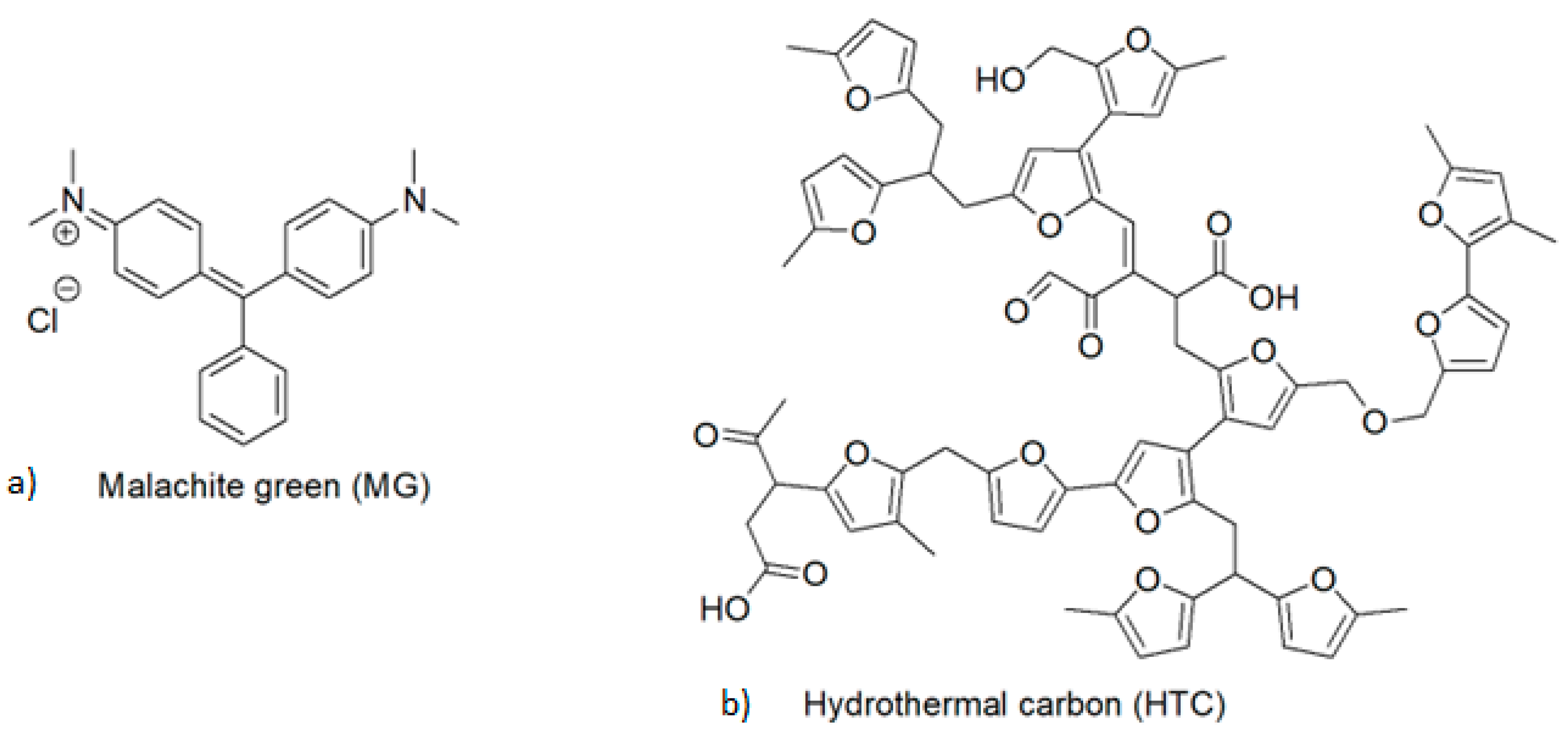
Removal of Malachite Green Using Hydrochar from PALM Leaves
Abstract
:1. Introduction
2. Experimental and Methodology
2.1. Equipment and Instrument
2.2. Biochar Preparation
2.2.1. Hydrothermal Carbonized Palm Leaves: HTC-PL Biochar
2.2.2. Activated Hydrothermal Carbonized Palm Leaves: AHTC-PL-208
2.3. Malachite Green Solution Preparation
2.4. Batch Experiments
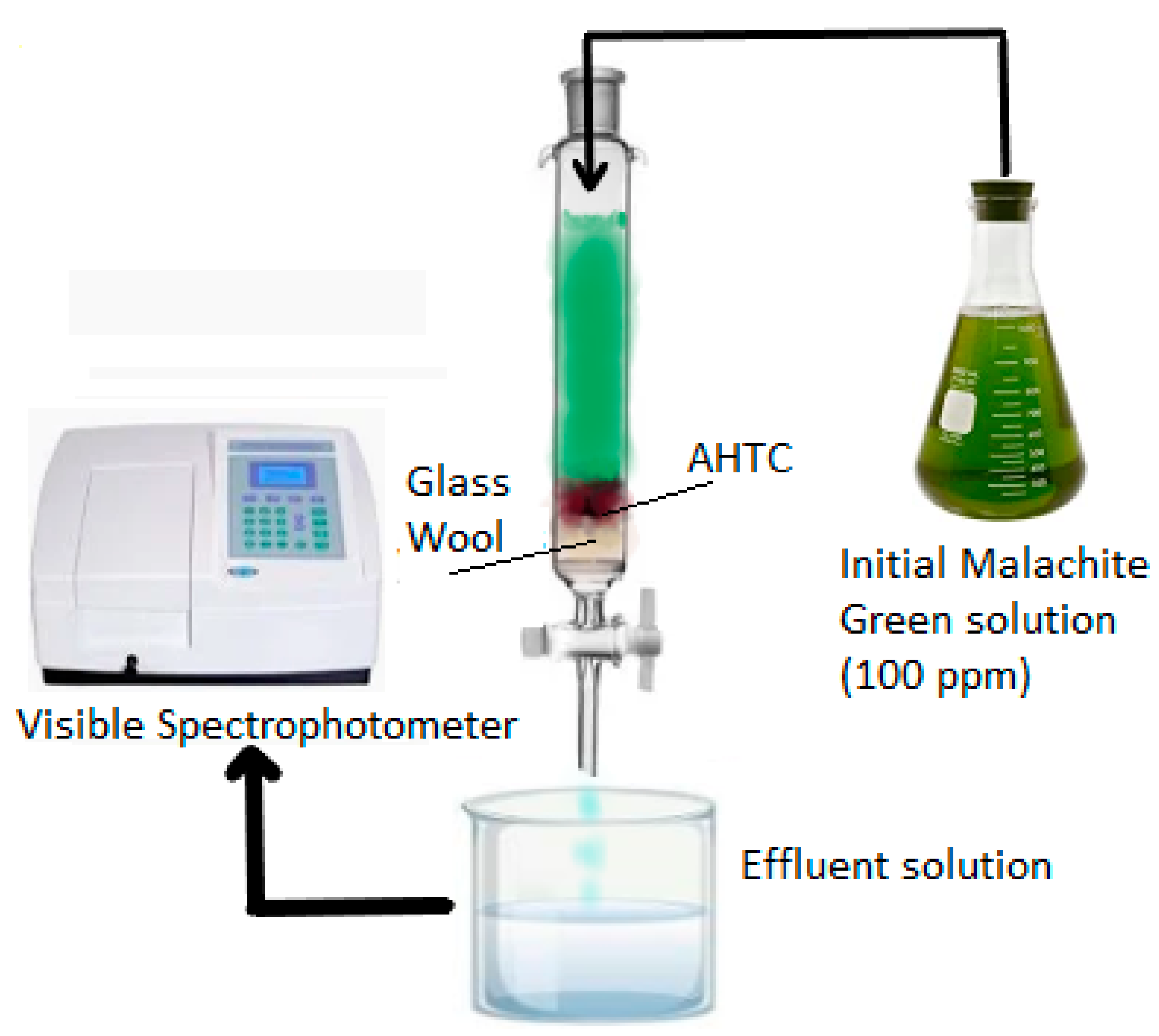
2.5. Column Study
2.6. Methodology
Adsorption Isotherm
3. Results and Discussion
3.1. Preparation of HTC Biochar
3.2. Properties of HTC Biochar
3.3. Thermal and TEM Analysis of HTC and AHTC
3.4. Effect of pH and Contact Time
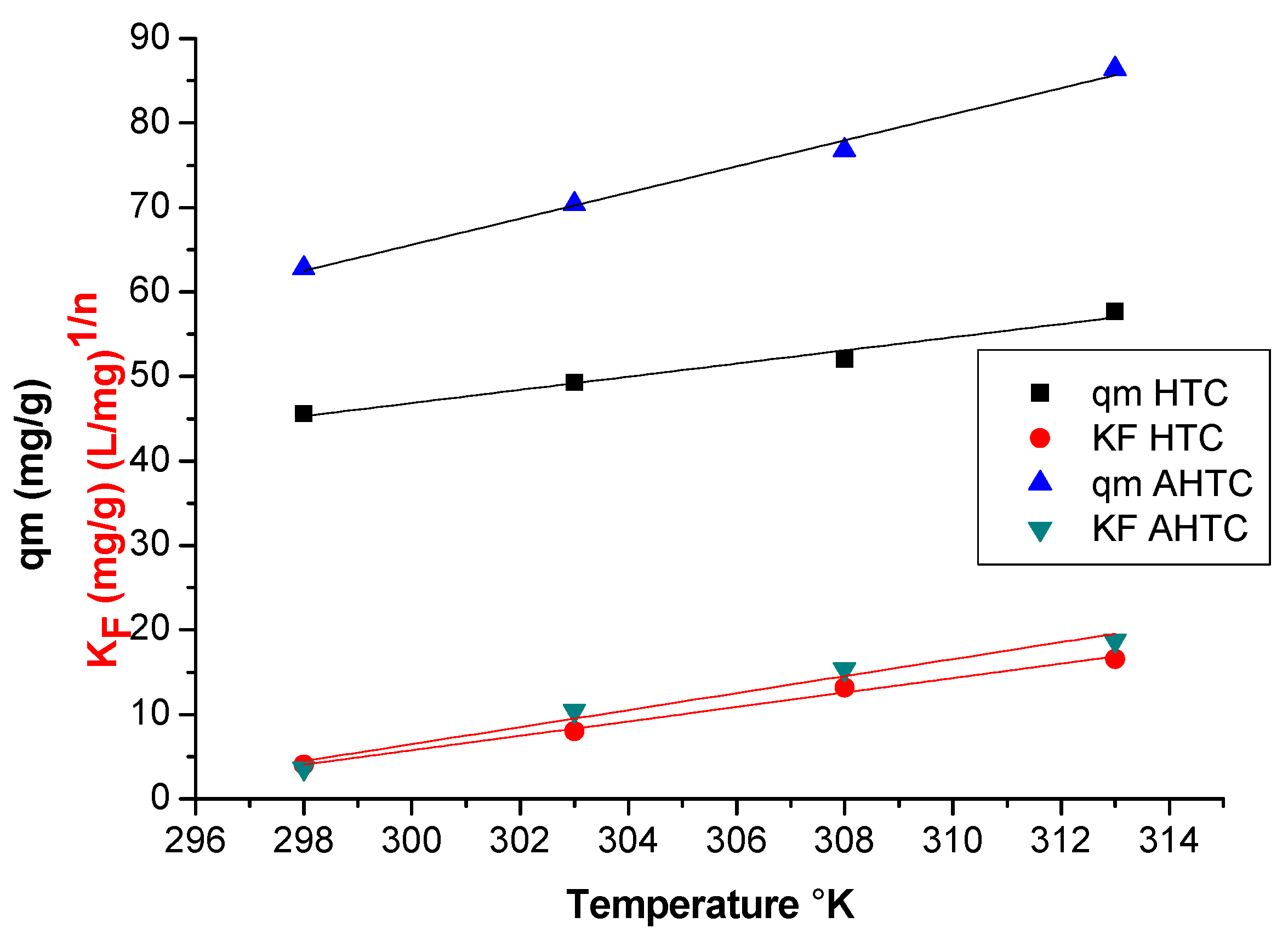
3.5. Isotherm Models Computations
3.6. Thermodynamics of Adsorption
3.7. Kinetics of Adsorption
Kinetics Models Computations
3.8. Comparison of Capacity of Prepared Biochar with Other Adsorbents
3.9. Adsorption in a Fixed Bed Column
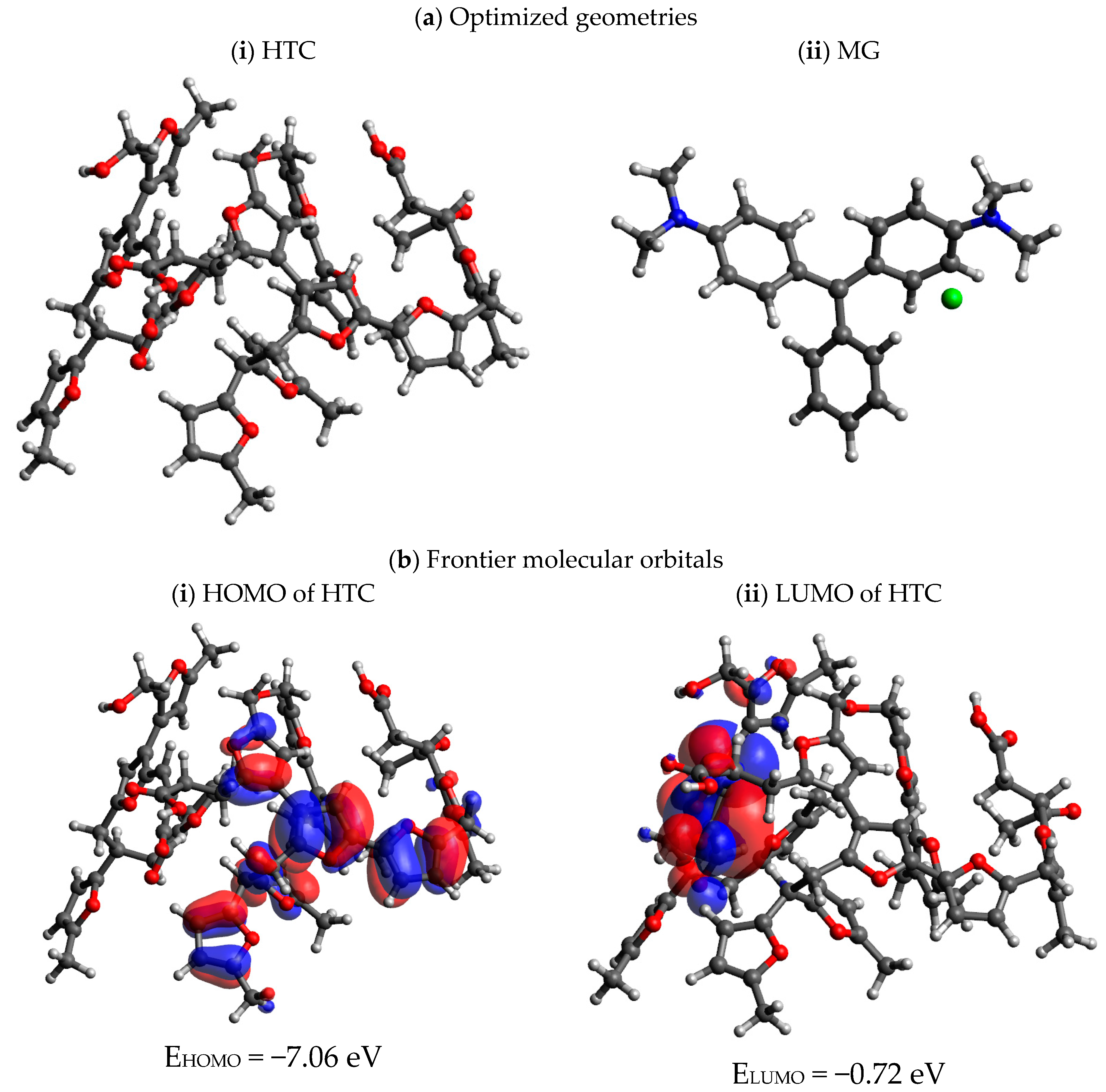
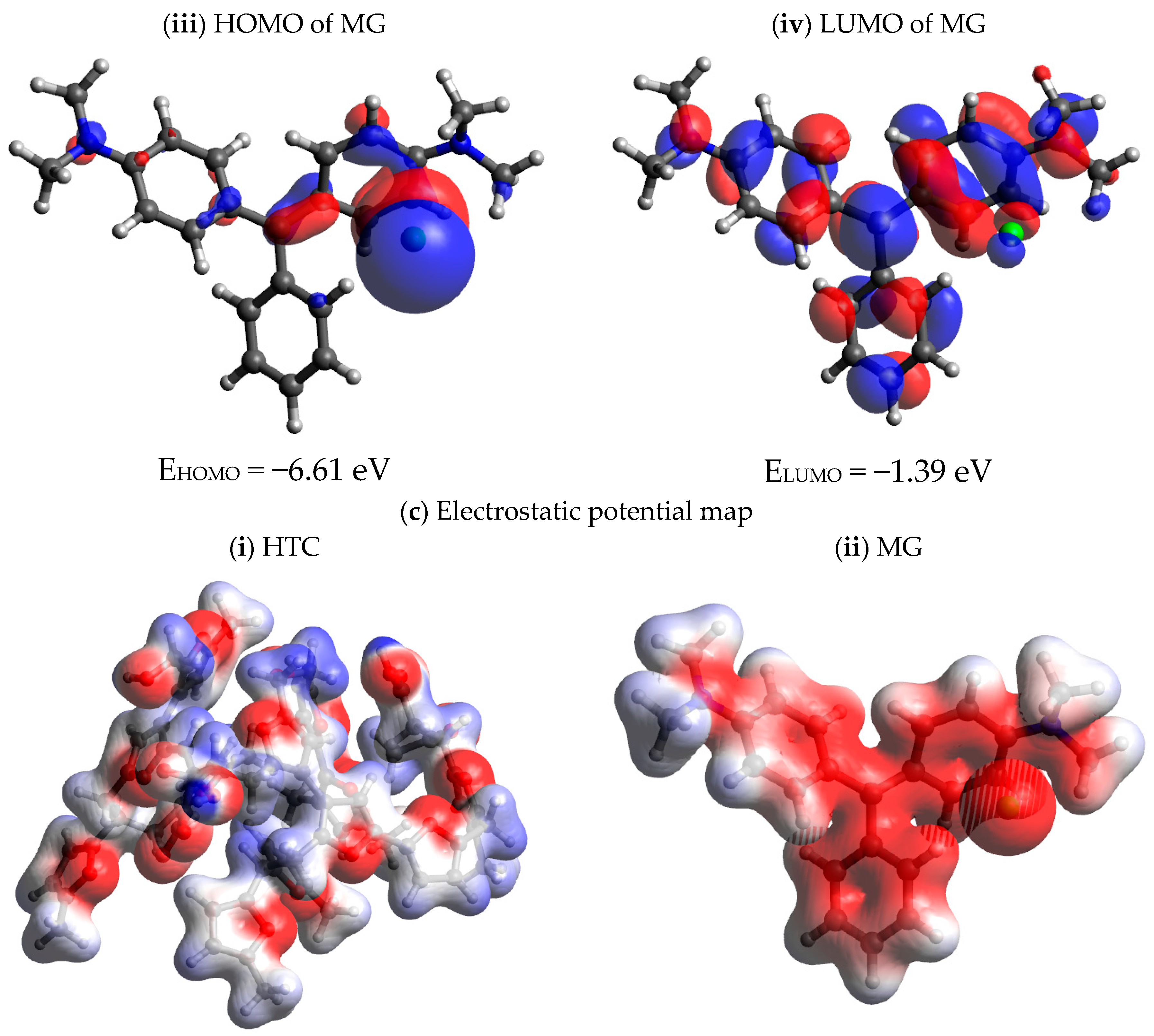
3.10. Computational Methods and Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahan, N.; Tahmid, M.; Shoronika, A.Z.; Fariha, A.; Roy, H.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. A Comprehensive Review on the Sustainable Treatment of Textile Wastewater: Zero Liquid Discharge and Resource Recovery Perspectives. Sustainability 2022, 14, 15398. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway, mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Baoventure, R.A.R. Adsorption modelling of textile dyes by sepiolite. Appl. Clay Sci. 2008, 42, 137–145. [Google Scholar] [CrossRef]
