Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
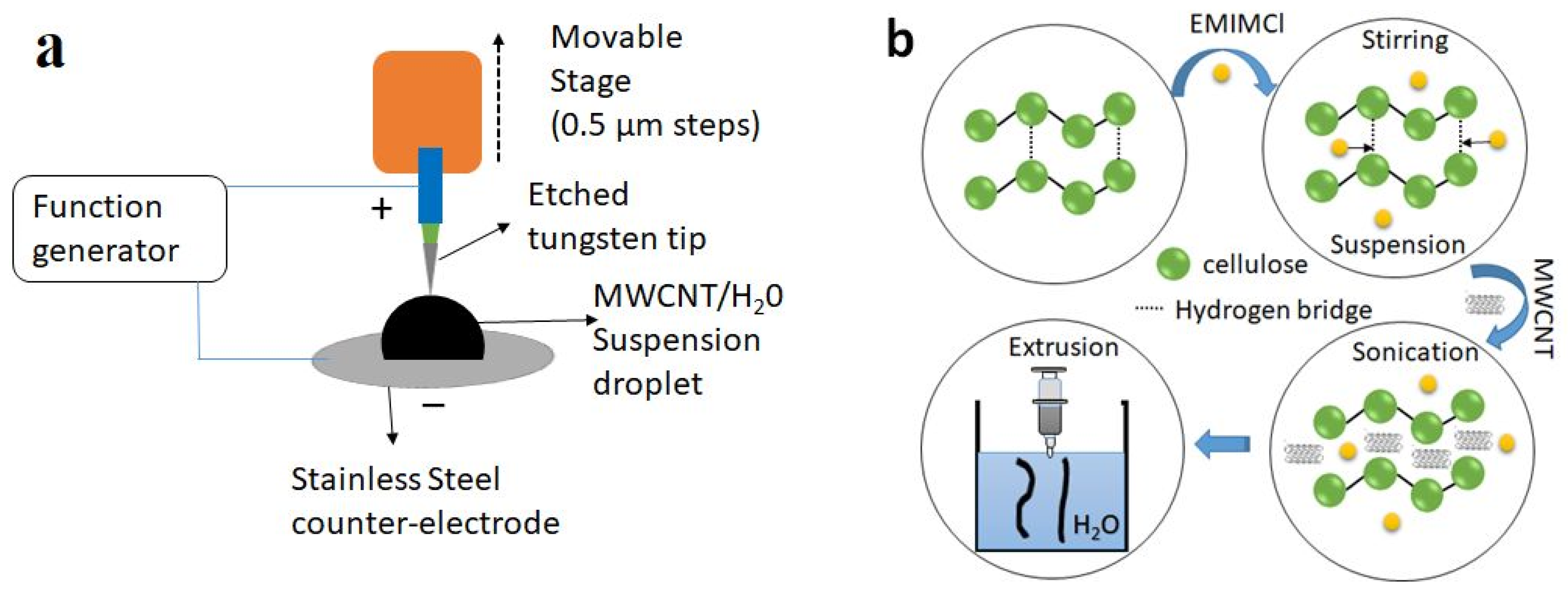
2.2. MWCNT (CNT) and Cellulose MWCNT (Cell-CNT) Composite Fiber
2.3. Characterizations
3. Results and Discussions
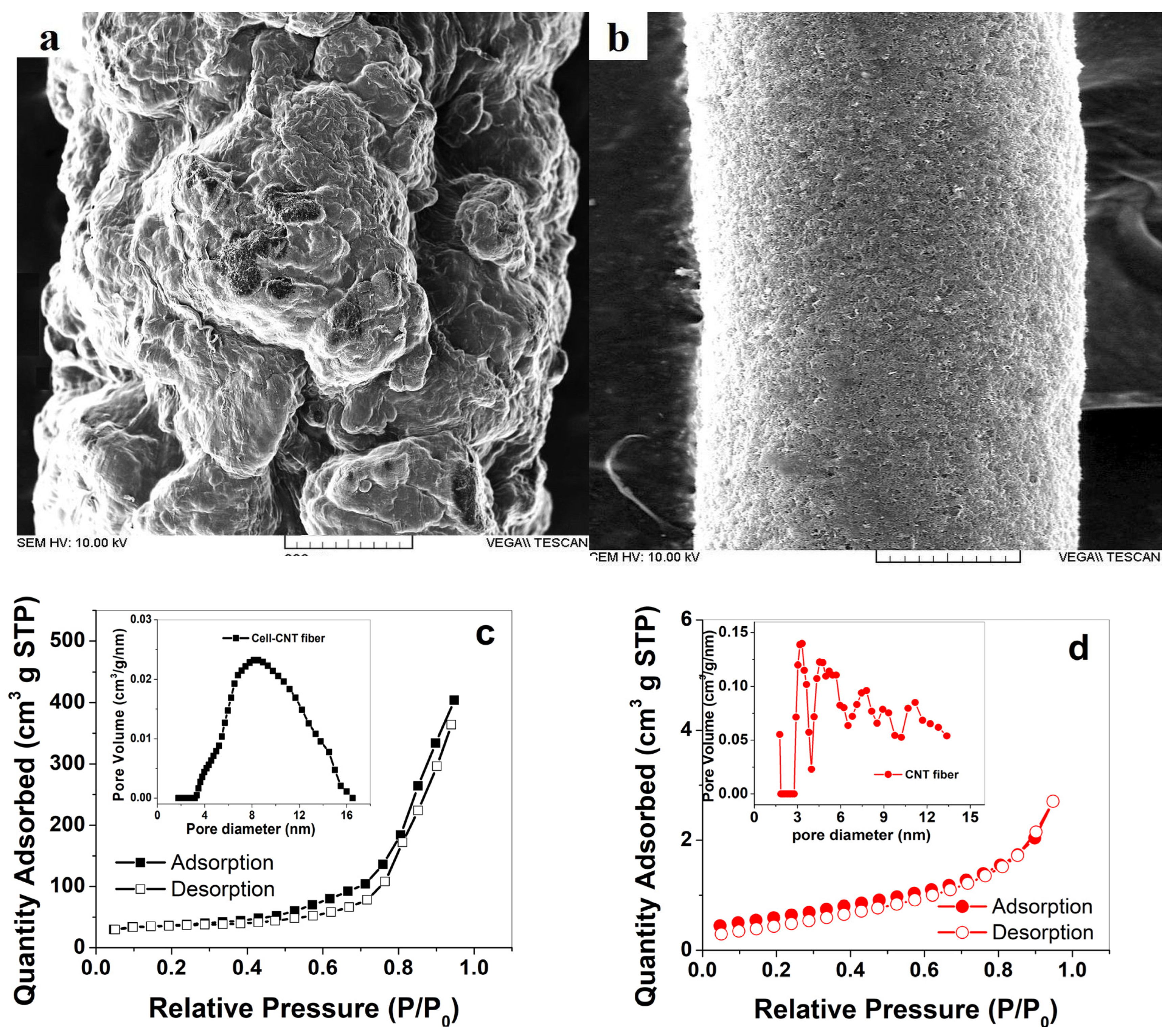
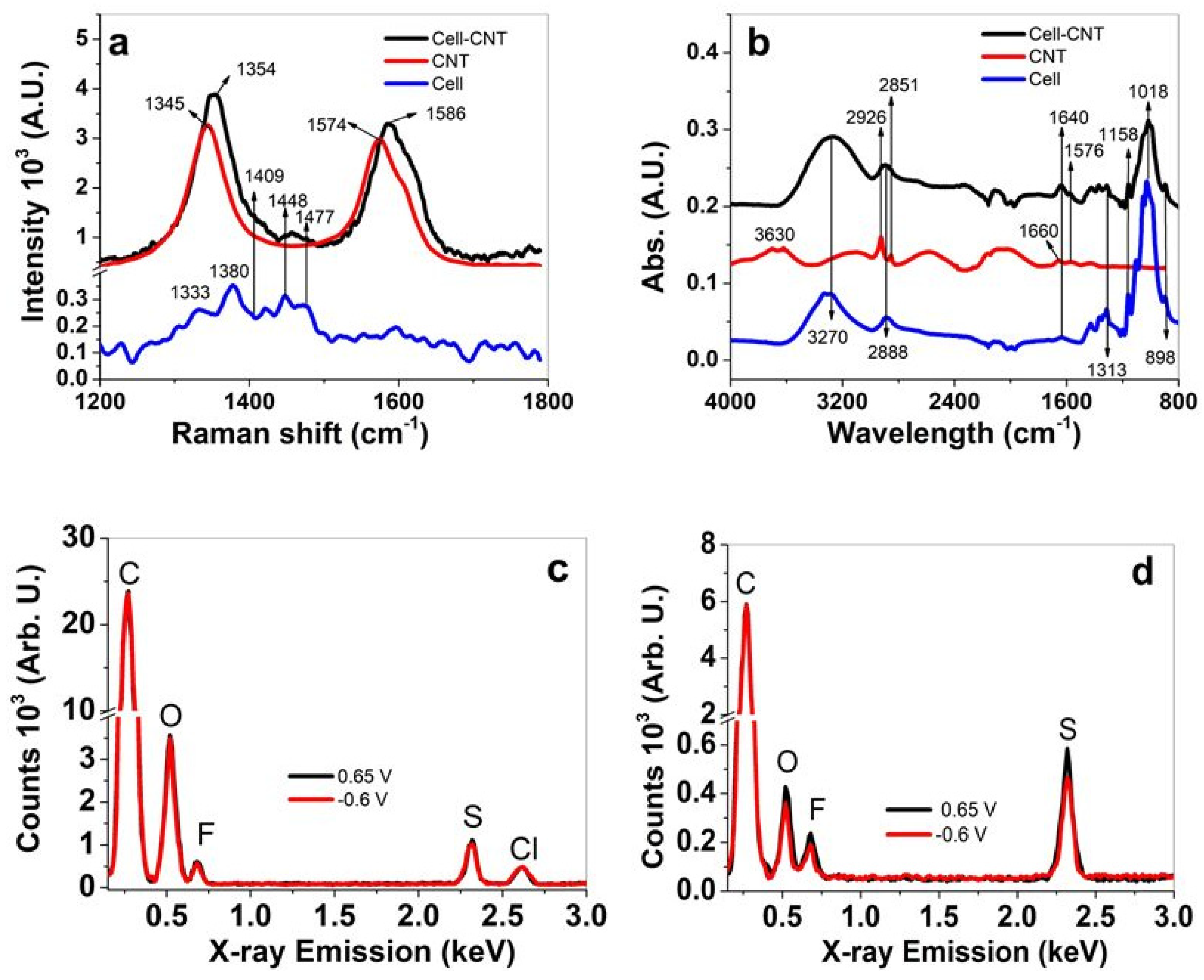
3.1. Characterizations of CNT and Cell-CNT Fibers
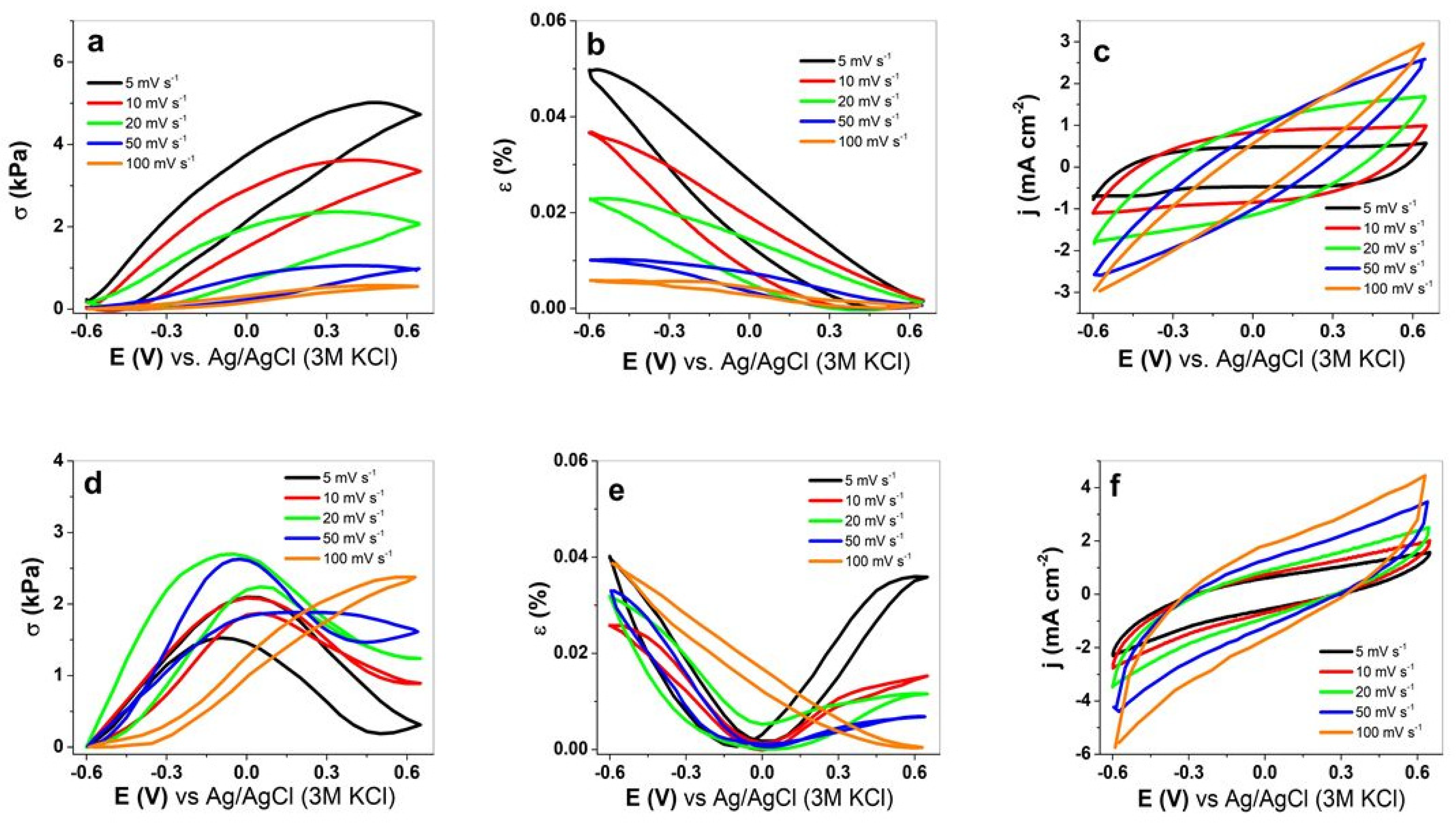
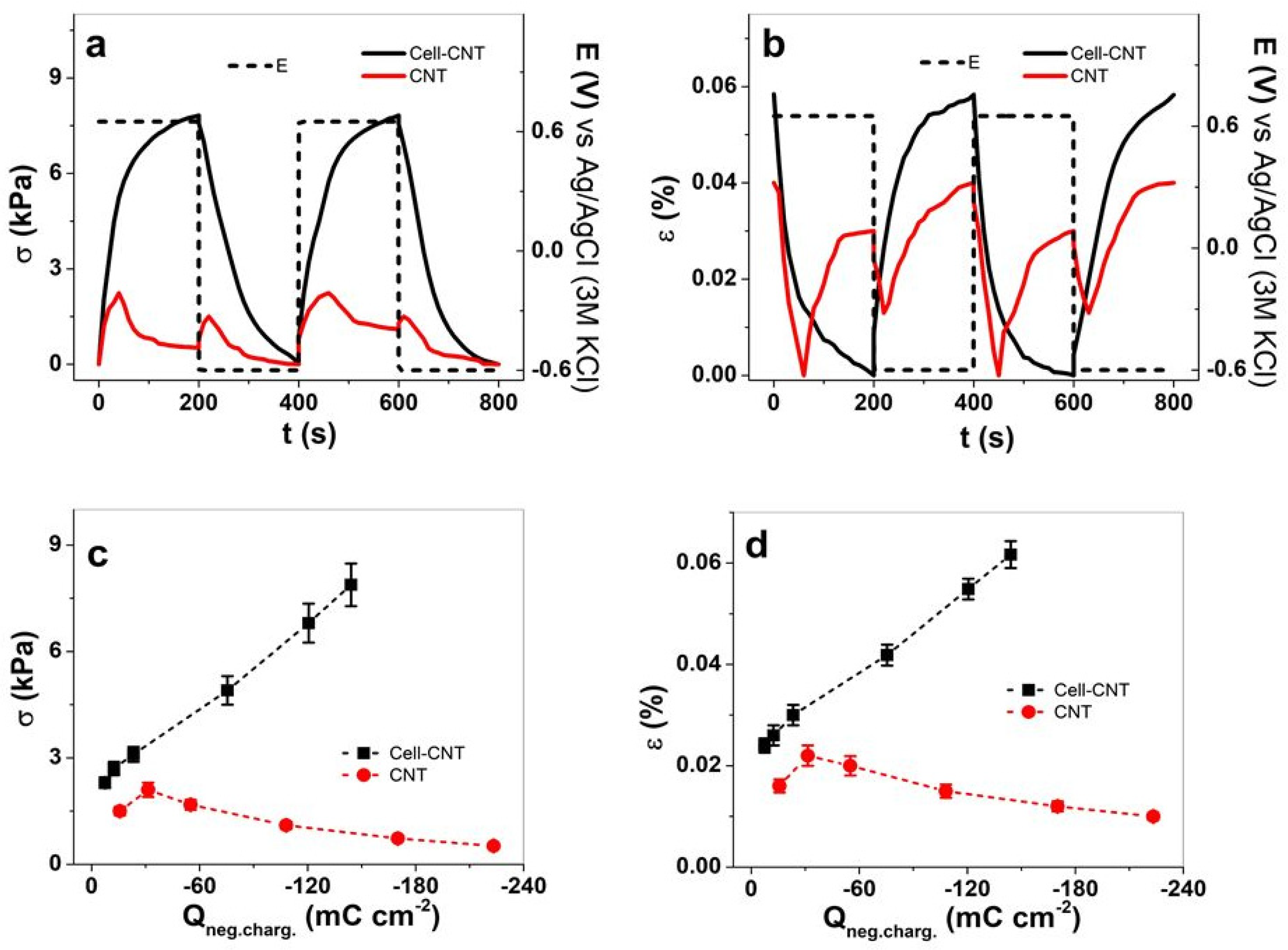
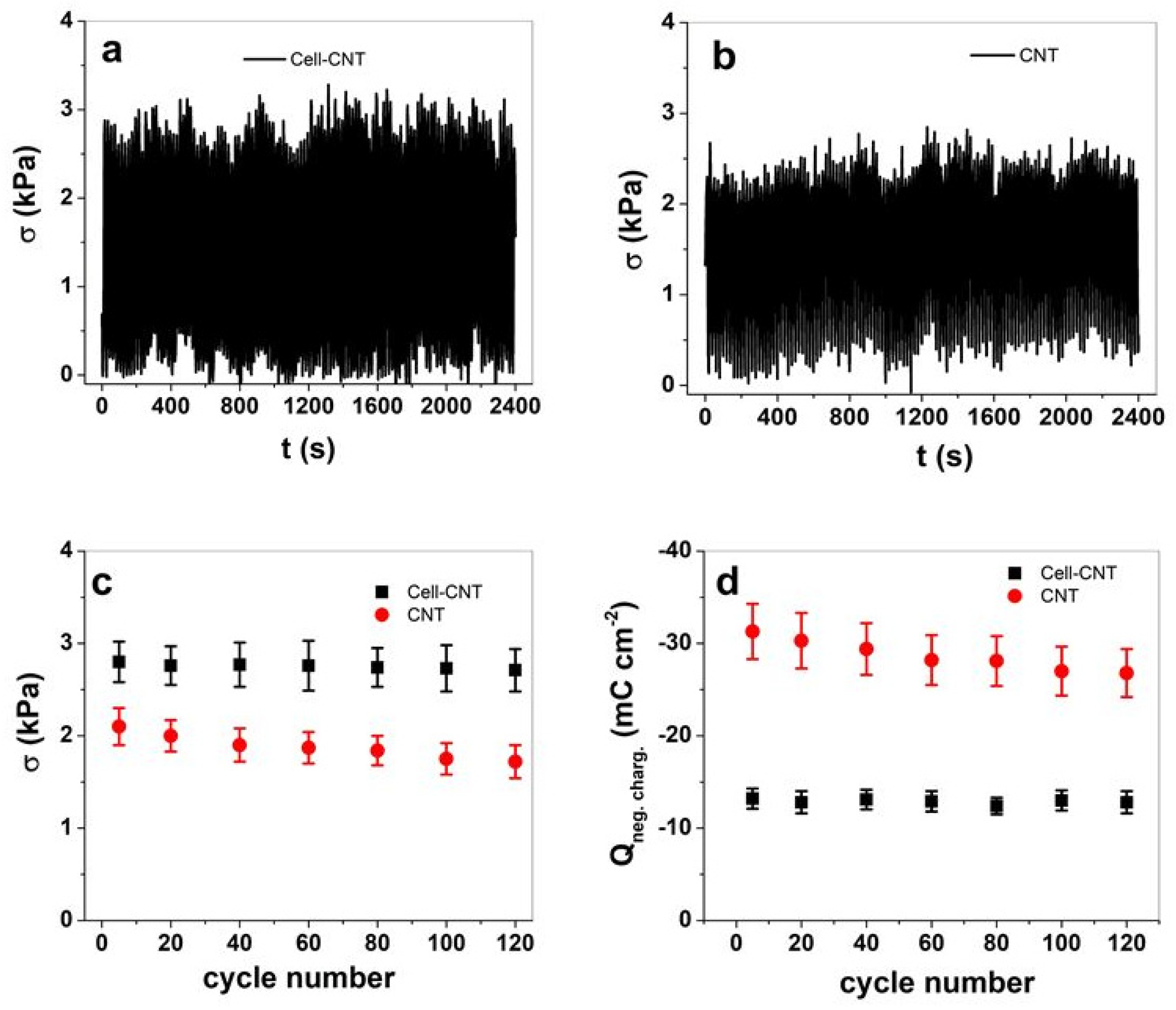
3.2. Isometric and Isotonic EMD Measurement
3.2.1. Cyclic Voltammetry
3.2.2. Square Wave Potential Steps
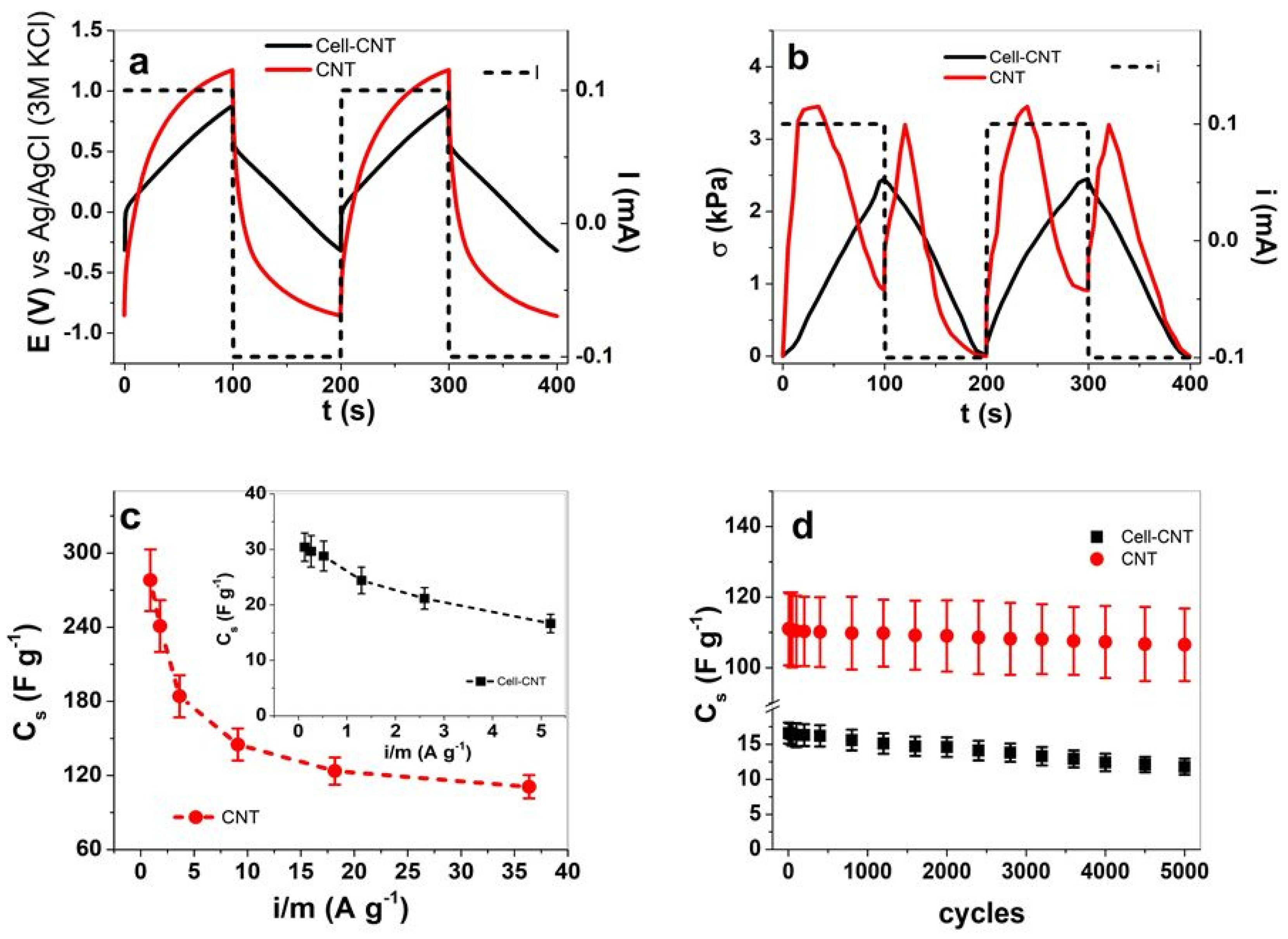
3.3. Energy Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Lee, Y.H.; Kim, S.W.; Seo, J.Y.; Lee, S.Y.; Nyholm, L. Why Cellulose-Based Electrochemical Energy Storage Devices? Adv. Mater. 2020, 33, 2000892. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-Based Li-Ion Batteries: A Review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-Performance Green Flexible Electronics Based on Biodegradable Cellulose Nanofibril Paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ummartyotin, S.; Manuspiya, H. A Critical Review on Cellulose: From Fundamental to an Approach on Sensor Technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Elhi, F.; Puust, L.; Kiefer, R.; Tamm, T. Electrolyte Contribution to the Multifunctional Response of Cellulose Carbon Nanotube Fibers. React. Funct. Polym. 2023, 182, 105480. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-Based Supercapacitors: Material and Performance Considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- Anju, V.P.; Manoj, M.; Mohanan, P.; Narayanankutty, S.K. A Comparative Study on Electromagnetic Interference Shielding Effectiveness of Carbon Nanofiber and Nanofibrillated Cellulose Composites. Synth. Met. 2019, 247, 285–297. