Adjusting pH of the Secondary Composting Materials to Further Enhance the Lignocellulose Degradation and Promote the Humification Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Statistical Analyses
3. Results and Discussion
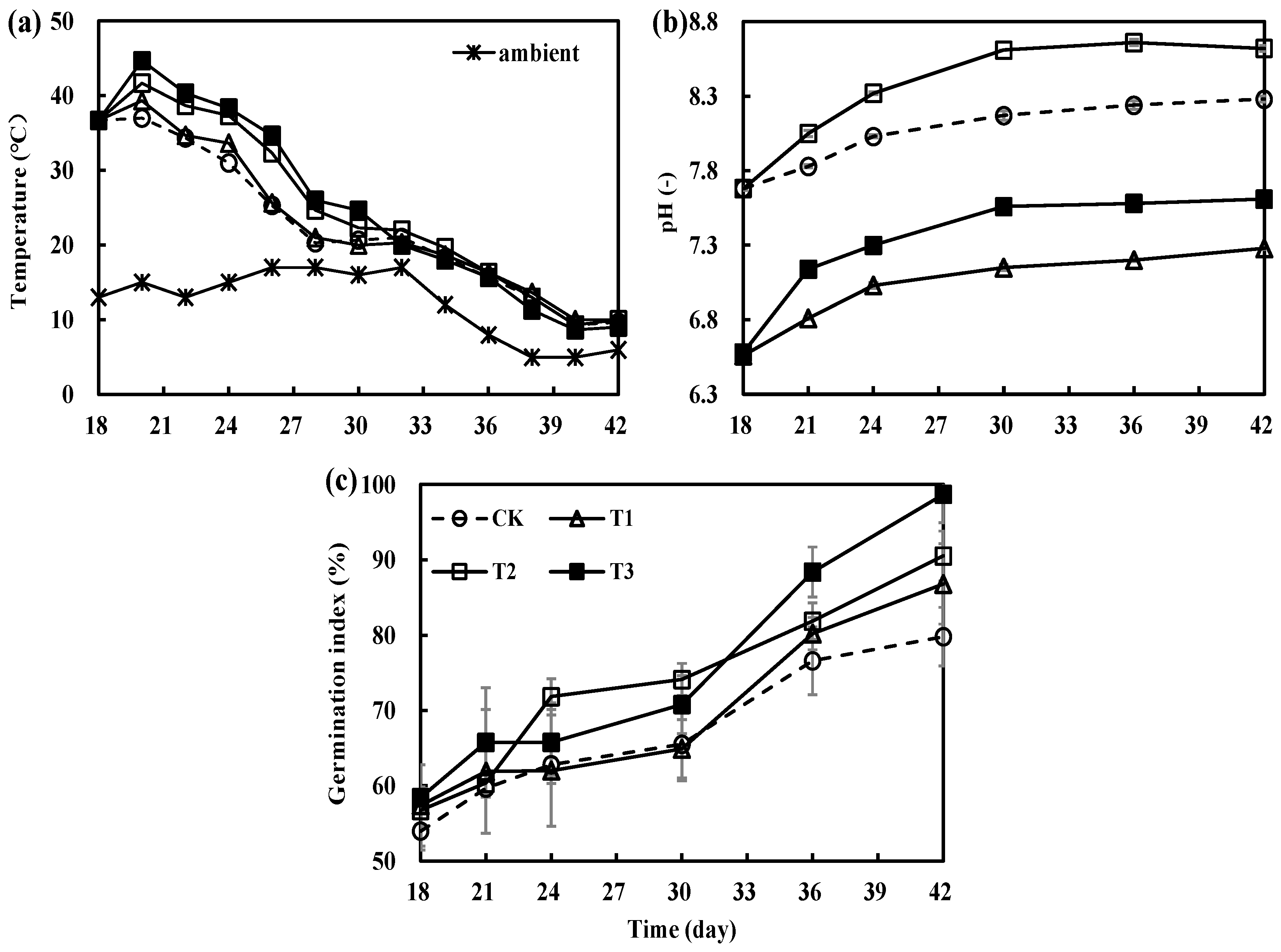
3.1. Variations in Physicochemical Parameters during the Secondary Fermentation of Composting
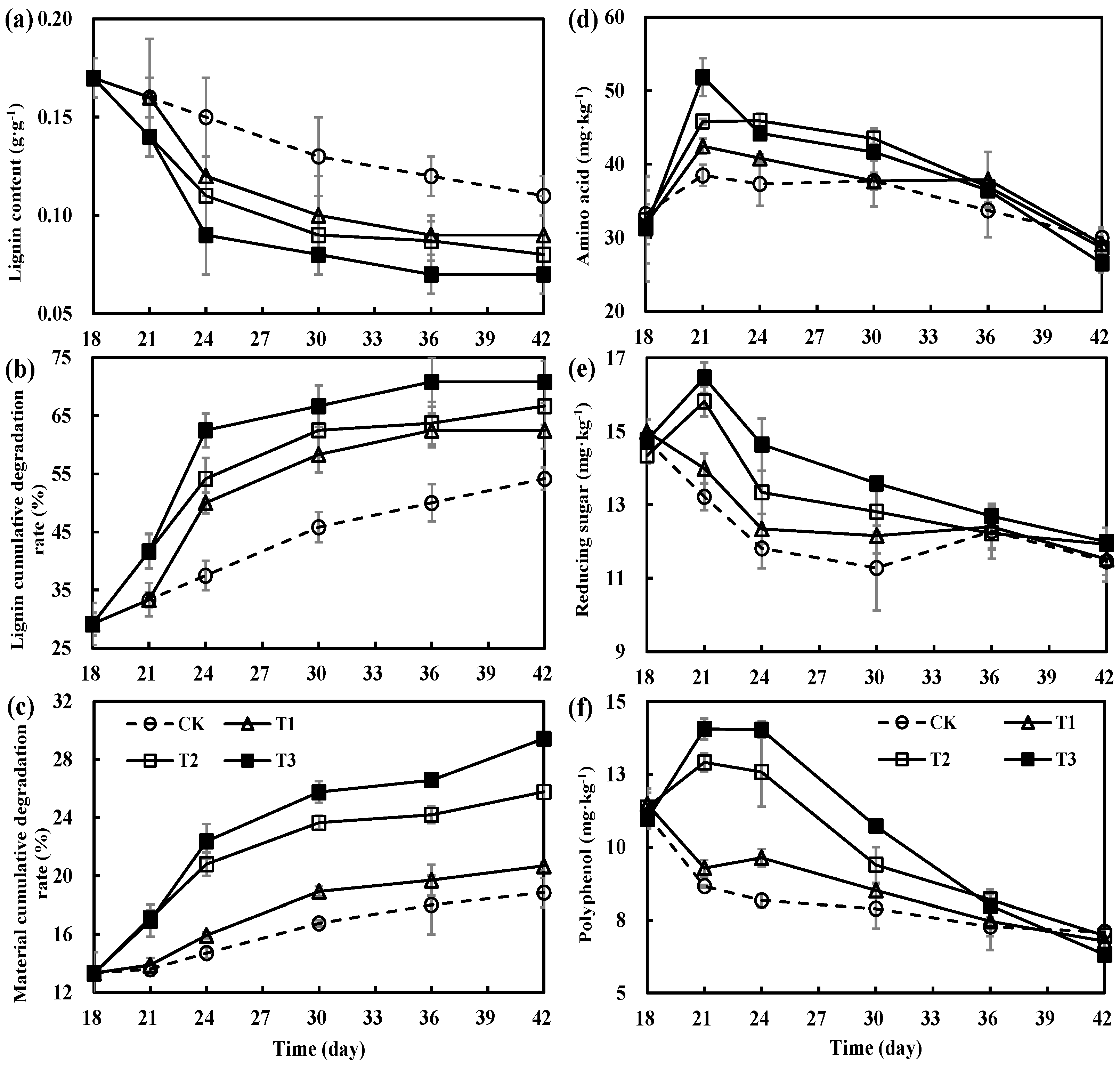
3.2. Variations in Decomposition of Organic Materials during the Secondary Fermentation of Composting
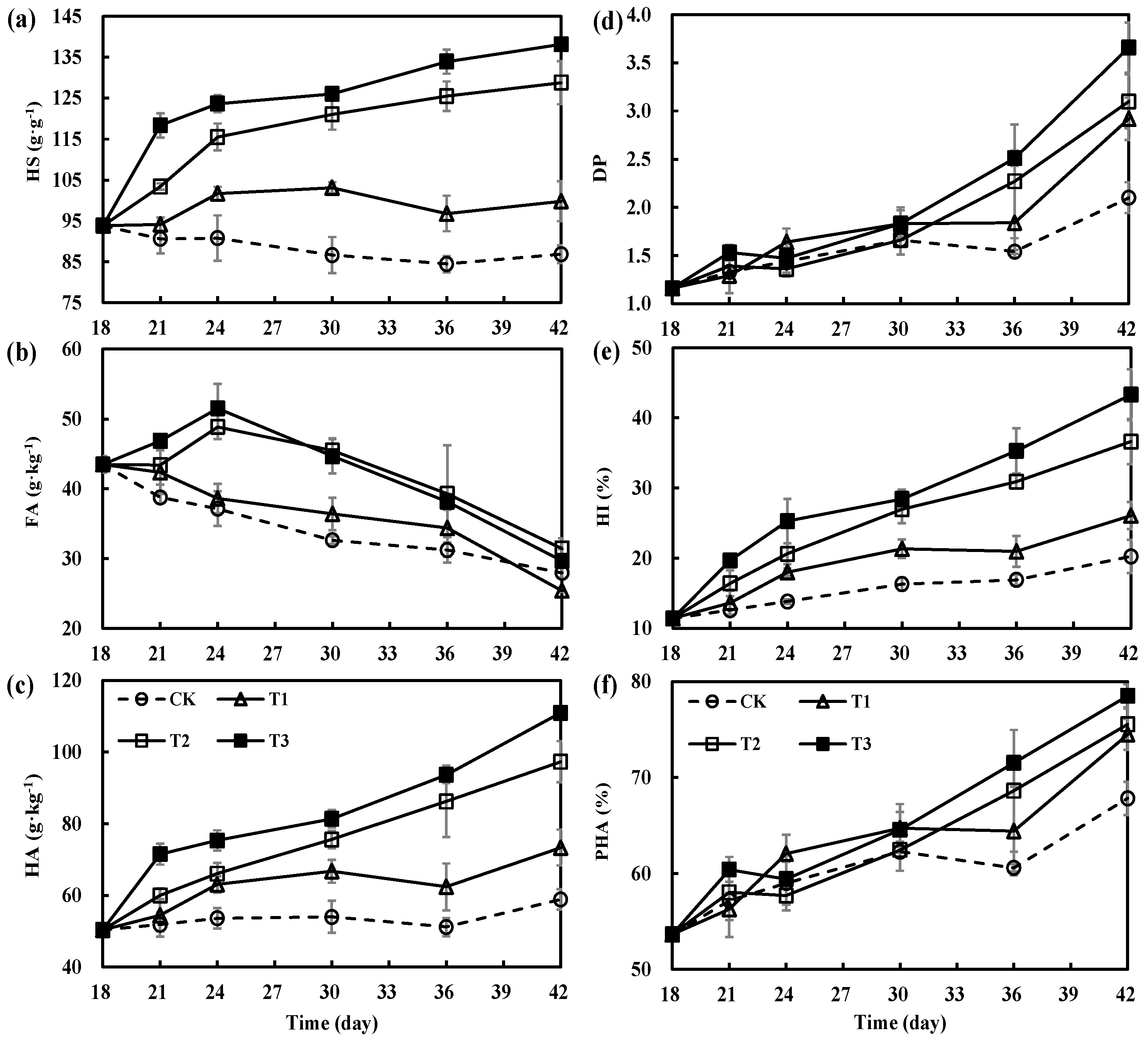
3.3. Variations in Humification Parameters during Secondary Fermentation of Composting
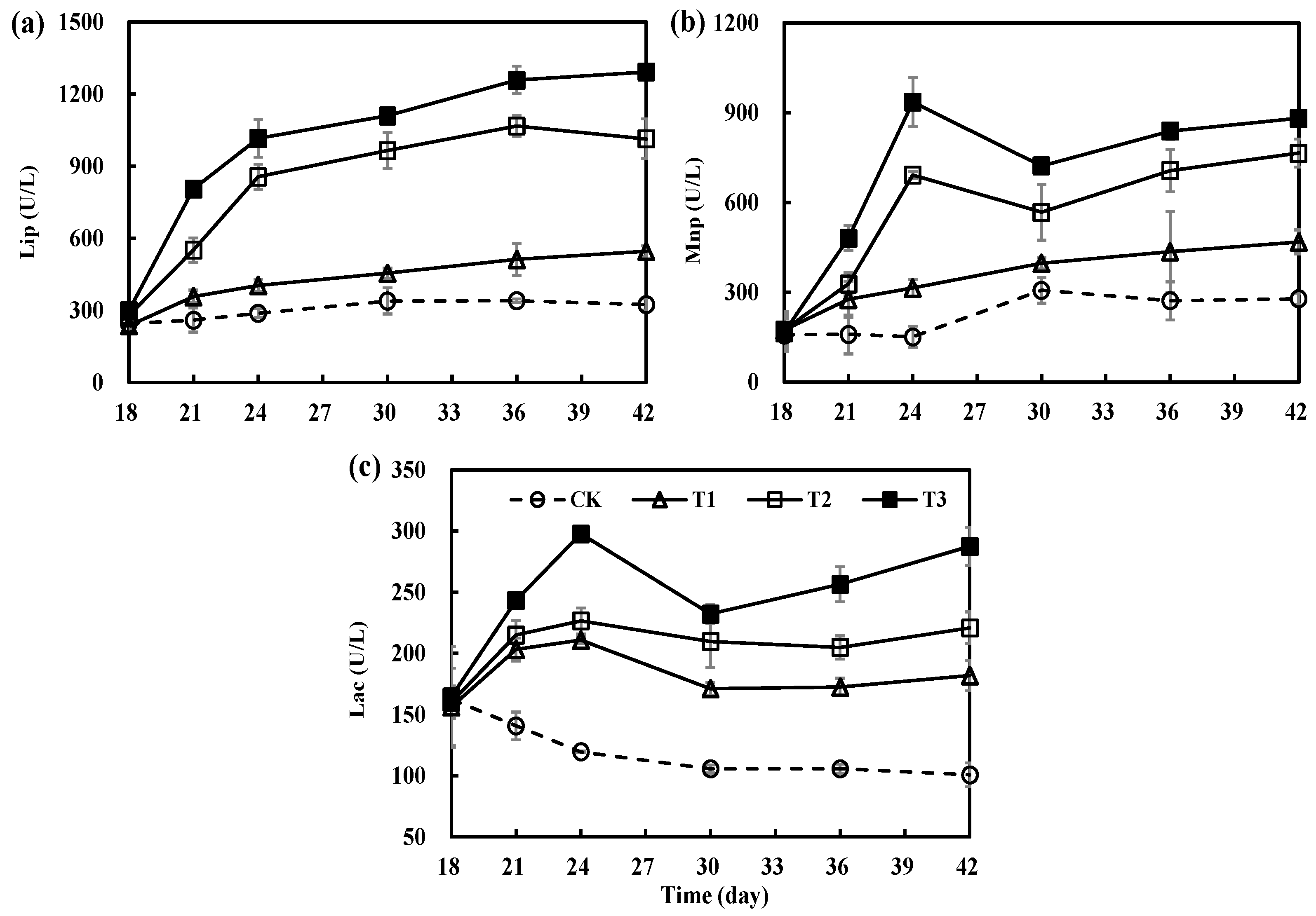
3.4. Variations in Key Degrading Enzymes during Secondary Fermentation of Composting
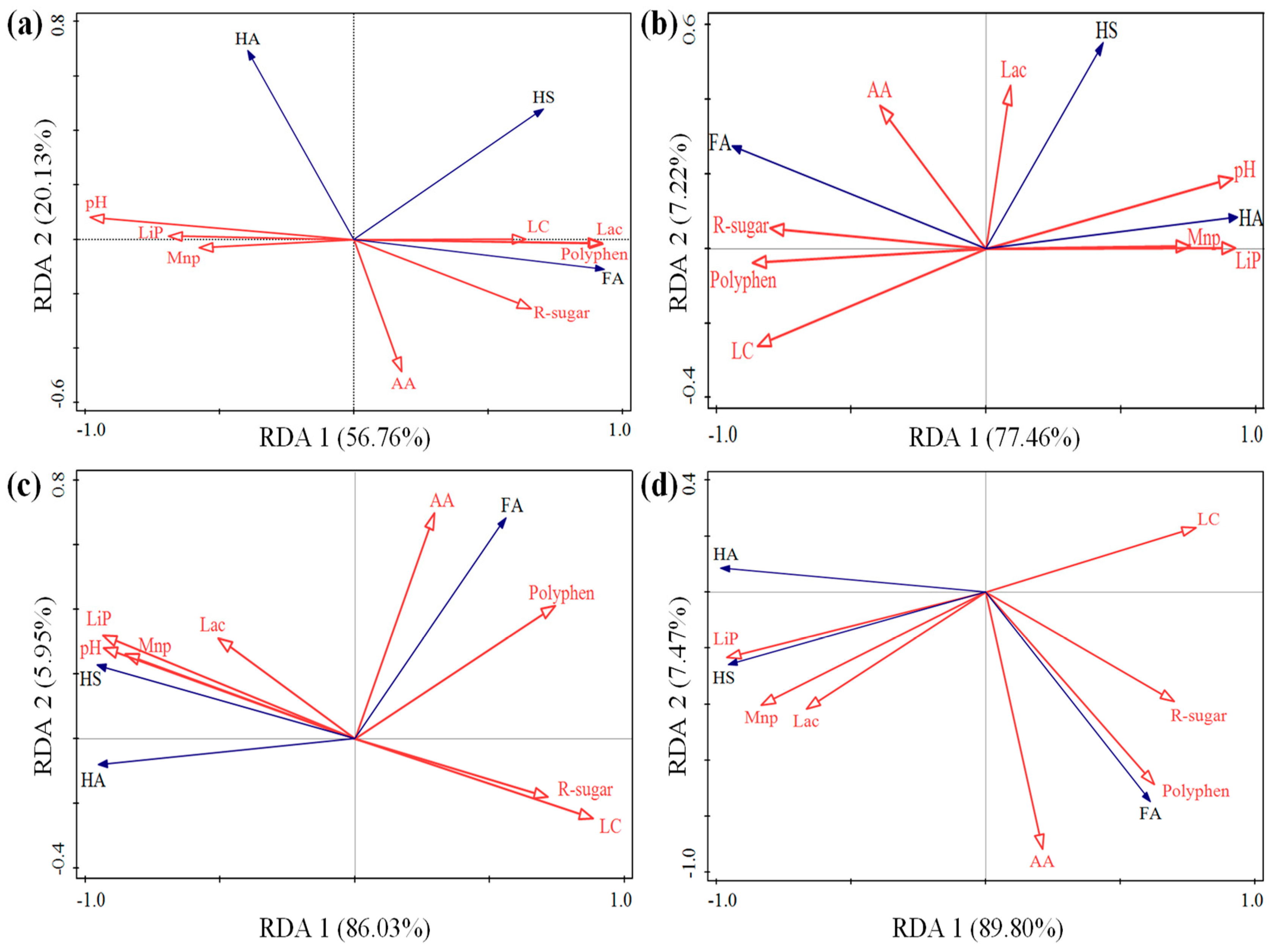
3.5. The Relationship between Physicochemical Parameters and Humification Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Xu, Z.; Li, Y.; Zhang, L.; Luo, W. Bacterial dynamics for gaseous emission and humification in bio-augmented composting of kitchen waste. Sci. Total Environ. 2021, 801, 149640. [Google Scholar] [CrossRef]
- López-González, J.A.; del Vargas-García, M.C.; López, M.J.; Suárez-Estrella, F.; del Jurado, M.M.; Moreno, J. Biodiversity and succession of mycobiota associated to agricultural lignocellulosic waste-based composting. Bioresour. Technol. 2015, 187, 305–313. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, X.; Awasthi, M.K.; Wang, Q.; Ren, X.; Li, R.; Chen, H.; Wang, M.; Liu, T.; Zhang, Z. Performance evaluation of gaseous emissions and Zn speciation during Zn-rich antibiotic manufacturing wastes and pig manure composting. Bioresour. Technol. 2018, 267, 688–695. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Zhang, W.; Zhou, H.; Chen, X.; Li, Y.; Wei, D.; Wei, Z. Roles of bacterial community in the transformation of organic nitrogen toward enhanced bioavailability during composting with different wastes. Bioresour. Technol. 2019, 285, 121326. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, X.; Li, M.; Zhu, Q.; Li, G.; Ma, C.; Li, Q.; Meng, J.; Liu, Y.; Li, Q. Impacts of red mud on lignin depolymerization and humic substance formation mediated by laccase-producing bacterial community during composting. J. Hazard. Mater. 2020, 20, 124557. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Mori, Y.; Ishida, A.; Nakajima, M.; Ito, N.; Hamada, M.; Yamashita, K.; Fujiwara, T.; Tonosaki, M.; Katayama, Y. Variation in Hemicellulose Structure and Assembly in the Cell Wall Associated with the Transition from Earlywood to Latewood in Cryptomeria japonica. J. Wood Chem. Technol. 2018, 38, 254–263. [Google Scholar] [CrossRef]
- Yang, W.T.; Zou, J.F.; Zhou, J.L.; Zeng, Q.R.; Liao, B.H. Impacts of rapeseed dregs on Cd availability in contaminated acid soil and Cd translocation and accumulation in rice plants. Environ. Sci. Pollut. Res. 2016, 23, 20853–20861. [Google Scholar] [CrossRef]
- Kulikowska, D.; Sindrewicz, S. Effect of barley straw and coniferous bark on humification process during sewage sludge composting. Waste Manag. 2018, 79, 207–213. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Sun, H.; Xu, Z. Analysis of humus formation and factors for driving the humification process during composting of different agricultural wastes. Front. Environ. Sci. 2022, 10, 1364. [Google Scholar] [CrossRef]
- Ait Baddi, G.; Cegarra, J.; Merlina, G.; Revel, J.C.; Hafidi, M. Qualitative and quantitative evolution of polyphenolic compounds during composting of an olive-mill waste–wheat straw mixture. J. Hazard. Mater. 2009, 165, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, D.; Ma, H. Darkening mechanism and kinetics of humification process in catechol-Maillard system. Chemosphere 2015, 130, 40–45. [Google Scholar] [CrossRef]
- He, X.-S.; Zhang, Y.-L.; Liu, Z.-H.; Wei, D.; Liang, G.; Liu, H.-T.; Xi, B.-D.; Huang, Z.-B.; Ma, Y.; Xing, B.-S. Interaction and coexistence characteristics of dissolved organic matter with toxic metals and pesticides in shallow groundwater. Environ. Pollut. 2020, 258, 113736. [Google Scholar] [CrossRef]