- Hammud, H.H.; Fayoumi, L.; Holail, H.; Mostafa, E.-S.M.E. Biosorption Studies of Methylene Blue by Mediterranean Algae Carolina and Its Chemically Modified Forms. Linear and Nonlinear Models’ Prediction Based on Statistical Error Calculation. Int. J. Chem. 2011, 3, 147–163. [Google Scholar] [CrossRef]
- Alfrhan, M.A.; Hammud, H.H.; Al-Omair, M.A.; El-Sonbati, M.A. Uptake of crystal violet from water by modified Khalas dates residues. Desalination Water Treat. 2020, 174, 361–375. [Google Scholar] [CrossRef]
- Hammud, H.H.; Shmait, A.; Hourani, N. Removal of Malachite Green from water using hydrothermally carbonized pine needles. RSC Adv. 2014, 5, 7909–7920. [Google Scholar] [CrossRef]
- Hammud, H.H.; El Hamaoui, B.; Noubani, N.H.; Feng, X.; Wu, Z.-S.; Mullen, K.; Ayub, K. Carbon-Cobalt Nanostructures as an Efficient Adsorbent of Malachite Green. Nanosci. Nanotechnol.-Asia 2018, 8, 263–280. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Karnati, R.K.; Hussain, S.G.; Mazher, J.; Prakasam, T. Cobalt–carbon/silica nanocomposites prepared by pyrolysis of a cobalt 2,2’-bipyridine terephthalate complex for remediation of cationic dyes. RSC Adv. 2020, 10, 17660–17672. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Hussain, S.G.; Prakasam, T. Novel Cobalt-Carbon@Silica Adsorbent. Sci. Rep. 2020, 10, 18652. [Google Scholar] [CrossRef] [PubMed]
- Hammud, H.H.; Karnati, R.K.; Alotaibi, N.; Hussain, S.G.; Prakasam, T. Cobalt–carbon silica nanoparticles for uptake of cationic and anionic dye from polluted water. Molecules 2021, 26, 7489. [Google Scholar] [CrossRef] [PubMed]