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Q.; Dang, W.; Zhou, Q.; Jiang, X.; Sun, X.; Wang, Z.; An, M.; Ni, Y. A Multifunctional Paper-Based Supercapacitor with Excellent Temperature Adaptability, Plasticity, Tensile Strength, Self-Healing, and High Thermoelectric Effects. J. Mater. Chem. A 2023, 11, 4769–4779. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.N. Recent Progress on Development of Electrolyte and Aerogel Electrodes Applied in Supercapacitors. J. Power Sources 2023, 560, 232698. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, Z.; Wu, J.; Zhang, J.; He, J. Regenerated-Cellulose/Multiwalled-Carbon-Nanotube Composite Fibers with Enhanced Mechanical Properties Prepared with the Ionic Liquid 1-Allyl-3-Methylimidazolium Chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable Preparation of Three-Dimensional Porous Structures of Reduced Graphene Oxide/Cellulose Composites and Their Application in Supercapacitors. Carbon N. Y. 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Raghunathan, S.P.; Narayanan, S.; Poulose, A.C.; Joseph, R. Flexible Regenerated Cellulose/Polypyrrole Composite Films with Enhanced Dielectric Properties. Carbohydr. Polym. 2017, 157, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Qu, K.; You, Y.; Huang, Z.; Liu, S.; Li, J.; Guo, Q.H. Overview of Cellulose-Based Flexible Materials for Supercapacitors. J. Mater. Chem. A 2021, 9, 7278–7300. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Nie, Y.; Wang, C.; Ji, X.; Zhou, L.; Pan, F.; Zhang, S. Preparation of MWCNTs-Graphene-Cellulose Fiber with Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 20013–20021. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of Cellulose with Ionic Liquids and Its Application: A Mini-Review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Hassan, S.H.; Voon, L.H.; Velayutham, T.S.; Zhai, L.; Kim, H.C.; Kim, J. Review of Cellulose Smart Material: Biomass Conversion Process and Progress on Cellulose-Based Electroactive Paper. J. Renew. Mater. 2018, 6, 1–25. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.; Xu, J.; Dang, W.; Sun, X.; An, M.; Ni, Y.; Mao, J. Kinetics Process for Structure-Engineered Integrated Gradient Porous Paper-Based Supercapacitors with Boosted Electrochemical Performance. Nano Res. 2023, in print. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A. A Novel Method for Coating of Carbon Nanotube on Cellulose Fiber Using 1,2,3,4-Butanetetracarboxylic Acid as a Cross-Linking Agent. Prog. Org. Coat. 2012, 74, 470–478. [Google Scholar] [CrossRef]
- Huniade, C.; Melling, D.; Vancaeyzeele, C.; Nguyen, G.T.-M.; Vidal, F.; Plesse, C.; Jager, E.W.H.; Bashir, T.; Persson, N.-K. Ionofibers: Ionically Conductive Textile Fibers for Conformal i-Textiles. Adv. Mater. Technol. 2022, 7, 2101692. [Google Scholar] [CrossRef]
- Kiefer, R.; Plaado, M.; Harjo, M.; Tamm, T. Tuning the Linear Actuation of Multiwall Carbon Nanotube Fibers with Carbide-Derived Carbon. Synth. Met. 2022, 288, 117099. [Google Scholar] [CrossRef]
- Tran, C.D.; Le-Cao, K.; Bui, T.T.; Dau, V.T. Dielectrophoresis Can Control the Density of CNT Membranes as Confirmed by Experiment and Dissipative Particle Simulation. Carbon N. Y. 2019, 155, 279–286. [Google Scholar] [CrossRef]