- Xiao, X.; Xi, B.-D.; He, X.-S.; Zhang, H.; Li, D.; Zhao, X.-Y.; Zhang, X.-H. Hydrophobicity-dependent electron transfer capacities of dissolved organic matter derived from chicken manure compost. Chemosphere 2019, 222, 757–765. [Google Scholar] [CrossRef] [PubMed]
- He, X.-S.; Xi, B.-D.; Zhang, Z.-Y.; Gao, R.-T.; Tan, W.-B.; Cui, D.-Y. Insight into the evolution, redox, and metal binding properties of dissolved organic matter from municipal solid wastes using two-dimensional correlation spectroscopy. Chemosphere 2014, 117, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Deng, F.; Wang, R.; Li, J.; Liu, X.; Li, D. Bioaugmentation on humification during co-composting of corn straw and biogas slurry. Bioresour. Technol. 2023, 374, 128756. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Li, Y.; Liu, Y.; Jiang, H.; Li, H.; Yuan, Y.; Chen, Y.; Zou, B. Improving the humification by additives during composting: A review. Waste Manag. 2023, 158, 93–106. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Huang, H.; Fan, C.; Zhu, Y.; Li, H.; Liu, Z.; Chen, M.; et al. Impact of Phanerochaete chrysosporium inoculation on indigenous bacterial communities during agricultural waste composting. Appl. Microbiol. Biotechnol. 2013, 97, 3159–3169. [Google Scholar] [CrossRef]
- Li, J.; Bao, H.; Xing, W.; Yang, J.; Liu, R.; Wang, X.; Lv, L.; Tong, X.; Wu, F. Succession of fungal dynamics and their influence on physicochemical parameters during pig manure composting employing with pine leaf biochar. Bioresour. Technol. 2020, 297, 122377. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Zhang, Z.; Zhang, G.; Li, Z.; Wang, L.; Zheng, J. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 2015, 36, 815–826. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, Z.; Liu, Y.; Duan, H. Enhanced humification of maize straw and canola residue during composting by inoculating Phanerochaete chrysosporium in the cooling period. Bioresour. Technol. 2019, 293, 122075. [Google Scholar] [CrossRef]
