Synthesis and Characterization of Zinc-Lead-Phosphate Glasses Doped with Europium for Radiation Shielding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Density and Oxygen Packing Density, Molar Volume, and Oxygen Volume Measurements
2.3. Measuring Infrared Absorption
2.4. Optical Transmission Measurements
2.5. Gamma-ray Transmission Measurement
2.6. Theoretical Background of γ-ray Shielding
2.6.1. Single-Valued Zeff and Neff
2.6.2. Energy-Dependent Zeff (E) and Neff (E)
2.7. Gamma-ray Kerma Coefficient Calculations
3. Results
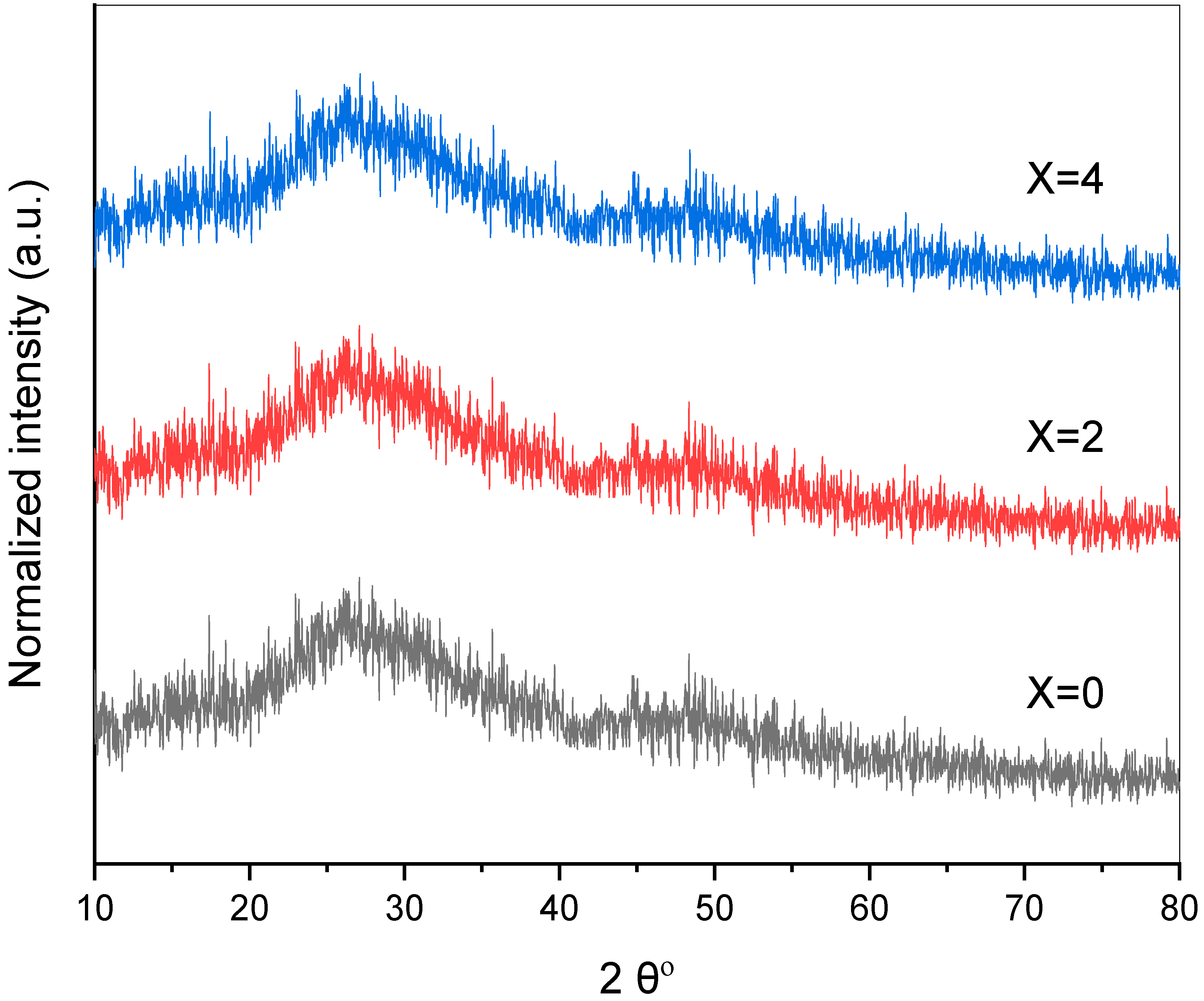
3.1. Structural and Physical Characteristics
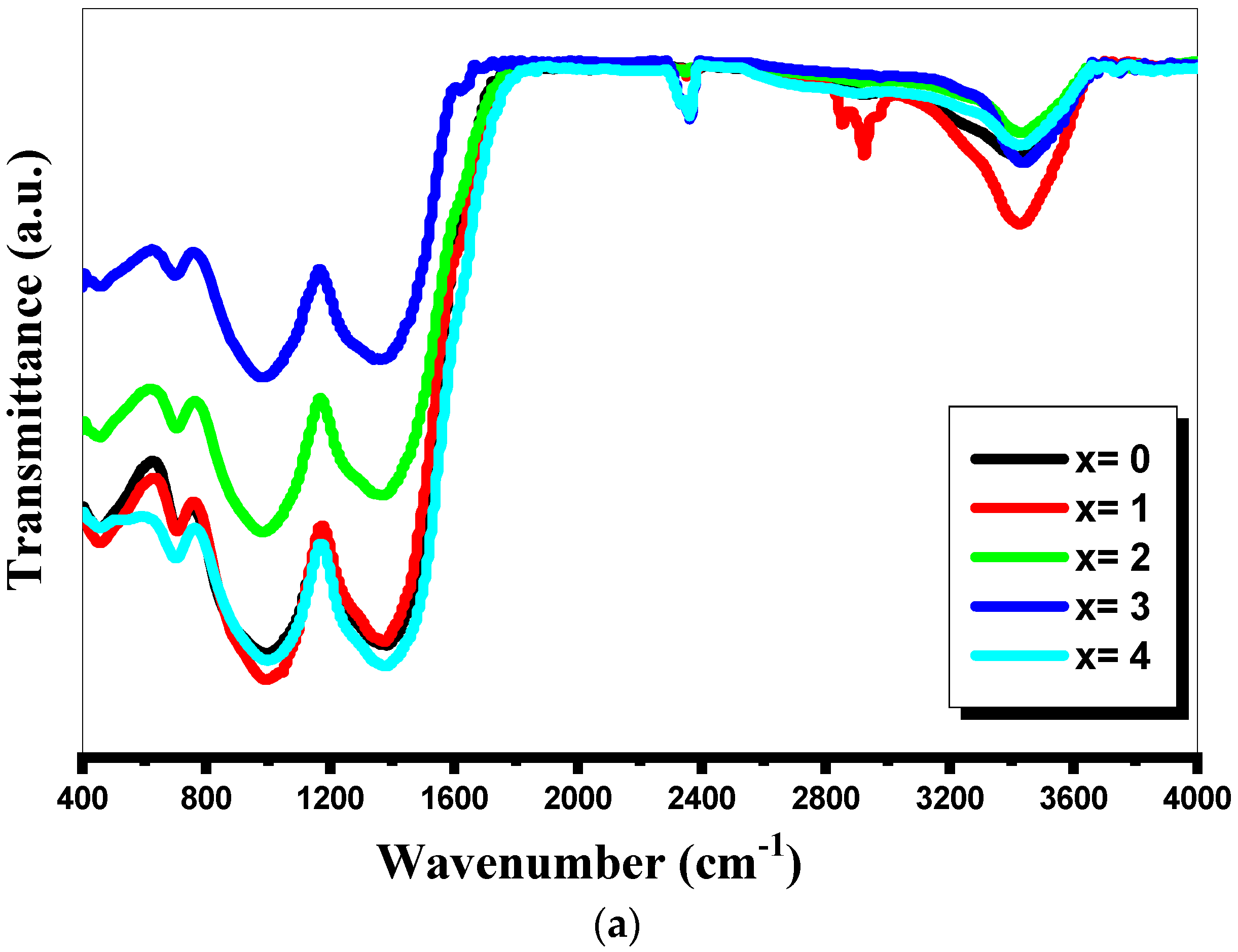
3.2. FTIR Absorption Spectra
3.3. Optical Characterization
3.4. Gamma-ray Attenuation Characteristics
3.4.1. Mass Attenuation Coefficients
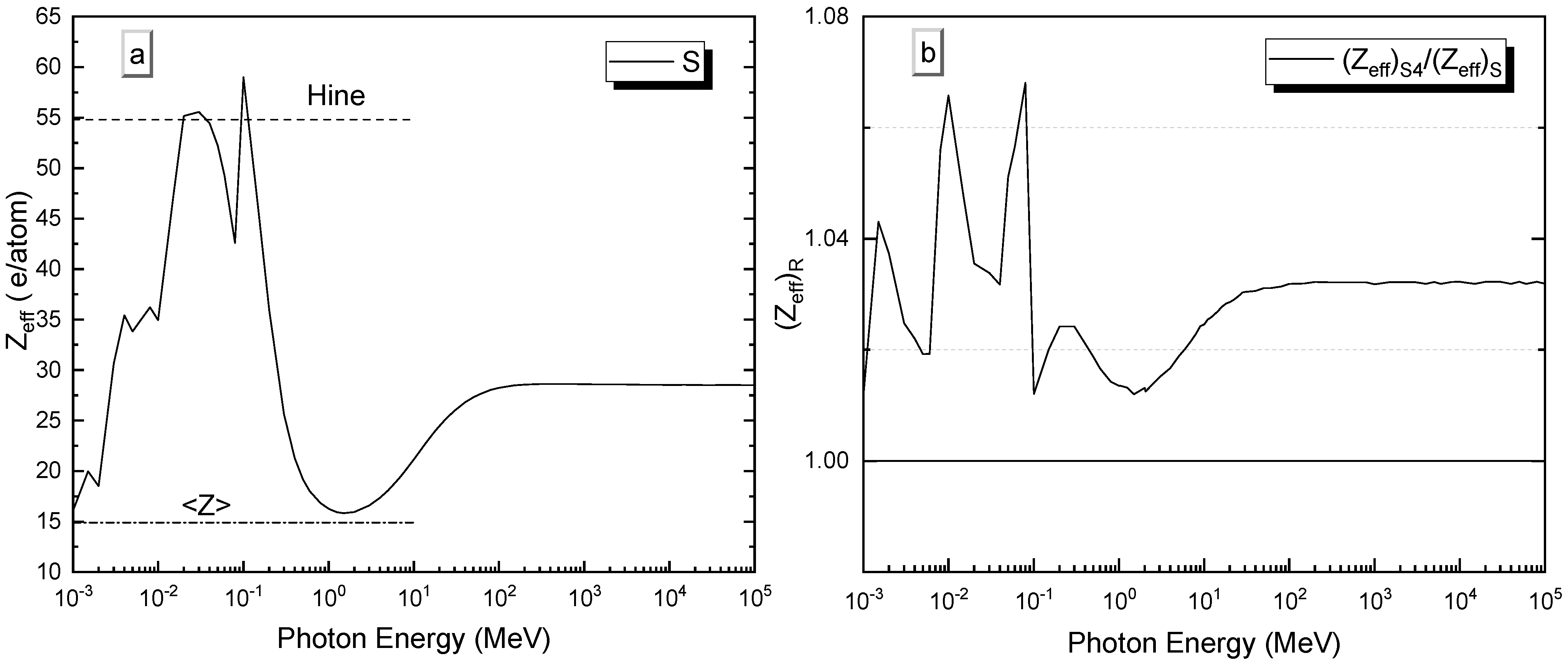
3.4.2. Effective Atomic Numbers and Effective Electron Densities
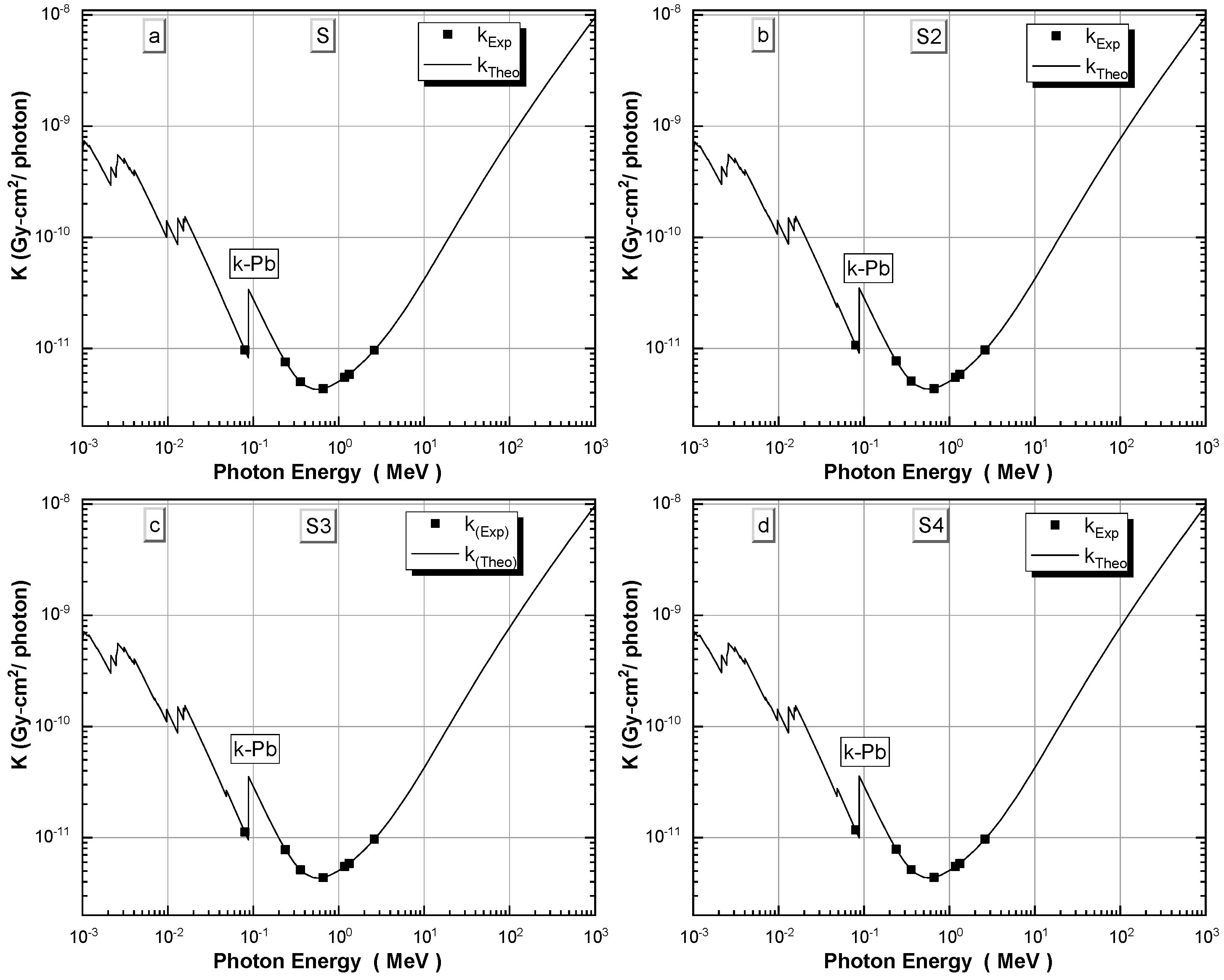
3.5. Experimental and Theoretical Kerma Coefficients of γ-ray
3.6. Fast Neutron Attenuation Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khor, S.; Talib, Z.; Yunus, W.M. Optical properties of ternary zinc magnesium phosphate glasses. Ceram. Int. 2012, 38, 935–940. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, P.; Singh, N. Fast ion conducting phosphate glasses and glass ceramic composites: Promising materials for solid state batteries. J. Non-Cryst. Solids 2012, 358, 2841–2846. [Google Scholar] [CrossRef]