- Plaado, M.; Kaasik, F.; Valner, R.; Lust, E.; Saar, R.; Saal, K.; Peikolainen, A.-L.; Aabloo, A.; Kiefer, R. Electrochemical Actuation of Multiwall Carbon Nanotube Fiber with Embedded Carbide-Derived Carbon Particles. Carbon N. Y. 2015, 94, 911–918. [Google Scholar] [CrossRef]
- Tang, J.; Gao, B.; Geng, H.; Velev, O.D.; Qin, L.C.; Zhou, O. Assembly of 1D Nanostructures into Sub-Micrometer Diameter Fibrils with Controlled and Variable Length by Dielectrophoresis. Adv. Mater. 2003, 15, 1352–1355. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Halim, M.M.; Halin, I.A. Dielectrophoretic Alignment of Carbon Nanotubes: Theory, Applications, and Future. Nanotechnology 2023, 34, 242001–242039. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; George, S.J.; Kulkarni, G.U. Electric Field Assisted Assembly of 1D Supramolecular Nanofibres for Enhanced Supercapacitive Performance. J. Mater. Chem. A 2020, 8, 13106–13113. [Google Scholar] [CrossRef]
- Kosidlo, U.; Omastova, M.; Micusik, M.; Ciric-Marjanovic, G.; Randriamahazaka, H.; Wallmersperger, T.; Aabloo, A.; Kolaric, I.; Bauernhansl, T. Nanocarbon Based Ionic Actuators-a Review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Oh, J.; Kozlov, M.; Fok, E.C.W.; Zhang, M.; Fang, S.; Baughman, R.H.; Madden, J.D.W. Electrochemical Actuation of Carbon Nanotube Yarns. Smart Mater. Struct. 2007, 16, S243–S249. [Google Scholar] [CrossRef]