- Zeng, G.; Yu, M.; Chen, Y.; Huang, D.; Zhang, J.; Huang, H.; Jiang, R.; Yu, Z. Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour. Technol. 2010, 101, 222–227. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Qi, H.; Zhao, X.; Yang, T.; Du, Y.; Zhang, H.; Wei, Z. Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Yang, T.; Wei, Z.; Li, Y.; Wei, Y.; Chen, X.; Wang, L. Effects of exogenous protein-like precursors on humification process during lignocellulose-like biomass composting: Amino acids as the key linker to promote humification process. Bioresour. Technol. 2019, 291, 121882. [Google Scholar] [CrossRef]
- Cai, G.; Li, J.; Zhou, M.; Zhu, G.; Li, Y.; Lv, N.; Wang, R.; Li, C.; Pan, X. Compost-derived indole-3-acetic-acid-producing bacteria and their effects on enhancing the secondary fermentation of a swine manure-corn stalk composting. Chemosphere 2022, 291, 132750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhu, Y.; Kan, Z.; Li, B.; Cao, Y.; Jin, H. Effects of two-stage microbial inoculation on organic carbon turnover and fungal community succession during co-composting of cattle manure and rice straw. Bioresour. Technol. 2021, 341, 125842. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, Y.; Zhang, C.; Wei, D.; Wu, J.; Zhao, X.; Hao, J.; Wei, Z. Driving effects of minerals on humic acid formation during chicken manure composting: Emphasis on the carrier role of bacterial community. Bioresour. Technol. 2019, 294, 122239. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A. Evaluation of two different aeration systems for composting two-phase olive mill wastes. Process Biochem. 2006, 41, 616–623. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.C.; Wu, Q.T.; Nagar, B.B. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Ge, M.; Shen, Y.; Ding, J.; Meng, H.; Zhou, H.; Zhou, J.; Cheng, H.; Zhang, X.; Wang, J.; Wang, H.; et al. New insight into the impact of moisture content and pH on dissolved organic matter and microbial dynamics during cattle manure composting. Bioresour. Technol. 2022, 344, 126236. [Google Scholar] [CrossRef]
