxide | 313.48 | [320] |
| Doxycycline | GO | 398.40 | [320] |
| Oxytetracycline | F–GO with MNPs | 45.0 | [321] |
| Chlortetracycline | F–GO with MNPs | 42.6 | [321] |
| Doxycycline | F–GO with MNPs | 35.5 | [321] |
| Tetracycline | GO | 381.77 | [322] |
| Ciprofloxacin | Single-layer GO | 379 | [323] |
| Ciprofloxacin | KOH-activated graphene | 194.6 | [324] |
| Ciprofloxacin | Porous graphene hydrogel | 235.6 | [325] |
| Material | Operationg Conditions | Capacitance | Ref. |
|---|---|---|---|
| SWNTs | KOH Solution | 180 F/g | [372] |
| PVA/SF-SWNTs | Facile solution-cast technique | 26.4 F/g | [373] |
| SWCNTs | 350 °C-oxidized | 15 to 135 F/g | [374] |
| CNTs | Polypyrrole treated | 170 F/g | [375] |
| CNTs | Manganese oxide (MnO2) | 140 F/g | [376] |
| SWCNTs/TiO2 | SWCNT/TiO2 nanocomposites as electrodes and PVA/H2SO4 as gel electrolyte | 144 F/g | [377] |
| SWCNTs/phthalocyanine polymer composite | Self-nitrogen-doping | 228 F/g | [378] |
| MnO2/CNTs | Nanocomposite MnO2 | 580 F/g | [379] |
| MWNTs | Chemical evaporation deposition | 340 mAh/g (reversible) | [380] |
| PANI/SnS2@CNTs | Radial orientation and high chemical/physical stability | 891 F g−1 | [381] |
| NiCo2S4/N-CNTs | 1428 F/g | [382] | |
| NiCo2S4 | 733.5 F/g | [382] | |
| S-PANI/OCNT and S-PANI/SCNTs | Doping of long-chain protonic acids during in situ polymerization | 316.8 F/g and 345.4 F/g | [383] |
| Cobalt hydroxide/porous graphene/functionalized MWNTs | Hydrothermal method | 1426 F/g | [384] |
| CNTs | Nanosized lithium manganese oxide (LMO) | 107 mAh/g (reversible) | [385] |
| Hybrid CNTs | 0.5 M H2SO4 electrolyte | 145 F/g | [386] |
| CNTs | V2O5 | 850 mAh/g | [387] |
| Ti3C2/CNTs/MnCo2S4 | Electrostatic self-assembly between positively charged multi-walled carbon nanotubes (CNTs) and negatively charged Ti3C2, followed by subsequent anchoring of bimetallic sulphide MnCo2S4 nanoparticles to Ti3C2/CNT hybrids via a two-step hydrothermal method | 823 F/g | [388] |
| Activated-carbon-coated MWCNTs | Nitrogen (N-AC-MWCNT) | 103.1 F/g | [389] |
| Materials | Synthesis Method | Electrolyte | Reference Electrode | Capacitance (F/g) | Ref. |
|---|---|---|---|---|---|
| F-rGO | Modified Hummers method | 1.1 M LiPF6 | Ag/AgCl | 210.0 | [401] |
| N-doping PG-Ni | Modified Hummers method | 1.0 mol/L KOH | Saturated calomel | 575.0 | [402] |
| MnO2 nanoparticles on reduced graphene oxide (RGO) | Modified Hummers method | GO particles (0.5 mg·mL−1) | Saturated calomel electrode | 432 | [403] |
| ACP/GOx | - | 0.8 g gel H3PO4 | Ag/AgCl (3 M KCl) | 16.5 | [404] |
| COF/rGO | Modified Hummers method | Aqueous 1 M H2SO4 | Ag/AgCl (3 M KCl) | 321.0 | [405] |
| MoS2/graphene | Surface methodology | 1 M H2SO4(aq) solution | -- | 76.0 | [406] |
| MW-GNPs | Modified Hummers method | Solid-state electrolyte (1.0 g of PVA in 10 mL of deionized water) | -- | 78.1 | [407] |
| Graphene/MnO2 | Sonication | 1 M Na2SO4 | Saturated calomel | 320.0 | [408] |
| Ni(OH)2/NG | Tube furnace calcination | 6 M KOH | Saturated calomel | 1350.0 | [409] |
| RG/NiO | Microwave | 5 M NaOH | -- | 617.0 | [410] |
| Fe2O3/NG | Hydrothermal | 1M Na2SO4 | Saturated calomel | 260.1 | [411] |
