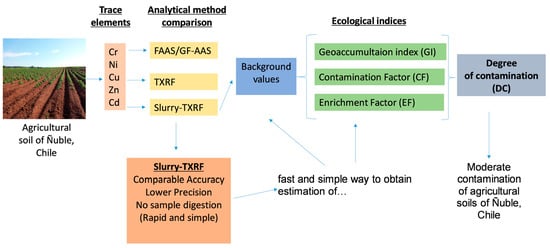
Rapid and Convenient Assessment of Trace Element Contamination in Agricultural Soils through Slurry-TXRF and Ecological Indices: The Ñuble Region, Chile as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
- Eroded metamorphic rock: This soil type exhibits the presence of shale, sandstone, phyllite, and slates. It has a clayey soil texture and slow water infiltration. Typically, this type of soil is found in high positions within areas characterized by steep or complex variable slopes, resulting in the formation of Catena due to the topography and drainage characteristics.
- Granite origin: Derived from granite and diorite rocks, this soil type has a clayey texture and low water infiltration. It is found in a hilly topography with complex and variable slopes, making it susceptible to water erosion.
- Fine alluvial sediment: This soil type is derived from the deposition of large amounts of fine alluvial sediments, which resulted from the fluvio-glacial sediments of the Andes Mountain glaciation during the Quaternary period. The thickness of the deposit varies considerably due to the influence of rivers in the area, which transport significant amounts of sediment, particularly fine material. These sediments have formed soils with a loamy–clayey texture and poor drainage characteristics [20].
2.2. Soil Sampling
2.3. Slurry Sample Preparation
2.4. Quantification of Element Concentrations and Method Validation
2.5. Exploratory Data Analysis
2.6. Background Values
2.7. Ecological Indices
3. Results
3.1. Method Comparison and Validation
3.2. Trace Elements Concentration
3.3. Exploratory Analysis: Correlation and Possible Sources
3.4. Background Values and Assessment of Potential Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining Biochar and Sewage Sludge for Immobilization of Heavy Metals in Mining Soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, T.; Zhang, Y.; Hou, Y.; Chang, Q. Health Risk Assessment of Heavy Metals in Agricultural Soils and Identification of Main Influencing Factors in a Typical Industrial Park in Northwest China. Chemosphere 2020, 252, 126591. [Google Scholar] [CrossRef]
- Wang, N.; Wu, X.; Liao, P.; Zhang, J.; Liu, N.; Zhou, Z.; Huang, H.; Zhang, L. Morphological Transformation of Heavy Metals and Their Distribution in Soil Aggregates during Biotransformation of Livestock Manure. Biocatal. Agric. Biotechnol. 2021, 32, 101963. [Google Scholar] [CrossRef]
- Xin, X.; Shentu, J.; Zhang, T.; Yang, X.; Baligar, V.C.; He, Z. Sources, Indicators, and Assessment of Soil Contamination by Potentially Toxic Metals. Sustainability 2022, 14, 15878. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Establishing Geochemical Background Variation and Threshold Values for 59 Elements in Australian Surface Soil. Sci. Total Environ. 2017, 578, 633–648. [Google Scholar] [CrossRef] [Green Version]
- Klockenkämper, R.; von Bohlen, A. Total-Reflection X-ray Fluorescence Analysis and Related Methods Total-Reflection X-ray Fluorescence Analysis and Related Methods, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2015; ISBN 978-1-118-46027-6. [Google Scholar]