- Tuomela, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Huang, W.; Liu, X.; Wen, P.; Wang, Y.; Yu, Z.; Zhou, S. Alkali lignin and sodium lignosulfonate additives promote the formation of humic substances during paper mill sludge composting. Bioresour. Technol. 2021, 320, 124361. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, N.; Xu, Y.; Wang, L.; Zheng, J.; Li, X. The microbial mechanisms of enhanced humification by inoculation with Phanerochaete chrysosporium and Trichoderma longibrachiatum during biogas residues composting. Bioresour. Technol. 2022, 351, 126973. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, L.; Zhao, B.; Li, Y.; Chen, Y.; Deng, Y.; Wang, Y. Humification process enhancement through relative abundance promotion of Talaromyces and Coprinopsis by inoculated Phanerochaete chrysosporium during the secondary fermentation of composting. Environ. Sci. Pollut. Res. 2023, 30, 9060–9065. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zeng, G.-M.; Chen, Y.-N.; Zhang, J.-C.; Yu, Y.; Li, H.; Liu, Z.-F.; Tang, L. Effects of inoculation with Phanerochaete chrysosporium on remediation of pentachlorophenol-contaminated soil waste by composting. Process Biochem. 2011, 46, 1285–1291. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of earthworm casts and zeolite on the two-stage composting of green waste. Waste Manag. 2015, 39, 119–129. [Google Scholar] [CrossRef]
- Tursun, T.; Mamut, R.; Abbas, A. Determination of Polysaccharides in Ramalina sinesis Jatta.by 3,5-Dinitrosalicylic Acid(DNS) Method. Food Sci. 2009, 30, 236–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Wang, R.; Lu, Q.; Wu, J.; Zhang, D.; Nie, Z.; Wei, Z. Effect of the addition of exogenous precursors on humic substance formation during composting. Waste Manag. 2018, 79, 462–471. [Google Scholar] [CrossRef]