- Alramadhan, S.A.; Hammud, H.H. Graphene Nickel Silica Supported Nanocomposites, Application in Water Treatment. Appl. Nanosci. 2020, 11, 273–291. [Google Scholar] [CrossRef]
- Jia, C.; Xu, Z.; Luo, D.; Xiang, H.; Zhu, M. Flexible Ceramic Fibers: Recent Development in Preparation and Application. Adv. Fiber Mater. 2022, 4, 573–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, K.; Wang, D.; Wang, G.; Chu, P.K.; Meng, Z.; Wang, X. Hierarchical CuO–ZnO/SiO2 Fibrous Membranes for Efficient Removal of Congo Red and 4-Nitrophenol from Water. Adv. Fiber Mater. 2022, 4, 1069–1080. [Google Scholar] [CrossRef]
- He, J.; Mo, P.; Luo, Y.-S.; Yang, P.-H. Strategies for Solving the Issue of Malachite Green Residues in Aquatic Products: A Review. Aquac. Res. 2023, 2023, 1–17. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, B.; Huang, Y.; Liu, Y.; Wu, Y.; Qu, R.; Tang, C. Pyrolysis temperature affects the physiochemi-cal characteristics of lanthanum-modified biochar derived from orange peels: Insights into the mechanisms of tetracycline adsorption by spectroscopic analysis and theoretical calculations. Sci. Total Environ. 2023, 862, 160860. [Google Scholar] [CrossRef]
- Zhao, M.; Li, S.; Wang, M.; Guan, X.; Zhao, R. Adsorption of water and formic acid molecules on the (104) surface of calcite: A theoretical study by DFT-D3. New J. Chem. 2023, 47, 8737–8743. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Zhangab, H.; Zhangd, F.; Huang, Q. Highly effective removal of malachite green from aqueous solution by hydrochar derived from phycocyanin-extracted algal bloom residues through hydrothermal carbonization. RSC Adv. 2017, 7, 5790–5799. