Effects of Ultrasound Treatment on the Physical and Chemical Properties of Ice Cream with a Strawberry Seed Oil Oleogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of the Oil
2.2. Obtaining and Analyzing the Oleogel
2.3. Recipe and Preparation of the Ice Cream
2.4. Ice Cream Charactrerization
2.4.1. Analysis of the Chemical Composition
2.4.2. Determination of the Content of Mineral Compounds
2.4.3. Free-Radical Scavenging Activity
2.4.4. Total Phenolic Content
2.4.5. Physical Properties of the Ice Cream
2.4.6. Measurements Using Fourier Transform Infrared Spectroscopy (FTIR)
2.4.7. Microstructure
2.5. Statistical Analysis
3. Results and Discussion
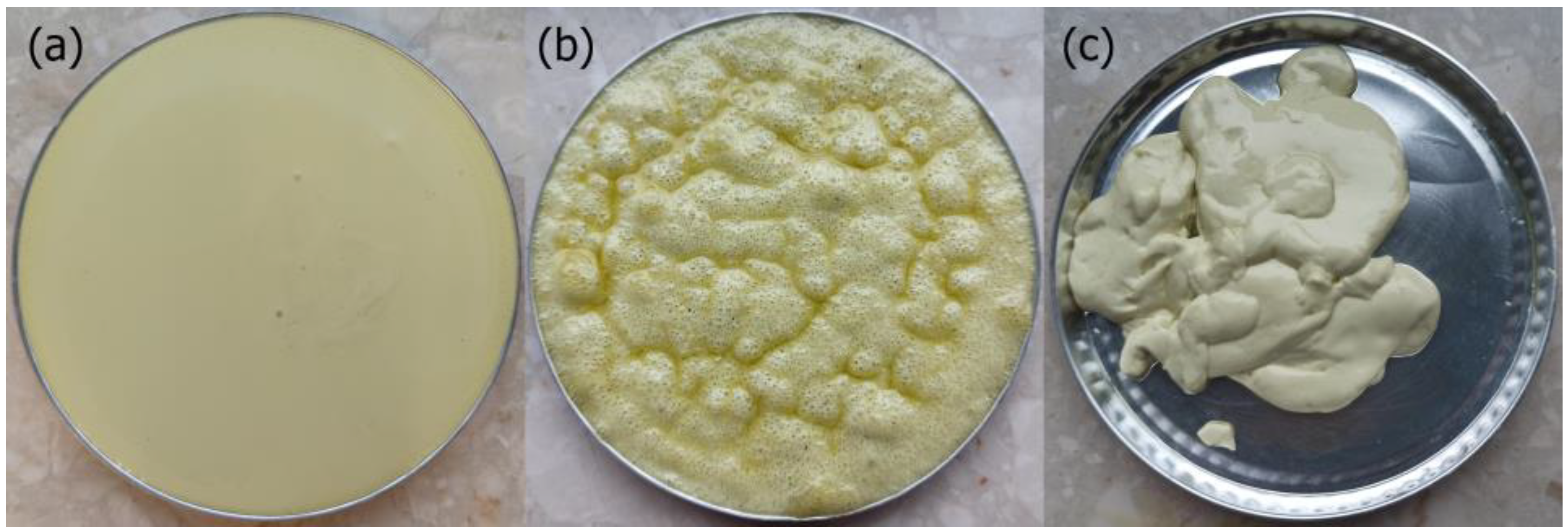
3.1. Properties of the Oleogels
3.2. Chemical Properties of the Ice Cream
3.3. Physical Properties of the Ice Cream
3.4. FTIR Spectroscopy
| FTIR | Type and Origin of Vibrations |
|---|---|
| Positioning of Band [cm−1] | |
| 3294 | νst(O–H) and –OH in H2O |
| 2952 | νs+as (C–H) in CH2 and CH3 groups, both in carbohydrates and in fatty acids |
| 2920 | |
| 2870 | |
| 2850 | |
| 1743 | ν (C=O) and ν (C=O)···OH |
| 1725 | |
| 1649/1644 | δvw (–OH) and νvw (–C=C–) and Amide I |
| 1544 | νvw (–C=C–) and Amide II |
| 1452 | δ (–O–CH) and δ (–C–C–H) |
| 1415 | δst (O–H) in C–OH group + δ (C–H) |
| 1376 | δ (–OH) in C–OH group and ν (–C–H, –CH3) and deformation |
| 1342 | |
| 1238 | νm (–C–O) or δm (–CH2–) ν (C–H) in carbohydrates and Amide III |
| 1198/1144 | ν (C–H) in carbohydrates and ν (C–O) in C–O–C group and νst (C–C) in the carbohydrate structure |
| 1097 | |
| 1048 | |
| 1030 | |
| 976 | νst (C–C) in the carbohydrate structure, δ (C–H) |
| 963 | |
| 916 | |
| 892 | ν (C–C) in the carbohydrate structure, δ (C–H) |
| 864 | |
| 815 | |
| 776 | |
| 698 |
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akdeniz, V.; Akalin, A.S. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of high-intensity ultrasound to improve food processing efficiency: A review. Foods 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Lopez, L.M.; Garcia-Galicia, I.A.; Tirado-Gallegos, J.M.; Sanchez-Vega, R.; Huerta-Jimenez, M.; Ashokkumar, M.; Alarcon-Rojo, A.D. Recent advances in the application of ultrasound in dairy products: Effect on functional, physical, chemical, microbiological and sensory properties. Ultrason. Sonochem. 2021, 73, 105467. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, V.; Ghasemi-Varnamkhasti, M.; González, L.A. Analytical measurements of ultrasound propagation in dairy products: A review. Trends Food Sci. Technol. 2017, 61, 38–48. [Google Scholar] [CrossRef]
- Bahram-Parvar, M. A review of modern instrumental techniques for measurements of ice cream characteristics. Food Chem. 2015, 188, 625–631. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]
- Baboli, Z.M.; Williams, L.; Chen, G. Design of a batch ultrasonic reactor for rapid pasteurization of juices. J. Food Eng. 2020, 268, 109736. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Muhammad, A.I.; Song, L.; Guo, M.; Liu, D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020, 320, 126614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, P.; Sun, D.W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020, 60, 104733. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Anema, S.G. Ultrasonication of reconstituted whole milk and its effect on acid gelation. Food Chem. 2017, 217, 593–601. [Google Scholar] [CrossRef]