- Lakhdar, A.; Falleh, H.; Ouni, Y.; Oueslati, S.; Debez, A.; Ksouri, R.; Abdelly, C. Municipal solid waste compost application improves productivity, polyphenol content, and antioxidant capacity of Mesembryanthemum edule. J. Hazard. Mater. 2011, 191, 373–379. [Google Scholar] [CrossRef]
- Rich, N.; Bharti, A.; Kumar, S. Effect of bulking agents and cow dung as inoculant on vegetable waste compost quality. Bioresour. Technol. 2018, 252, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.-C.; Hafidi, M. Chemical and spectroscopic analysis of organic matter transformation during composting of sewage sludge and green plant waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]
- Ma, C.; Hu, B.; Wei, M.-B.; Zhao, J.-H.; Zhang, H.-Z. Influence of matured compost inoculation on sewage sludge composting: Enzyme activity, bacterial and fungal community succession. Bioresour. Technol. 2019, 294, 122165. [Google Scholar] [CrossRef] [PubMed]
- Latifah, O.; Ahmed, O.H.; Susilawati, K.; Majid, N.M. Compost maturity and nitrogen availability by co-composting of paddy husk and chicken manure amended with clinoptilolite zeolite. Waste Manag. Res. 2015, 33, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, B.; Xi, C.; Luo, X.; Yuan, X.; Wang, X.; Zhu, W.; Wang, H.; Cui, Z. Effect of pig manure on the chemical composition and microbial diversity during co-composting with spent mushroom substrate and rice husks. Bioresour. Technol. 2018, 251, 22–30. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Karthikeyan, O.P.; Selvam, A. Biological nutrient transformation during composting of pig manure and paper waste. Environ. Technol. 2017, 38, 754–761. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Yang, Y.; Ma, R.; Shen, Y.; Li, G.; Yuan, J. Superphosphate, biochar, and a microbial inoculum regulate phytotoxicity and humification during chicken manure composting. Sci. Total Environ. 2022, 824, 153958. [Google Scholar] [CrossRef]