- Azizabadi, N.; Azar, P.A.; Tehrani, M.S.; Derakhshi, P. Synthesis and characteristics of gel-derived SiO2-CaO-P2O5-SrO-Ag2O-ZnO bioactive glass: Bioactivity, biocompatibility, and antibacterial properties. J. Non-Cryst. Solids 2021, 556, 120568. [Google Scholar] [CrossRef]
- Wahab, E.A.; El-Maaref, A.; Shaaban, K.; Börcsök, J.; Abdelawwad, M. Lithium cadmium phosphate glasses doped Sm3+ as a host material for near-IR laser applications. Opt. Mater. 2021, 111, 110638. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Luo, M.; Dong, X.; Du, T.; Wang, Y. Low melting glasses in ZnO-Fe2O3-P2O5 system with high chemical durability and thermal stability for sealing or waste immobilization. J. Non-Cryst. Solids 2017, 469, 62–69. [Google Scholar] [CrossRef]
- Niraula, G.; Coaquira, J.A.; Aragon, F.H.; Villar, B.M.G.; Mello, A.; Garcia, F.; Muraca, D.; Zoppellaro, G.; Vargas, J.M.; Sharma, S.K. Tuning the shape, size, phase composition and stoichiometry of iron oxide nanoparticles: The role of phosphate anions. J. Alloys Compd. 2021, 856, 156940. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Electronic oxide polarizability and optical basicity of simple oxides. I. J. Appl. Phys. 1996, 79, 1736–1740. [Google Scholar] [CrossRef]
- El-Egili, K.; Doweidar, H.; Moustafa, Y.; Abbas, I. Structure and some physical properties of PbO-P2O5 glasses. Phys. B Condens. Matter 2003, 339, 237–245. [Google Scholar] [CrossRef]