- Zisu, B.; Schleyer, M.; Chandrapala, J. Application of ultrasound to reduce viscosity and control the rate of age thickening of concentrated skim milk. Int. Dairy J. 2013, 31, 41–43. [Google Scholar] [CrossRef]
- Erfanian, A.; Rasti, B. Effects of sonication condition on milk-soymilk yogurt properties. Int. Food Res. J. 2019, 26, 1823–1834. [Google Scholar]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. A comparison of selected quality characteristics of yoghurts prepared from thermosonicated and conventionally heated milks. Food Chem. 2010, 119, 1108–1113. [Google Scholar] [CrossRef]
- Türker, A.D.; Dogan, M. Effects of ultrasound homogenization on the structural and sensorial attributes of ice cream: Optimization with Taguchi and data envelopment analysis. Food Meas. 2021, 15, 4888–4898. [Google Scholar] [CrossRef]
- Kot, A.; Kamińska-Dwórznicka, A.; Jakubczyk, E. Study on the influence of ultrasound homogenisation on the physical properties of vegan ice cream mixes. Appl. Sci. 2022, 12, 8492. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Oleogels prepared with low molecular weight gelators: Texture, rheology and sensory properties, a review. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on food-grade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 7659–7676. [Google Scholar] [CrossRef] [PubMed]
- Banaś, K.; Harasym, J. Natural gums as oleogelators. Int. J. Mol. Sci. 2021, 22, 12977. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B. Recent developments of oleogel utilizations in bakery products. Crit. Rev. Food Sci. Nutr. 2019, 60, 2460–2479. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B. Utilization of Beeswax Oleogel-Shortening Mixtures in Gluten-Free Bakery Products. J. Am. Oil Chem. Soc. 2019, 96, 545–554. [Google Scholar] [CrossRef]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; de Walle, D.V.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef]
- Moriano, M.E.; Alamprese, C. Organogels as novel ingredients for low saturated fat ice creams. LWT 2017, 86, 371–376. [Google Scholar] [CrossRef]
- Nazarewicz, S.; Kozłowicz, K.; Kobus, Z.; Gładyszewska, B.; Matwijczuk, A.; Ślusarczyk, L.; Skrzypek, T.; Sujka, M.; Kozłowicz, N. The Use of Ultrasound in Shaping the Properties of Ice Cream with Oleogel Based on Oil Extracted from Tomato Seeds. Appl. Sci. 2022, 12, 9165. [Google Scholar] [CrossRef]
- Airoldi, R.; da Silva, T.L.T.; Ract, J.N.R.; Foguel, A.; Colleran, H.L.; Ibrahim, S.A.; da Silva, R.C. Potential use of carnauba wax oleogel to replace saturated fat in ice cream. J. Am. Oil Chem. Soc. 2022, 99, 1085–1099. [Google Scholar] [CrossRef]
- Jing, X.; Chen, Z.; Tang, Z.; Tao, Y.; Huang, Q.; Wu, Y.; Zhang, H.; Li, X.; Liang, J.; Liu, Z.; et al. Preparation of camellia oil oleogel and its application in an ice cream system. LWT 2022, 169, 113985. [Google Scholar] [CrossRef]