- Bilo, F.; Borgese, L.; Pardini, G.; Marguí, E.; Zacco, A.; Dalipi, R.; Federici, S.; Bettinelli, M.; Volante, M.; Bontempi, E.; et al. Evaluation of Different Quantification Modes for a Simple and Reliable Determination of Pb, Zn and Cd in Soil Suspensions by Total Reflection X-ray Fluorescence Spectrometry. J. Anal. At. Spectrom. 2019, 34, 930–939. [Google Scholar] [CrossRef]
- Anemana, T.; Óvári, M.; Szegedi, Á.; Uzinger, N.; Rékási, M.; Tatár, E.; Yao, J.; Streli, C.; Záray, G.; Mihucz, V.G. Optimization of Lignite Particle Size for Stabilization of Trivalent Chromium in Soils. Soil Sediment Contam. Int. J. 2020, 29, 272–291. [Google Scholar] [CrossRef] [Green Version]
- Allegretta, I.; Ciasca, B.; Pizzigallo, M.D.R.; Lattanzio, V.M.T.; Terzano, R. A Fast Method for the Chemical Analysis of Clays by Total-Reflection x-Ray Fluorescence Spectroscopy (TXRF). Appl. Clay Sci. 2019, 180, 105201. [Google Scholar] [CrossRef]
- De La Calle, I.; Cabaleiro, N.; Romero, V.; Lavilla, I.; Bendicho, C. Sample Pretreatment Strategies for Total Reflection X-ray Fluorescence Analysis: A Tutorial Review. Spectrochim. Acta—Part B At. Spectrosc. 2013, 90, 23–54. [Google Scholar] [CrossRef]
- Meyer, A.; Grotefend, S.; Gross, A.; Wätzig, H.; Ott, I. Total Reflection X-ray Fluorescence Spectrometry as a Tool for the Quantification of Gold and Platinum Metallodrugs: Determination of Recovery Rates and Precision in the Ppb Concentration Range. J. Pharm. Biomed. Anal. 2012, 70, 713–717. [Google Scholar] [CrossRef]
- Wellenreuther, G.; Fittschen, U.E.A.; Achard, M.E.S.; Faust, A.; Kreplin, X.; Meyer-Klaucke, W. Optimizing Total Reflection X-ray Fluorescence for Direct Trace Element Quantification in Proteins I: Influence of Sample Homogeneity and Reflector Type. Spectrochim. Acta—Part B At. Spectrosc. 2008, 63, 1461–1468. [Google Scholar] [CrossRef]
- Borgese, L.; Zacco, A.; Bontempi, E.; Pellegatta, M.; Vigna, L.; Patrini, L.; Riboldi, L.; Rubino, F.M.; Depero, L.E. Use of Total Reflection X-ray Fluorescence (TXRF) for the Evaluation of Heavy Metal Poisoning Due to the Improper Use of a Traditional Ayurvedic Drug. J. Pharm. Biomed. Anal. 2010, 52, 787–790. [Google Scholar] [CrossRef]
- Shaw, B.J.; Semin, D.J.; Rider, M.E.; Beebe, M.R. Applicability of Total Reflection X-ray Fluorescence (TXRF) as a Screening Platform for Pharmaceutical Inorganic Impurity Analysis. J. Pharm. Biomed. Anal. 2012, 63, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Marguí, E.; Floor, G.H.; Hidalgo, M.; Kregsamer, P.; Román-Ross, G.; Streli, C.; Queralt, I. Analytical Possibilities of Total Reflection X-ray Spectrometry (TXRF) for Trace Selenium Determination in Soils. Anal. Chem. 2010, 82, 7744–7751. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Wenzel, T.; Whitehouse, M.J.; Loose, M.; Zack, T.; Barth, M.; Worgard, L.; Krasz, V.; Eby, G.N.; Stosnach, H.; et al. The Volatile Inventory (F, Cl, Br, S, C) of Magmatic Apatite: An Integrated Analytical Approach. Chem. Geol. 2012, 291, 241–255. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Galli, A.; Gondola, M.; Martini, M. Comparison between XRF, TXRF, and PXRF Analyses for Provenance Classification of Archaeological Bricks. X-ray Spectrom. 2013, 42, 262–267. [Google Scholar] [CrossRef]