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Hanbali, M.; Holail, H.; Hammud, H. Remediation of lead by pretreated red algae: Adsorption isotherm, kinetic, column modeling and simulation studies. Green Chem. Lett. Rev. 2014, 7, 342–358. [Google Scholar] [CrossRef]
- Cecen, F.; Aktaş, Ö. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1–388. [Google Scholar]
- Do, D.D. Adsorption Analysis: Equilibrium and Kinetics, Series on Chemical Engineering (2); Imperial College Press: London, UK, 1998; pp. 1–913. [Google Scholar]
- Doğan, M.; Alkan, M.; Onganer, Y. Adsorption of Methylene Blue from Aqueous Solution onto Perlite. Water Air Soil Pollut. 2000, 120, 229–248. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. J. Hazard. Mater. 2010, 179, 178–186. [Google Scholar] [CrossRef]
- Deniz, F.; Karaman, S. Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Mohammad, A.; Prasad, B.; Mishra, I.M. Adsorption of Zn using factory tea waste:kinetics equilibrium and thermodynamics. Clean Soil Water Air 2008, 36, 320–329. [Google Scholar] [CrossRef]
- Ramnani, S.; Sabharwal, S. Adsorption behavior of Cr(VI) onto radiation crosslinked chitosan and its possible application for the treatment of wastewater containing Cr(VI). React. Funct. Polym. 2006, 66, 902–909. [Google Scholar] [CrossRef]
- Nwabanna, J.T.; Igokwe, P.k. Kinetic Modelling of Heavy Metals Adsorption on Fixed Bed column. Int. J. Environ. Res. 2012, 6, 945–952. [Google Scholar]