- Zulim Botega, D.C.; Marangoni, A.G.; Smith, A.K.; Goff, H.D. The potential application of rice bran wax oleogel to replace solid fat and enhance unsaturated fat content in ice cream. J. Food Sci. 2013, 7, C1334–C1339. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.B.; Munk, D.M.; Gustavsson, F.; Risbo, J. Using Ethylcellulose to Structure Oil Droplets in Ice Cream Made with High Oleic Sunflower Oil. J. Food Sci. 2018, 83, 2520–2526. [Google Scholar] [CrossRef]
- Silva-Avellaneda, E.; Bauer-Estrada, K.; Prieto-Correa, R.E.; Quintanilla-Carvajal, M.X. The effect of composition, microfluidization and process parameters on formation of oleogels for ice cream applications. Sci. Rep. 2021, 11, 7161. [Google Scholar] [CrossRef]
- 12966-2:2017; Animal and Vegetable Fats and Oils Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO International Organization for Standardization: Geneva, Switzerland, 2017.
- Żbikowska, A.; Kupiec, M.; Onacik-Gür, S. Wpływ karagenu na teksturę i stabilność oleożeli hydroksypropylometylocelulozowych. Acta Agrophysica 2017, 24, 553–561. [Google Scholar]
- Yılmaz, E.; Öğütcü, M. Properties and stability of hazelnut oil organogels with beeswax and monoglyceride. J. Am. Oil Chem. Soc. 2014, 91, 1007–1017. [Google Scholar] [CrossRef]
- Da Pieve, S.; Calligaris, S.; Co, E.; Nicoli, M.C.; Marangoni, A.G. Shear nanostructuring of monoglyceride organogels. Food Biophys. 2010, 5, 211–217. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Novotny, J.; Gebauer, S.; Baer, D. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 2012, 92, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunová, S.; Ivanišová, E.; Žiarovská, J.; Zamiešková, L.; Felsöciová, S.; Petkoska, A.T.; Nedelkoska, D.N.; Kačániová, M. Differences between microbiota, phytochemical, antioxidant profile and DNA fingerprinting of cabernet sauvignon grape from Slovakia and Macedonia. Slovak J. Food Sci. 2020, 14, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Savanović, D.M.; Grujić, R.D.; Savanović, J.M.; Mandić, S.U.; Rakita, S.M. Analysis of frozen chicken meat using differential scanning calorimetry. Food Feed Res. 2018, 45, 129–137. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Baba, A.S.; Misran, M. Effect of vegetable milks on the physical and rheological properties of ice cream. Food Sci. Technol. Res. 2014, 20, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Eskandari, M.H.; Niakosari, M.; Bedeltavana, A. The effect of inulin on the physicochemical properties and sensory attributes of low-fat ice cream. Int. Dairy J. 2016, 57, 52–55. [Google Scholar] [CrossRef]
- Güven, M.; Karaca, O.B. The effect of varying sugar content and fruit concentration on the physical properties of vanilla and fruit ice-cream-type frozen yogurts. Int. J. Dairy Technol. 2002, 55, 27–31. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, H.K.; Kumar, N.; Kaur, M. The effect of inulin as a fat replacer on the quality of low-fat ice cream. Int. J. Dairy Technol. 2015, 68, 374–380. [Google Scholar] [CrossRef]
- Skrzypek, T.; Kazimierczak, W.; Zięba, E.; Olszewski, J.; Ferenc, K.; Zabielski, R. Chapter 2—How to Get A Proper 2D and 3D Image? In Atlas of the Pig Gut, Research and Techniques from Birth to Adulthood; Zabielski, R., Skrzypek, T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–36. [Google Scholar] [CrossRef]