| Biomass | Production Temperature (K) | BET Surface Area (m2/g) | Activating Agent | Adsorption Capacity (mmol/g) | Ref. |
|---|---|---|---|---|---|
| Bamboo | 1073 | 2000 | KOH | 7.00 | [425] |
| Fir bark | 973 | 1242 | KOH | 6.10 | [426] |
| Longan shells | 1073 | 3260 | KOH | 5.60 | [427] |
| Eucalyptus sawdust | 1073 | 2850 | KOH | 5.20 | [428] |
| Paulownia sawdust | 1073 | 1555 | KOH | 7.14 | [429] |
| Wheat | 1073 | 2192 | KOH | 5.70 | [430] |
| Black locust | 1103 | 2511 | KOH | 5.86 | [431] |
| Celtuce leaves | 1073 | 3404 | KOH | 6.04 | [432] |
| Pulverized semi-coke | 973 | 120.5–590.2 | HCl | 1.80 | [433] |
| Commercial activated carbon | 1073 | 1374 | ZnCl2 | - | [434] |
| Soya beans | 673 | 710–1193 | KOH | 4.24 | [435] |
| Activated carbon | 200–1000 °C | - | 53 mg/g | [436] | |
| African palm shells | 1073 | 473–1361 | Cu(NO3)2 | 103–217 mg/g | [437] |
| Granular activated carbon (GAC) | - | 954 | 3-aminopropyl-triethoxysilane | 34.6 | [438] |
| GAC | - | 954 | 3-aminopropyl-triethoxysilane | 79.5 Cin:50%, T:25 °C | [438] |
| Adsorbents | Modification Chemicals | Adsorption (mg/g) | Conditions | Ref. |
|---|---|---|---|---|
| CNTs | 3-aminopropyl-triethoxysilane | 43.3 | Influent concentration mgL−1: 15%, T:20 °C | [454] |
| CNTs | 3-aminopropyl-triethoxysilane | 114.0 | Cin:50%, T:20 °C | [454] |
| SWCNT | - | 87 | Cin:99%, T:35 °C | [455] |
| Chitosan/MWCNTs | Chitosan | 3 mg/g | At 45°C, 1.1 bar | [456] |
| CNTs | 3-aminopropyl-triethoxysilane | 40.9 | Cin: 10%, T: 25 °C | [438] |
| CNTs | 3-aminopropyl-triethoxysilane | 96.3 | Cin: 50%, T: 25 °C | [438] |
| CNTs | Tetraethylenepentamine (TEPA) | 3.87 mmol/g | 2 v.% of CO2 and 2 v.% of H2O at 40 °C | [452] |
| MWCNT/Cd-nanozeolite composites | Polyethylenimine | 5.7 mmol/g | At 25 °C, 1 bar | [457] |
| MWCNTs | Polyethylenimine (PEI) | 93 mg/gfiber | PEI load at 20 wt.-% | [458] |
| MWCNTs | Polyaspartamide (PAA), | 99.5% | 100 °C | [459] |
| Modified carbon nanotubes (MCNTs) | Tetraethylenepentamine | 5 mmol CO2/g | Containing 10 vol% CO2 in N2 with 1 vol% H2O | [460] |
| Microcrystalline cellulose | Amidoxime | 3.85 mmol/g | 120 °C, 1.01325 bar | [461] |
| CNTs | Pebax-1657 membrane | 5 wt% CNTs | [462] | |
| Functionalized carbon nanotubes | Carbon nanofibers (CNFs) | 6.3 mmol g−1 | At 1.0 bar and 298 K | [463] |
| Amine-loaded carbon nanotubes | Temperature/vacuum swing adsorption | 67% after TVSA | [463] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Zh. Bekmyrza, K.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. https://doi.org/10.3390/su15118815
Reza MS, Afroze S, Kuterbekov K, Kabyshev A, Zh. Bekmyrza K, Haque MN, Islam SN, Hossain MA, Hassan M, Roy H, et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability. 2023; 15(11):8815. https://doi.org/10.3390/su15118815
Chicago/Turabian StyleReza, Md Sumon, Shammya Afroze, Kairat Kuterbekov, Asset Kabyshev, Kenzhebatyr Zh. Bekmyrza, Md Naimul Haque, Shafi Noor Islam, Md Aslam Hossain, Mahbub Hassan, Hridoy Roy, and et al. 2023. "Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review" Sustainability 15, no. 11: 8815. https://doi.org/10.3390/su15118815