- Marquardt, D.W. An algorithm for least-squares of non-linear parameters. J. Soc. Appl. Ind. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Libra, A.J.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- McKendry, P. Review paper Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hammud, H.H.; Karnati, R.K.; Al Shafee, M.; Fawaz, Y.; Holail, H. Activated hydrochar from palm leaves as efficient lead adsorbent. Chem. Eng. Commun. 2019, 208, 197–209. [Google Scholar] [CrossRef]
- Demirbaş, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, X. Removal of malachite green from water by Firmiana simplex wood fiber. Electron. J. Biotechnol. 2009, 12, 1–10. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Pimentel, P.M.; Melo, M.A.F.; Melo, D.M.A.; Assucao, A.L.C.; Henrique, D.M.; Silva, C.N., Jr.; Gonzalez, G. Kinetics and thermodynamics of Cu(II)adsorption on oil shale wastes. Fuel Process. Tech. 2008, 89, 62–67. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, L.; Zhao, M.; Dai, L.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: Experimental and simulation. Biochar 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Hasany, S.; Bhanger, M. Sorption Profile of Cd(II) Ions onto Beach Sand from Aqueous Solution. J. Hazard. Mater. 2007, 141, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.P.; Boute, J. Intraparticle Diffusion-Adsorption Model to Describe Liquid/Solid Adsorption Kinetics. Rev. Mex. Ing. Quim. 2016, 15, 161–173. [Google Scholar]