- Muñoz, F.; Rocherullé, J.; Ahmed, I.; Hu, L. Phosphate Glasses. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 553–594. [Google Scholar] [CrossRef]
- Elalaily, N.A.; Zahran, A.H.; Sallam, O.I.; Eldin, F.M.E. Structure and electrical conductivity of ɤ-irradiated lead-phosphate glass containing MoO3. Appl. Phys. A 2019, 125, 128. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, P.; Ghosh, S. Structural properties of Er3+ doped lead zinc phosphate glasses. Mater. Sci. Eng. B 2019, 240, 116–120. [Google Scholar] [CrossRef]
- Sidek, H.; El-Mallawany, R.; Matori, K.; Halimah, M. Effect of PbO on the elastic behavior of ZnO-P2O5 glass systems. Results Phys. 2016, 6, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Shaaban, H.M.; Abdallah, A. Spectroscopic studies of ZnO borate-tellurite glass doped with Eu2O3. J. Mater. Res. Technol. 2018, 7, 240–247. [Google Scholar] [CrossRef]
- Chen, G.; Stump, N.; Haire, R.; Peterson, J. Study of the phase behavior of Eu2O3 under pressure via luminescence of Eu3+. J. Alloys Compd. 1992, 181, 503–509. [Google Scholar] [CrossRef]
- Zagrai, M.; Suciu, R.-C.; Rada, S.; Pică, M.; Pruneanu, S. Structural and optical properties of Eu3+ ions in lead glass for photonic applications. J. Non-Cryst. Solids 2021, 569, 120988. [Google Scholar] [CrossRef]
- Sharvani, K.N.; Ganesh, P.S.; Kaewkhao, J.; Intachai, N.; Kothan, S.; Rachniyom, W.; Pasha, A.; Rajaramakrishna, R. Optical and structural properties of Eu3+ doped MgO-Li2O-Na2O-BaO-B2O3 glasses for scintillating glass applications. Radiat. Phys. Chem. 2022, 199, 110295. [Google Scholar] [CrossRef]