- Kola, O.; Parıldı, E.; Keçeli, N.; Akkaya, M.R. Fatty Acids Composition and Bioactive Substances of Cold Pressed Oils from Strawberry Seed Çilek Tohumundan Soğuk Sıkımla Elde Edilen Yağların Biyoaktif Bileşenleri ve Yağ Asidi Kompozisyonu. Turk. J. Eng. Res. Educ. 2022, 1, 62–70. [Google Scholar]
- da Silva, A.C.; Jorge, N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1600024. [Google Scholar] [CrossRef]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native Oils from Apple, Blackcurrant, Raspberry, and Strawberry Seeds as a Source of Polyenoic Fatty Acids, Tocochromanols, and Phytosterols: A Health Implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef] [Green Version]
- Sikora, E.; Michorczyk, P.; Olszańska, M.; Ogonowski, J. Supercritical CO2 extract from strawberry seeds as a valuable component of mild cleansing compositions. Int. J. Cosmet. Sci. 2015, 37, 574–578. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Mawson, R.; Versteeg, K.; Barbosa-Cánovas, G.V. Composition properties, physicochemical characteristics and shelf life of whole milk after thermal and thermo-sonication treatments. J. Food Qual. 2009, 32, 283–302. [Google Scholar] [CrossRef]
- Villamiel, M.; de Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Akca, S.; Akpinar, A. The Effects of grape, pomegranate, sesame seed powder and their oils on probiotic ice cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Nascimento, E.D.A.; Melo, E.D.A.; Lima, V.L.A.G.D. Ice cream with functional potential added grape agro-industrial waste. J. Culin. Sci. Technol. 2018, 16, 128–148. [Google Scholar] [CrossRef]
- Sayar, E.; Şengül, M.; Ürkek, B. Antioxidant capacity and rheological, textural properties of ice cream produced from camel’s milk with blueberry. J. Food Process. Preserv. 2022, 46, e16346. [Google Scholar] [CrossRef]
- Storck, C.R.; Basso, C.; Favarin, F.R.; Rodrigues, A.C. Microbiological quality and composition of flour from fruit juice production residues with different granulometries. Braz. J. Food Technol. 2015, 18, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Haghani, S.; Hadidi, M.; Pouramin, S.; Adinepour, F.; Hasiri, Z.; Moreno, A.; Munekata, P.E.S.; Lorenzo, J.M. Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants 2021, 10, 1777. [Google Scholar] [CrossRef]
- Kotan, T.E. Mineral composition and some quality characteristics of ice creams manufactured with the addition of blueberry. Gıda 2018, 43, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, S.; Saeedabadian, A. Influences of lactose hydrolysis of milk and sugar reduction on some physical properties of ice cream. J. Food Sci. Technol. 2015, 52, 367–374. [Google Scholar] [CrossRef]
- Carvalho, C.C.; Bodini, R.B.; Sobral, P.J.; Oliveira, A.L. Ice creams made from cow’s and goat’s milks with different fat concentrations: Physical-chemical and sensory properties. Food Sci. Technol. 2022, 42, e79721. [Google Scholar] [CrossRef]