- Boparai, H.K.; Joseph, M.; O’Caroll, D.M. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nanozero Valent Iron Particles. J. hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Kumar, K.V.; Sivanesan, S. Isotherms for Malachite Green onto rubber wood (Hevea brasiliensis) sawdust: Comparison of linear and non-linear methods. Dye. Pigment. 2007, 72, 124–129. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf. A 2005, 264, 17–28. [Google Scholar] [CrossRef]
- Pathy, A.; Krishnamoorthy, N.; Chang, S.X.; Paramasivan, B. Malachite green removal using algal biochar and its composites with kombucha SCOBY: An integrated biosorption and phycoremediation approach. Surf. Interfaces 2022, 30, 101880. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Khan, R.A.; Rafique, R.F.; Khalid, K. Batch and fixed bed adsorption studies of lead (II) cations from aqueous solutions onto granular activated carbon derived from Mangostana garcinia shell. BioResources 2012, 7, 2895–2915. [Google Scholar]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics.Part 1. A theoretical model for respirator catridge service time. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Rodríguez, A.; Álvarez, S.; García, J. Modeling and Elimination of Atenolol on Granular Activated Carbon in Fixed Bed Column. Int. J. Environ. Res. 2012, 6, 961–968. [Google Scholar] [CrossRef]
- Yahaya, N.; Abustan, I.; Latif, M.M.; Bello, O.; Ahmad, M. Fixed-bed Column study for Cr(II) removal from aqueous solutions using rice husk based activated carbon. Int. J. Eng. Technol. 2011, 11, 248–252. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Peterson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian: Wallingford, CT, USA, 2016.