- Zhong, B.; An, X.; An, W.; Xiao, X.; Zhang, Q. Effect of bioaugmentation on lignocellulose degradation and antibiotic resistance genes removal during biogas residues composting. Bioresour. Technol. 2021, 340, 125742. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus chemistry; genesis, composition, reactions. Soil Sci. 1982, 135, 129–130. [Google Scholar] [CrossRef]
- McCann, M.C.; Carpita, N.C. Biomass recalcitrance: A multi-scale, multi-factor, and conversion-specific property. J. Exp. Bot. 2015, 66, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, X.; Liu, Y.; Li, H.; Liu, H. Effect of microbiological inoculants DN-1 on lignocellulose degradation during co-composting of cattle manure with rice straw monitored by FTIR and SEM. Environ. Prog. Sustain. Energy 2016, 35, 345–351. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Ni, J.; Xie, D. Effect of four crop straws on transformation of organic matter during sewage sludge composting. J. Integr. Agric. 2016, 15, 232–240. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Zhao, M.; Rong, H.; Zhang, K. Influence of inoculating white-rot fungi on organic matter transformations and mobility of heavy metals in sewage sludge based composting. J. Hazard. Mater. 2018, 344, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, H.; Huang, X.; Dan, W.; Yue, Z.; Wei, Z.; Qian, L.; Zhang, R.; Tong, T. How does manganese dioxide affect humus formation during bio-composting of chicken manure and corn straw? Bioresour. Technol. 2018, 269, 169–178. [Google Scholar] [CrossRef]
- Xie, X.; Gao, X.; Pan, C.; Wei, Z.; Zhao, Y. Assessment of Multiorigin Humin Components Evolution and Influencing Factors During Composting. J. Agric. Food Chem. 2019, 67, 4184–4192. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Yue, Z.; Xue, Y. Assessment of humification degree of dissolved organic matter from different composts using fluorescence spectroscopy technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Yao, X.; Zhou, M.; Li, W.; Liu, Z.; Hu, B. Inoculation enhances directional humification by increasing microbial interaction intensity in food waste composting. Chemosphere 2023, 322, 138191. [Google Scholar] [CrossRef]