- Natali, M.; Zanella, A.; Rankovic, A.; Banas, D.; Cantaluppi, C.; Abbadie, L.; Lata, J.C. Assessment of Trace Metal Air Pollution in Paris Using Slurry-TXRF Analysis on Cemetery Mosses. Environ. Sci. Pollut. Res. 2016, 23, 23496–23510. [Google Scholar] [CrossRef] [Green Version]
- Serra Stepke, I.; Castillo, P.; Hidalgo, M.; Fuentealba, O.; Cerda, R.; Salazar, F. Itata Valley: How Traditional Farming and Geomorphological Characteristics Can Became from a Limiting Factor to a Key Factor in Competitiveness. In Proceedings of the Fifth International Congress on Mountain and Steep Slope Viticulture, Conegliano, Italy, 29 March–1 April 2017. [Google Scholar]
- Stolpe, B.N. Descripciones de Los Principales Suelos de La VIII Región de Chile; Universidad de Concepción: Concepción, Chile, 2006. [Google Scholar]
- Servicio Agrícola y Ganadero de Chile (SAG). Protocolo de Toma de Muestras de Suelo; Servicio Agrícola y Ganadero de Chile (SAG): Santiago, Chile, 2022. [Google Scholar]
- Moya-Riffo, A.; Bennun, L.; Sanhueza, V.; Santibañez, M. A Procedure for Overlapping Deconvolution and the Determination of Its Confidence Interval for Arsenic and Lead Signals in TXRF Spectral Analysis. X-ray Spectrom. 2013, 42, 93–99. [Google Scholar] [CrossRef]
- González, G.M.; Castillo, R.D.P.; Neira, J.Y. Multivariate Calibration for the Improvement of the Quantification of Cadmium in the Presence of Potassium as Interferent by Total Reflection X-ray Fluorescence. X-ray Spectrom. 2019, 48, 700–707. [Google Scholar] [CrossRef]
- Zagal, E.; Sadzawka, R.A. Protocolo de Métodos de Análisis Para Suelos y Lodos; SAG Servicio Agricola y Ganadero: Santiago, Chile, 2007. [Google Scholar]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate Statistical and GIS-Based Approach to Identify Heavy Metal Sources in Soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef]
- Ungaro, F.; Ragazzi, F.; Cappellin, R.; Giandon, P. Arsenic Concentration in the Soils of the Brenta Plain (Northern Italy): Mapping the Probability of Exceeding Contamination Thresholds. J. Geochem. Explor. 2008, 96, 117–131. [Google Scholar] [CrossRef]
- Desaules, A. Critical Evaluation of Soil Contamination Assessment Methods for Trace Metals. Sci. Total Environ. 2012, 426, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Redon, P.O.; Bur, T.; Guiresse, M.; Probst, J.L.; Toiser, A.; Revel, J.C.; Jolivet, C.; Probst, A. Modelling Trace Metal Background to Evaluate Anthropogenic Contamination in Arable Soils of South-Western France. Geoderma 2013, 206, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Seijo, A.; Andrade, M.L.; Vega, F.A. Origin and Spatial Distribution of Metals in Urban Soils. J. Soils Sediments 2017, 17, 1514–1526. [Google Scholar] [CrossRef]
- Tume, P.; González, E.; Reyes, F.; Fuentes, J.P.; Roca, N.; Bech, J.; Medina, G. Sources Analysis and Health Risk Assessment of Trace Elements in Urban Soils of Hualpen, Chile. Catena 2019, 175, 304–316. [Google Scholar] [CrossRef]
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing Heavy Metal Contamination in Sado Estuary Sediment: An Index Analysis Approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution Indices as Useful Tools for the Comprehensive Evaluation of the Degree of Soil Contamination–A Review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [Green Version]