- Warren, M.M.; Hartel, R.W. Effects of emulsifier, overrun and dasher speed on ice cream microstructure and melting properties. J. Food Sci. 2018, 83, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Dertli, E.; Toker, O.S.; Durak, M.Z.; Yilmaz, M.T.; Tatlısu, N.B.; Sagdic, O.; Cankurt, H. Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: Rheological, molecular, microstructural and sensory characterization. Carbohydr. Polym. 2016, 136, 427–440. [Google Scholar] [CrossRef]
- Pulungan, M.H.; Santoso, E.S.M. Ice cream cup production using purple sweet potato (Ipomoea batatas L. Poir) as a substitute ingredient. Ind. J. Teknol. Dan Manaj. Agroindustri 2020, 9, 184–194. [Google Scholar] [CrossRef]
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–195. [Google Scholar] [CrossRef]
- Shukri, W.H.Z.; Hamzah, E.N.H.; Halim, N.R.A.; Isa, M.I.N.; Sarbon, N.M. Effect of different types of hydrocolloids on the physical and sensory properties of ice cream with fermented glutinous rice (tapai pulut). Int. Food Res. J. 2014, 21, 1777–1787. [Google Scholar]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of probiotics in multi-polysaccharide microcapsules by electro-hydrodynamic atomization and incorporation into ice-cream formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Vladimír, M.; Matwijczuk, A.P.; Niemczynowicz, A.; Kycia, R.A.; Karcz, D.; Gładyszewska, B.; Ślusarczyk, L.; Burg, P. Chemometric approach to characterization of the selected grape seed oils based on their fatty acids composition and FTIR spectroscopy. Sci. Rep. 2021, 11, 19256. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, D.; Dhiman, A.K. Encapsulation of soy isoflavone extract by freeze drying, its stability during storage and development of isoflavone enriched yoghurt. J. Food Sci. Technol. 2022, 59, 4945–4955. [Google Scholar] [CrossRef] [PubMed]



| Fatty Acid | Content [%] | |
|---|---|---|
| Saturated fatty acid | Myristic acid C14:0 | 0.05 |
| Palmitic acid C16:0 | 4.88 | |
| Margaric (heptadecanoic) acid C17:0 | 0.05 | |
| Stearic acid C18:0 | 2.18 | |
| Arachidic (eicosanoic) acid C20:0 | 0.55 | |
| Unsaturated fatty acid | Linoleic acid (n-6) C18:2 | 47.37 |
| Linolenic acid (n-3) C18:3 | 17.64 | |
| Palmitoleic acid C16:1 | 0.20 | |
| Oleic acid (n-9) C18:1 | 24.11 | |
| cis-5 Eicosenoic acid C20:1 | 0.21 |
| Properties | PT5 | PT6 | PT7 | UT5 | UT6 | UT7 |
|---|---|---|---|---|---|---|
| Dry matter | 36.80 ab ± 0.14 | 36.89 ab ± 0.16 | 36.59 a ± 0.34 | 37.19 ab ± 0.47 | 37.43 b ± 0.16 | 37.29 ab ± 0.31 |
| pH | 6.29 d ± 0.01 | 6.34 ab ± 0.01 | 6.40 c ± 0.01 | 6.32 a ± 0.01 | 6.36 b ± 0.01 | 6.40 c ± 0.01 |
| Protein | 13.91 b ± 0.06 | 13.15 d ± 0.05 | 12.16 a ± 0.04 | 13.90 b ± 0.03 | 12.90 c ± 0.09 | 12.26 a ± 0.07 |
| Fat | 10.56 a ± 0.08 | 11.88 d ± 0.03 | 12.75 e ± 0.03 | 9.93 b ± 0.03 | 10.45 a ± 0.03 | 11.07 c ± 0.03 |
| Ash | 3.16 c ± 0.02 | 2.82 b ± 0.03 | 2.65 a ± 0.04 | 3.05 c ± 0.06 | 2.87 b ± 0.06 | 2.68 a ± 0.06 |
| Carbohydrate | 48.60 a ± 0.13 | 48.77 a ± 0.01 | 50.53 b ± 0.07 | 56.82 e ± 0.08 | 54.35 d ± 0.07 | 52.58 c ± 0.25 |
| Dietary fiber | 20.48 f ± 0.06 | 19.97 e ± 0.05 | 19.22 d ± 0.04 | 12.92 a ± 0.05 | 16.04 b ± 0.06 | 18.13 c ± 0.10 |
| Caloric value | 386 b ± 0.24 | 395 a ± 0.16 | 404 d ± 0.21 | 398 c ± 0.44 | 395 a ± 0.20 | 395 a ± 0.28 |
| TPC (mg GAE·(100 g)−1) | 1.00 b ± 0.04 | 0.98 b ± 0.06 | 0.78 c ± 0.02 | 0.99 b ± 0.01 | 1.28 a ± 0.05 | 0.86 c ± 0.07 |