- Denningtom, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009.
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Olusegun, S.; Rodrigues, G.; Freitas, E.; Lara, L.; Rocha, W.; Mohallem, N. Sequestrating anionic and cationic dyes from wastewater using spray dried biopolymeric magnetic composite: Experimental and theoretical studies. J. Hazard. Mater. 2019, 380, 120872. [Google Scholar] [CrossRef]
- El Haouti, R.; Ouachtak, H.; El Guerdaoui, A.; Amedlous, A.; Amaterz, E.; Haounati, R.; Addi, A.A.; Akbal, F.; El Alem, N.; Taha, M.L. Cationic dyes adsorption by Na-Montmorillonite Nano Clay: Experimental study combined with a theoretical investigation using DFT-based descriptors and molecular dynamics simulations. J. Mol. Liq. 2019, 290, 111139. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Huang, X.; Wu, Q.; Li, M.; Xie, Q.; Liu, Z.; Zou, X. Adsorption of sulfonamides on polyamide microplastics in an aqueous solution: Behavior, structural effects, and its mechanism. Chem. Eng. J. 2023, 454, 140452. [Google Scholar] [CrossRef]
- Politzer, P.; Abu-Awwad, F. A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor. Chem. Acta 1998, 99, 83. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Pearson, R.G. The electronic chemical potential and chemical hardness. J. Mol. Struct. Theochem. 1992, 255, 261–270. [Google Scholar] [CrossRef]





| Batch ID | Tmax (°C) | Ash (%) db | Mass Recovery% | Volatile Matter VM (%) db | Fixed Carbon FC (%) db | Fixed Carbon Yield yFC (%) db | Carbon C (%) db | HHV 1 (MJ/kg) db | ηcoal 2 (%) db |
|---|---|---|---|---|---|---|---|---|---|
| PL-186 | 186 | 3.5 | 30.97 | 75.38 | 21.12 | 16.03 | 43.5 | 20.95 | 79.6 |
| PL-201 | 201 | 3.2 | 30.08 | 74.92 | 21.88 | 16.05 | 45.5 | 21.71 | 79.7 |
| PL-207 | 207 | 2.93 | 29.90 | 74.36 | 22.71 | 16.59 | 47.5 | 22.10 | 80.8 |
| PL-212 | 212 | 2.5 | 29.48 | 73.85 | 23.65 | 17.01 | 50.4 | 23.36 | 84.0 |
| PL-225 | 244 | 2 | 29.30 | 73.49 | 24.51 | 17.52 | 50.9 | 24.81 | 88.7 |
| (A) Sample | T °C | Non-Linear Langmuir Model | Non-Linear Freundlich Model | |||||||||
| qm (mg/g) | RL | KL (L/mg) | SSE | χ2 | R2 | KF (mg/g) (L/mg) 1/n | n | SSE | χ2 | R2 | ||
| HTC | 25 °C | 45.59 | 0.562 | 0.026 | 34.27 | 6.85 | 0.94 | 4.06 | 2.17 | 57.8805 | 11.575 | 0.894 |
| 30 °C | 49.28 | 0.386 | 0.043 | 24.06 | 8.02 | 0.95 | 8.01 | 2.58 | 208.285 | 41.657 | 0.817 | |
| 35 °C | 52.03 | 0.287 | 0.067 | 16.59 | 9.32 | 0.91 | 13.18 | 4.06 | 49.249 | 12.312 | 0.888 | |
| 40 °C | 57.66 | 0.262 | 0.076 | 54.06 | 9.01 | 0.95 | 16.55 | 4.02 | 12.854 | 6.571 | 0.990 | |
| AHTC | 25 °C | 62.80 | 0.503 | 0.033 | 60.09 | 15.02 | 0.91 | 3.58 | 1.65 | 142.571 | 28.514 | 0.870 |
| 30 °C | 70.40 | 0.448 | 0.041 | 96.23 | 16.04 | 0.94 | 10.41 | 2.43 | 119.876 | 19.979 | 0.930 | |
| 35 °C | 76.78 | 0.342 | 0.048 | 43.85 | 7.31 | 0.97 | 15.35 | 2.91 | 50.960 | 8.192 | 0.946 | |
| 40 °C | 86.41 | 0.284 | 0.063 | 46.70 | 9.34 | 0.96 | 18.70 | 3.13 | 53.530 | 10.883 | 0.933 | |
| (B) Sample | Non-Linear Temkin | Non-Linear D-R | ||||||||||
| Kt (L/mg) | B (J/mol) | R2 | β | E (KJ/mol) | qm (mg/g) | R2 | ||||||