- Aǧca, N.; Özdel, E. Assessment of Spatial Distribution and Possible Sources of Heavy Metals in the Soils of Sariseki-Dörtyol District in Hatay Province (Turkey). Environ. Earth Sci. 2014, 71, 1033–1047. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Müller, G. Index of Geoaccumulation in Sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of Selected Element Concentrations and Enrichment Ratios in Background and Anthropogenically Impacted Roadside Areas. Arch. Environ. Contam. Toxicol. 2000, 38, 428–438. [Google Scholar] [CrossRef]
- Loska, K.; Wiechulła, D.; Korus, I. Metal Contamination of Farming Soils Affected by Industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Fu, C.; Guo, J.; Pan, J.; Qi, J.; Zhou, W. Potential Ecological Risk Assessment of Heavy Metal Pollution in Sediments of the Yangtze River within the Wanzhou Section, China. Biol. Trace Elem. Res. 2009, 129, 270–277. [Google Scholar] [CrossRef]
- Bilo, F.; Borgese, L.; Cazzago, D.; Zacco, A.; Bontempi, E.; Guarneri, R.; Bernardello, M.; Attuati, S.; Lazo, P.; Depero, L.E. TXRF Analysis of Soils and Sediments to Assess Environmental Contamination. Environ. Sci. Pollut. Res. 2014, 21, 13208–13214. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, A. Analysis of Various Solid Samples by Synchronous Fluorescence Spectroscopy and Related Methods: A Review. Talanta 2020, 216, 120944. [Google Scholar] [CrossRef] [PubMed]
- Rinot, O.; Borisover, M.; Levy, G.J.; Eshel, G. Fluorescence Spectroscopy: A Sensitive Tool for Identifying Land-Use and Climatic Region Effects on the Characteristics of Water-Extractable Soil Organic Matter. Ecol. Indic. 2021, 121, 107103. [Google Scholar] [CrossRef]
- Costa Ferreira, S.L.; dos Anjos, J.P.; Assis Felix, C.S.; da Silva Junior, M.M.; Palacio, E.; Cerda, V. Speciation Analysis of Antimony in Environmental Samples Employing Atomic Fluorescence Spectrometry—Review. TrAC Trends Anal. Chem. 2019, 110, 335–343. [Google Scholar] [CrossRef]
- Haas, G.; Geier, U.; Frieben, B.; Köpke, U. Estimation of Environmental Impact of Conversion to Organic Agriculture in Hamburg Using the Life-Cycle-Assessment Method; Institut für Organischen Landbau—Universität Bonn: Bonn, Germany, 2005. [Google Scholar]
- Esmaeilzadeh, M.; Jaafari, J.; Mohammadi, A.A.; Panahandeh, M.; Javid, A.; Javan, S. Investigation of the Extent of Contamination of Heavy Metals in Agricultural Soil Using Statistical Analyses and Contamination Indices. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1125–1136. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic Life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Pandita, S.; Sharma, A.; Bakshi, P.; Sharma, P.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Ecological and Human Health Risks Appraisal of Metal(Loid)s in Agricultural Soils: A Review. Geol. Ecol. Landsc. 2021, 5, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Xue, N.; Han, Z. A Meta-Analysis of Heavy Metals Pollution in Farmland and Urban Soils in China over the Past 20 Years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]






| Slurry-TXRF | ||||||