- Kim, P.; Lieberl, C.M. Nanotube Nanotweezers. Science 1999, 286, 2148–2150. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Asaka, K. Faradaic and Capacitive Components of the CNT Electrochemical Responses. Front. Mater. 2016, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, R.; Elhi, F.; Peikolainen, A.-L.; Tamm, T. Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte. Polymers 2021, 13, 4439. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A Critical Review of the Biological Mechanisms Underlying the in Vivo and in Vitro Toxicity of Carbon Nanotubes: The Contribution of Physico-Chemical Characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Elhi, F.; Peikolainen, A.L.; Kiefer, R.; Tamm, T. Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte. Materials 2020, 13, 3213. [Google Scholar] [CrossRef] [PubMed]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [Green Version]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kiefer, R.; Elhi, F.; Peikolainen, A.-L.; Puust, L.; Tamm, T. The Importance of Potential Range Choice on the Electromechanical Response of Cellulose-Carbon Nanotube Fibers. Synth. Met. 2022, 283, 116966. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/Carbon Nanotube Nano Composite Fibers—A Review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef]
- Plaado, M.; Mononen, R.M.; Lhmus, R.; Kink, I.; Saal, K. Formation of Thick Dielectrophoretic Carbon Nanotube Fibers. Nanotechnology 2011, 22, 305711. [Google Scholar] [CrossRef] [Green Version]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Cellulose/Carbon Nanotube/MnO2 Composite Electrodes with High Mass Loadings for Symmetric Supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, L.R. Properties That Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and Nonspecific Molecular Interactions of Polymers with Multiwalled Carbon Nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Feldner, A.; Fischer, S. FT Raman Spectroscopic Investigation of Cellulose Acetate. Cellulose 2011, 18, 995–1003. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Atalla, R.H. Raman Spectroscopy. In Surface Analysis of Paper; Conners, T.E., Banerjee, S., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 152–181. ISBN 9780429279997. [Google Scholar]
- Lucas, M.; Wagner, G.L.; Nishiyama, Y.; Hanson, L.; Samayam, I.P.; Schall, C.A.; Langan, P.; Rector, K.D. Reversible Swelling of the Cell Wall of Poplar Biomass by Ionic Liquid at Room Temperature. Bioresour. Technol. 2011, 102, 4518–4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.Y.; Yoo, D.; Shin, Y.; Seo, G. FTIR Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Van Trinh, P.; Anh, N.N.; Thang, B.H.; Quang, L.D.; Hong, N.T.; Hong, N.M.; Khoi, P.H.; Minh, P.N.; Hong, P.N. Enhanced Thermal Conductivity of Nanofluid-Based Ethylene Glycol Containing Cu Nanoparticles Decorated on a Gr-MWCNT Hybrid Material. RSC Adv. 2017, 7, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Bodirlau, R.; Teaca, C.-A.; Spiridon, I. Influence of Ionic Liquid on Hydrolyzed Cellulose Material: FT-IR Spectroscopy and TG-DTG-DSC Analysis. Int. J. Polym. Anal. Charact. 2010, 15, 460–469. [Google Scholar] [CrossRef]
- Spiridon, I.; Teacă, C.-A.; Bodîrlău, R. Pretreatment with Ionic Liquids. BioResources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Langkilde, F.W.; Svantesson, A. Identification of Celluloses with Fourier-Transform (FT) Mid-Infrared, FT-Raman and near-Infrared Spectrometry. J. Pharm. Biomed. Anal. 1995, 13, 409–414. [Google