- Teresa, P.E.; Naseer, K.; Piotrowski, T.; Marimuthu, K.; Aloraini, D.A.; Almuqrin, A.H.; Sayyed, M. Optical properties and radiation shielding studies of europium doped modifier reliant multi former glasses. Optik 2021, 247, 168005. [Google Scholar] [CrossRef]
- Esawii, H.A.; Salama, E.; El-Ahll, L.S.; Moustafa, M.; Saleh, H.M. High impact tungsten-doped borosilicate glass composite for gamma and neutron transparent radiation shielding. Prog. Nucl. Energy 2022, 150, 104321. [Google Scholar] [CrossRef]
- Ehab, M.; Salama, E.; Ashour, A.; Attallah, M.; Saleh, H.M. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability 2022, 14, 13298. [Google Scholar] [CrossRef]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Esawii, H.A. Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy 2021, 137, 103764. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.; Saleh, H.M.; Omar, K.M.; Sayyed, M.; El-Khatib, A.M.; Elsafi, M. Implementation of waste silicate glass into composition of ordinary cement for radiation shielding applications. Nucl. Eng. Technol. 2022, 54, 1456–1463. [Google Scholar] [CrossRef]
- Reda, S.M.; Saleh, H.M. Calculation of the gamma radiation shielding efficiency of cement-bitumen portable container using MCNPX code. Prog. Nucl. Energy 2021, 142, 104012. [Google Scholar] [CrossRef]