| HTC | 25 °C | 0.206 | 230.24 | 0.974 | 0.0079 | 7.375 | 30.95 | 0.949 | ||||
| 30 °C | 0.622 | 221.85 | 0.910 | 0.0057 | 7.382 | 49.12 | 0.995 | |||||
| 35 °C | 1.027 | 244.47 | 0.909 | 0.0078 | 7.396 | 53.13 | 0.836 | |||||
| 40 °C | 1.762 | 248.45 | 0.989 | 0.0081 | 7.201 | 53.95 | 0.945 | |||||
| AHTC | 25 °C | 0.119 | 118.33 | 0.944 | 0.0081 | 7.866 | 58.42 | 0.830 | ||||
| 30 °C | 0.394 | 142.65 | 0.986 | 0.0082 | 7.803 | 65.27 | 0.976 | |||||
| 35 °C | 0.377 | 145.57 | 0.967 | 0.0085 | 7.652 | 66.17 | 0.9927 | |||||
| 40 °C | 0.792 | 146.74 | 0.958 | 0.0084 | 7.700 | 82.56 | 0.938 | |||||
| Thermodynamic Parameters | Activation Thermodynamic Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Temperature °C | ΔH° (KJ/mol) | ΔS° (J/K·mol) | ΔG° (KJ/mol) | ΔH≠ (KJ/mol) | ΔS≠ (J/K·mol) | ΔG≠ (KJ/mol) | Ea (KJ/mol) |
| HTC | 298 | 39.79 | 132.18 | 0.404 | 5.06 | −312.75 | 96.69 | 7.55 |
| 303 | −0.257 | 98.25 | ||||||
| 308 | −0.918 | 99.82 | ||||||
| 313 | −1.579 | 101.38 | ||||||
| AHTC | 298 | 54.24 | 185.28 | −0.976 | 29.05 | −238.30 | 98.87 | 31.55 |
| 303 | −1.902 | 100.06 | ||||||
| 308 | −2.829 | 101.25 | ||||||
| 313 | −3.755 | 102.45 | ||||||
| Sample | Temperature °C | qe (mg/g) (Exp.) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/mg·min) | R2 | |||
| HTC | 25 | 29.97 | 21.82 | 0.0085 | 0.93 | 23.70 | 0.0021 | 0.99 |
| 30 | 37.62 | 23.08 | 0.0092 | 1.00 | 32.05 | 0.0022 | 0.99 | |
| 35 | 39.30 | 27.59 | 0.0094 | 0.88 | 30.58 | 0.0024 | 0.98 | |
| 40 | 39.59 | 28.54 | 0.0099 | 0.84 | 30.30 | 0.0025 | 0.98 | |
| AHTC | 25 | 34.39 | 21.42 | 0.0046 | 0.93 | 37.74 | 0.0008 | 0.99 |
| 30 | 41.55 | 21.12 | 0.0067 | 0.95 | 37.74 | 0.0012 | 0.99 | |
| 35 | 43.23 | 24.80 | 0.0076 | 0.83 | 40.49 | 0.0013 | 0.99 | |
| 40 | 46.67 | 31.65 | 0.0387 | 1.00 | 52.08 | 0.0015 | 1.00 | |
| Chemical Reactivity Parameters | HTC | MG |
|---|---|---|
| EHOMO (eV) | 22,127.060 | 22,126.610 |
| ELUMO (eV) | 22,120.720 | 22,121.390 |
| EHOMO–LUMO (eV) | 6.340 | 5.220 |
| Ionization Potential (I, eV) | 7.06 | 6.61 |
| Electron Affinity (A, eV) | 0.72 | 1.39 |
| Electronic Chemical Potential (μ, eV) | 22,123.89 | 22,124.00 |
| Global Hardness (η, eV) | 3.17 | 2.61 |
| Global Softness (S, eV) | 0.32 | 0.38 |
| Global Electrophilicity (ω, eV) | 23.98 | 20.88 |
| Dipole moment (D) | 4.00 | 11.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Hammoud, M.H.; Hussein, A.A.; Fawaz, Y.B.; Abdul Hamid, M.H.S.; Sheikh, N.S. Removal of Malachite Green Using Hydrochar from PALM Leaves. Sustainability 2023, 15, 8939. https://doi.org/10.3390/su15118939
Hammud HH, Hammoud MH, Hussein AA, Fawaz YB, Abdul Hamid MHS, Sheikh NS. Removal of Malachite Green Using Hydrochar from PALM Leaves. Sustainability. 2023; 15(11):8939. https://doi.org/10.3390/su15118939
Chicago/Turabian StyleHammud, Hassan H., Mohamad H. Hammoud, Aqeel A. Hussein, Youssef B. Fawaz, Malai Haniti Sheikh Abdul Hamid, and Nadeem S. Sheikh. 2023. "Removal of Malachite Green Using Hydrochar from PALM Leaves" Sustainability 15, no. 11: 8939. https://doi.org/10.3390/su15118939