Scholar] [CrossRef]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-Derived Carbon in Polypyrrole Changing the Elastic Modulus with a Huge Impact on Actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, M.I.; Muralidharan, A.; Pratt, L.R.; Rempe, S.B. Assessment of Simple Models for Molecular Simulation of Ethylene Carbonate and Propylene Carbonate as Solvents for Electrolyte Solutions. Top. Curr. Chem. 2018, 376, 7. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Karatrantos, A.V.; Ohba, T.; Cai, Q. The Effect of Different Organic Solvents and Anion Salts on Sodium Ion Storage in Cylindrical Carbon Nanopores. Phys. Chem. Chem. Phys. 2019, 21, 22722–22731. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.L.; Stevenson, K.J. Anomalous Electrochemical Dissolution and Passivation of Iron Growth Catalysts in Carbon Nanotubes. Langmuir 2007, 23, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Martinez, J.G.; Foroughi, J.; Spinks, G.M.; Jager, E.W.H. Artificial Muscles from Hybrid Carbon Nanotube-Polypyrrole-Coated Twisted and Coiled Yarns. Macromol. Mater. Eng. 2020, 305, 2000421. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G. Carbon Nanotube and Graphene Fiber Artificial Muscles. Nanoscale Adv. 2019, 1, 4592–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, L.; Martinez, J.G.; Otero, T.F. Creeping and Structural Effects in Faradaic Artificial Muscles. J. Solid State Electrochem. 2015, 19, 2683–2689. [Google Scholar] [CrossRef] [Green Version]