- Bayoumi, T.; Reda, S.; Saleh, H. Assessment study for multi-barrier system used in radioactive borate waste isolation based on Monte Carlo simulations. Appl. Radiat. Isot. 2012, 70, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Wagh, A.; Raviprakash, Y.; Kamath, S.D. Gamma rays interactions with Eu2O3 doped lead fluoroborate glasses. J. Alloys Compd. 2017, 695, 2781–2798. [Google Scholar] [CrossRef]
- Yin, S.; Wang, H.; Wang, S.; Zhang, J.; Zhu, Y. Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System. Crystals 2022, 12, 178. [Google Scholar] [CrossRef]
- Amjad, R.J.; Sahar, M.; Ghoshal, S.; Dousti, M.R.; Arifin, R. Synthesis and characterization of Dy3+ doped zinc-lead-phosphate glass. Opt. Mater. 2013, 35, 1103–1108. [Google Scholar] [CrossRef]
- Dousti, M.R.; Amjad, R.J. Spectroscopic properties of Tb3+-doped lead zinc phosphate glass for green solid state laser. J. Non-Cryst. Solids 2015, 420, 21–25. [Google Scholar] [CrossRef]
- Dousti, M.R.; Ghoshal, S.; Amjad, R.J.; Sahar, M.; Nawaz, F.; Arifin, R. Structural and optical study of samarium doped lead zinc phosphate glasses. Opt. Commun. 2013, 300, 204–209. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, S.; Zhou, X. Energy transfer from Ce3+ to Tb3+ and Eu3+ in zinc phosphate glasses. J. Rare Earths 2010, 28, 40–42. [Google Scholar] [CrossRef]
- Segawa, H.; Hirosaki, N.; Ohki, S.; Deguchi, K.; Shimizu, T. Exploration of zinc phosphate glasses dispersed with Eu-doped SiAlON for white LED applications. Opt. Mater. 2013, 35, 2677–2684. [Google Scholar] [CrossRef]