- Lopez, M.J.; Vargas-García, M.D.C.; Suárez-Estrella, F.; Nichols, N.N.; Dien, B.S.; Moreno, J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment. Enzym. Microb. Technol. 2007, 40, 794–800. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-R.; Baldrian, P.; Murugesan, K.; Chang, Y.-S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Madan, S.; Sahoo, J.; Ali, M.; Pathania, R.; Kazmi, A.A. Diversity of bacterial isolates during full scale rotary drum composting. Waste Manag. 2013, 33, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
| Raw Material | Moisture Content (%) | Total C (g·kg−1) | Total N (g·kg−1) | C/N |
|---|---|---|---|---|
| Maize straw | 9.89 ± 0.77% | 485.8 ± 4.76 | 5.98 ± 0.25 | 81.24 ± 5.56 |
| Canola residue | 7.17 ± 0.29% | 452.86 ± 6.32 | 56.37 ± 0.84 | 8.03 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Wang, Y.; Li, L.; Ma, L.; Deng, Y.; Xu, Z. Adjusting pH of the Secondary Composting Materials to Further Enhance the Lignocellulose Degradation and Promote the Humification Process. Sustainability 2023, 15, 9032. https://doi.org/10.3390/su15119032
Zhao B, Wang Y, Li L, Ma L, Deng Y, Xu Z. Adjusting pH of the Secondary Composting Materials to Further Enhance the Lignocellulose Degradation and Promote the Humification Process. Sustainability. 2023; 15(11):9032. https://doi.org/10.3390/su15119032
Chicago/Turabian StyleZhao, Bing, Yuyun Wang, Lan Li, Liting Ma, Yaqin Deng, and Zhi Xu. 2023. "Adjusting pH of the Secondary Composting Materials to Further Enhance the Lignocellulose Degradation and Promote the Humification Process" Sustainability 15, no. 11: 9032. https://doi.org/10.3390/su15119032
APA StyleZhao, B., Wang, Y., Li, L., Ma, L., Deng, Y., & Xu, Z. (2023). Adjusting pH of the Secondary Composting Materials to Further Enhance the Lignocellulose Degradation and Promote the Humification Process. Sustainability, 15(11), 9032. https://doi.org/10.3390/su15119032