- Michardière, A.S.; Mateo-Mateo, C.; Derré, A.; Correa-Duarte, M.A.; Mano, N.; Poulin, P. Carbon Nanotube Microfiber Actuators with Reduced Stress Relaxation. J. Phys. Chem. C 2016, 120, 6851–6858. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, N.; Gadekar, P.; Kandasubramanian, B.; Kaka, F. Advanced Polymer-Based Materials and Mesoscale Models to Enhance the Performance of Multifunctional Supercapacitors. J. Energy Storage 2023, 58, 106337. [Google Scholar] [CrossRef]
- Pang, Z.; Sun, X.; Wu, X.; Nie, Y.; Liu, Z.; Yue, L. Fabrication and Application of Carbon Nanotubes/Cellulose Composite Paper. Vacuum 2015, 122, 135–142. [Google Scholar] [CrossRef]
- Felhősi, I.; Keresztes, Z.; Marek, T.; Pajkossy, T. Properties of Electrochemical Double-Layer Capacitors with Carbon-Nanotubes-on-Carbon-Fiber-Felt Electrodes. Electrochim. Acta 2020, 334, 135548. [Google Scholar] [CrossRef] [Green Version]
| Fibers | k [mg μm−1] | Y [kPa] | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Cell-CNT | 875 ± 42 | 746 ± 31 | 485 ± 28 | 413 ± 26 |
| CNT | 1 ± 0.1 | 4 ± 0.2 | 55.5 ± 3.2 | 222 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyen, N.Q.; Elhi, F.; Le, Q.B.; Kiefer, R. Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite. Sustainability 2023, 15, 9227. https://doi.org/10.3390/su15129227
Khuyen NQ, Elhi F, Le QB, Kiefer R. Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite. Sustainability. 2023; 15(12):9227. https://doi.org/10.3390/su15129227
Chicago/Turabian StyleKhuyen, Nguyen Quang, Fred Elhi, Quoc Bao Le, and Rudolf Kiefer. 2023. "Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite" Sustainability 15, no. 12: 9227. https://doi.org/10.3390/su15129227
APA StyleKhuyen, N. Q., Elhi, F., Le, Q. B., & Kiefer, R. (2023). Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite. Sustainability, 15(12), 9227. https://doi.org/10.3390/su15129227