- Zakaly, H.M.; Rashad, M.; Tekin, H.; Saudi, H.; Issa, S.A.; Henaish, A. Synthesis, optical, structural and physical properties of newly developed dolomite reinforced borate glasses for nuclear radiation shielding utilizations: An experimental and simulation study. Opt. Mater. 2021, 114, 110942. [Google Scholar] [CrossRef]
- Sikora, P.; El-Khayatt, A.M.; Saudi, H.; Chung, S.-Y.; Stephan, D.; Elrahman, M.A. Evaluation of the effects of bismuth oxide (Bi2O3) micro and nanoparticles on the mechanical, microstructural and γ-ray/neutron shielding properties of Portland cement pastes. Constr. Build. Mater. 2021, 284, 122758. [Google Scholar] [CrossRef]
- Gerward, L.; Guilbert, N.; Jensen, K.B.; Levring, H. WinXCom—A program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 2004, 71, 653–654. [Google Scholar] [CrossRef]
- Murty, R.C. Effective Atomic Numbers of Heterogeneous Materials. Nature 1965, 207, 398–399. [Google Scholar] [CrossRef]
- Mayneord, W.V. The significance of the röntgen. Acta Int. Union Against Cancer 1937, 3, 271–282. [Google Scholar]
- Sellakumar, P.; Samuel, E.J.J.; Supe, S.S. Water equivalence of polymer gel dosimeters. Radiat. Phys. Chem. 2007, 76, 1108–1115. [Google Scholar] [CrossRef]
- Hine, G.J. Secondary electron emission and effective atomic numbers. Nucleon. US Ceased Publ. 1952, 10, 1. [Google Scholar]
- Tsai, C.M.; Cho, Z.H. Physics of contrast mechanism and averaging effect of linear attenuation coefficients in a computerized transverse axial tomography (CTAT) transmission scanner. Phys. Med. Biol. 1976, 21, 544–559. [Google Scholar] [CrossRef]