| Certified (mg kg−1) | Results (mg kg−1) | RSD | t-Test p (0.05) | AnalyticalSensitivity ([mg kg−1]−1) | LOD (mg kg−1) | |
| Cd | 58.4 ± 1.99 | 61.7 ± 7.0 | 11.3 | 0.476 | 15.2 | 0.091 |
| Cr | 43.8 ± 2.42 | 48.9 ± 11.1 | 22.7 | 0.480 | 6.85 | 0.289 |
| Cu | 5.68 ± 0.467 | 6.92 ± 1.59 | 23.0 | 0.265 | 30.1 | 0.061 |
| Fe | 8320 ± 646 | 7801 ± 1700 | 21.8 | 0.647 | 0.89 | 0.153 |
| Ni | 6.63 ± 0.508 | 7.83 ± 1.44 | 18.4 | 0.245 | 29.6 | 0.079 |
| Zn | 74.8 ± 3.02 | 66.6 ± 10.9 | 16.4 | 0.249 | 12.5 | 0.051 |
| Digestion-TXRF | ||||||
| Cd | 58.4 ± 1.99 | 56.5 ± 5.2 | 9.20 | 0.586 | 108.3 | 0.013 |
| Cr | 43.8 ± 2.42 | 38.2 ± 5.9 | 15.4 | 0.203 | 162.5 | 0.029 |
| Cu | 5.68 ± 0.467 | 5.21 ± 0.89 | 17.1 | 0.463 | 443.0 | 0.008 |
| Fe | 8320 ± 646 | 7531 ± 1352 | 18.0 | 0.413 | 13.4 | 0.017 |
| Ni | 6.63 ± 0.508 | 6.21 ± 1.21 | 19.5 | 0.609 | 256.9 | 0.010 |
| Zn | 74.8 ± 3.02 | 67.5 ± 6.7 | 9.92 | 0.160 | 220.8 | 0.007 |
| GF-AAS a/F-AAS b | ||||||
| Cd a | 58.4 ± 1.99 | 59.6 ± 0.7 | 1.17 | 0.380 | 21.4 | 0.032 |
| Cr a | 43.8 ± 2.42 | 45.8 ± 2.5 | 5.49 | 0.378 | 8.43 | 0.933 |
| Cu b | 5.68 ± 0.467 | 6.8 ± 0.73 | 10.7 | 0.091 | 0.790 | 17.6 |
| Fe b | 8320 ± 646 | 8243 ± 745 | 9.04 | 0.899 | 0.152 | 3.40 |
| Ni b | 6.63 ± 0.508 | 6.15 ± 0.73 | 11.9 | 0.403 | 0.723 | 2.34 |
| Zn b | 74.8 ± 3.02 | 71.5 ± 4.5 | 6.29 | 0.351 | 0.625 | 1.30 |
| Median | Min | Percentile | Max | MAD a | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | |||||
| Cr | 63.68 | 32.11 | 35.86 | 47.31 | 63.73 | 91.06 | 133.22 | 165.10 | 6.15 |
| Ni | 9.57 | 3.77 | 4.80 | 6.53 | 9.76 | 13.27 | 19.64 | 22.50 | 0.89 |
| Cu | 30.95 | 12.52 | 14.12 | 18.68 | 31.34 | 41.35 | 57.00 | 62.31 | 0.95 |
| Zn | 49.14 | 20.06 | 26.73 | 37.89 | 49.26 | 59.17 | 87.92 | 115.30 | 1.75 |
| Cd | 0.56 | 0.23 | 0.25 | 0.34 | 0.56 | 0.74 | 1.03 | 1.12 | 0.03 |
| Variance | Percent (%) | Cumulative (%) | |
|---|---|---|---|
| PC1 | 52,386.45 | 90.4016 | 90.4016 |
| PC2 | 3729.35 | 6.4356 | 96.8372 |
| PC3 | 1751.07 | 3.0218 | 99.8590 |
| PC4 | 81.67 | 0.1409 | 99.9999 |
| PC5 | 0.05 | 0.0001 | 100.0000 |
| Cr | Ni | Cu | Zn | Cd | |
|---|---|---|---|---|---|
| Background Upper Limit (mg kg−1) | 47.6 | 6.82 | 17.0 | 30.7 | 0.284 |
| Outliers | 35 | 34 | 44 | 42 | 44 |
| Percentage | 72.92 | 70.83 | 91.67 | 87.50 | 91.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-González, G.; Medina, Y.; Muñoz, E.; Fuentes, P. Rapid and Convenient Assessment of Trace Element Contamination in Agricultural Soils through Slurry-TXRF and Ecological Indices: The Ñuble Region, Chile as a Case Study. Sustainability 2023, 15, 9190. https://doi.org/10.3390/su15129190
Medina-González G, Medina Y, Muñoz E, Fuentes P. Rapid and Convenient Assessment of Trace Element Contamination in Agricultural Soils through Slurry-TXRF and Ecological Indices: The Ñuble Region, Chile as a Case Study. Sustainability. 2023; 15(12):9190. https://doi.org/10.3390/su15129190
Chicago/Turabian StyleMedina-González, Guillermo, Yelena Medina, Enrique Muñoz, and Patricio Fuentes. 2023. "Rapid and Convenient Assessment of Trace Element Contamination in Agricultural Soils through Slurry-TXRF and Ecological Indices: The Ñuble Region, Chile as a Case Study" Sustainability 15, no. 12: 9190. https://doi.org/10.3390/su15129190