| Radical-Scavenging Activity (DPPH mg TEAC·(100 g)−1) | 1.24 b ± 0.02 | 1.29 b ± 0.04 | 1.42 a ± 0.06 | 1.20 b ± 0.04 | 1.16 b ± 0.06 | 0.94 c ± 0.05 |
| PT5 | PT6 | PT7 | UT5 | UT6 | UT7 | |
|---|---|---|---|---|---|---|
| K | 4059.80 | 4008.35 | 3994.50 | 4231.10 | 4042.90 | 3913.60 |
| Na | 1426.80 | 1404.00 | 1403.60 | 1478.70 | 1405.90 | 1345.10 |
| Ca | 4362.00 | 4018.00 | 5057.70 | 3637.80 | 3550.70 | 3974.40 |
| Mg | 458.90 | 448.24 | 458.20 | 422.30 | 418.50 | 418.70 |
| P | 3812.90 | 3315.30 | 3162.00 | 3466.40 | 3403.40 | 3204.00 |
| Fe | 45.20 | 45.80 | 30.10 | 45.50 | 37.00 | 16.90 |
| Mn | 0.10 | 0.20 | 0.20 | 0.40 | 0.10 | 0.10 |
| Zn | 10.10 | 9.70 | 8.90 | 9.70 | 9.30 | 9.10 |
| Cu | 1.00 | 0.80 | 0.70 | 0.30 | 0.70 | 0.70 |
| Co | 0.40 | 0.40 | 0.50 | 0.20 | 0.40 | 0.40 |
| Properties | PT5 | PT6 | PT7 | UT5 | UT6 | UT7 |
|---|---|---|---|---|---|---|
| Freezing point [°C] | −5.32 a ± 0.27 | −5.09 a ± 0.14 | −4.67 a ± 0.45 | −5.06 a ± 0.18 | −4.58 a ± 0.49 | −4.50 a ± 0.45 |
| Freezable water [%] | 39.02 c ± 0.48 | 40.48 a ± 0.49 | 41.45 ab ± 0.38 | 40.38 a ± 0.35 | 40.99 a ± 0.57 | 42.51 b ± 0.49 |
| Enthalpy of fusion [J·g−1] | 130.32 c ± 1.61 | 135.19 a ± 1.64 | 138.46 ab ± 1.26 | 134.86 a ± 1.15 | 136.91 a ± 1.91 | 141.97 b ± 1.62 |
| Overrun [%] | 38.94 c ± 0.40 | 41.24 d ± 0.47 | 45.64 e ± 1.09 | 31.60 a ± 0.86 | 31.45 a ± 0.38 | 26.01 b ± 0.57 |
| Complete melting time [min.] | 23.52 a ± 0.06 | 29.69 c ± 0.28 | 27.37 b ± 0.08 | 33.25 f ± 0.06 | 30.48 e ± 0.03 | 30.08 d ± 0.04 |
| Hardness [N] | 2.04 a ± 0.38 | 3.81 a ± 1.00 | 9.98 d ± 3.59 | 25.70 c ± 2.78 | 20.93 bc ± 1.12 | 17.71 b ± 1.72 |
| Apparent viscosity [mPa·s] | 332.63 b ± 0.64 | 369.00 c ± 1.00 | 395.67 d ± 0.58 | 518.63 f ± 0.55 | 427.30 e ± 0.61 | 273.63 a ± 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarewicz, S.; Kozłowicz, K.; Gładyszewska, B.; Rząd, K.; Matwijczuk, A.; Kobus, Z.; Ivanišová, E.; Harangozo, L.; Skrzypek, T. Effects of Ultrasound Treatment on the Physical and Chemical Properties of Ice Cream with a Strawberry Seed Oil Oleogel. Sustainability 2023, 15, 8975. https://doi.org/10.3390/su15118975
Nazarewicz S, Kozłowicz K, Gładyszewska B, Rząd K, Matwijczuk A, Kobus Z, Ivanišová E, Harangozo L, Skrzypek T. Effects of Ultrasound Treatment on the Physical and Chemical Properties of Ice Cream with a Strawberry Seed Oil Oleogel. Sustainability. 2023; 15(11):8975. https://doi.org/10.3390/su15118975
Chicago/Turabian StyleNazarewicz, Sybilla, Katarzyna Kozłowicz, Bożena Gładyszewska, Klaudia Rząd, Arkadiusz Matwijczuk, Zbigniew Kobus, Eva Ivanišová, Lubos Harangozo, and Tomasz Skrzypek. 2023. "Effects of Ultrasound Treatment on the Physical and Chemical Properties of Ice Cream with a Strawberry Seed Oil Oleogel" Sustainability 15, no. 11: 8975. https://doi.org/10.3390/su15118975
APA StyleNazarewicz, S., Kozłowicz, K., Gładyszewska, B., Rząd, K., Matwijczuk, A., Kobus, Z., Ivanišová, E., Harangozo, L., & Skrzypek, T. (2023). Effects of Ultrasound Treatment on the Physical and Chemical Properties of Ice Cream with a Strawberry Seed Oil Oleogel. Sustainability, 15(11), 8975. https://doi.org/10.3390/su15118975