- Puumalainen, P.; Olkkonen, H.; Sikanen, P. Assessment of fat content of liver by a photon scattering technique. Int. J. Appl. Radiat. Isot. 1977, 28, 785–787. [Google Scholar] [CrossRef]
- Manninen, S.; Koikkalainen, S. Determination of the effective atomic number using elastic and inelastic scattering of γ-rays. Int. J. Appl. Radiat. Isot. 1984, 35, 965–968. [Google Scholar] [CrossRef]
- Akkurt, I.; El-Khayatt, A. The effect of barite proportion on neutron and gamma-ray shielding. Ann. Nucl. Energy 2013, 51, 5–9. [Google Scholar] [CrossRef]
- El-Khayatt, A. Semi-empirical determination of gamma-ray kerma coefficients for materials of shielding and dosimetry from mass attenuation coefficients. Prog. Nucl. Energy 2017, 98, 277–284. [Google Scholar] [CrossRef]
- De, M.; Jana, S.; Mitra, S. Structural and spectroscopic characteristics of Eu3+ embedded titanium lead phosphate glasses for red luminescence. Solid State Sci. 2021, 114, 106560. [Google Scholar] [CrossRef]
- Saudi, H.A.; El-Kameesy, S.U. Investigation of modified zinc borate glasses doped with BaO as a nuclear radiation-shielding material. Radiat. Detect. Technol. Methods 2018, 2, 44. [Google Scholar] [CrossRef]
- Znášik, P.; Jamnick, M. Non-Crystalline Solids Preparation, infrared spectra and structure of glasses in the system CuC1-Cu2O-(P2O5 + MoO3). J. Non-Cryst. Solids 1992, 146, 74–80. [Google Scholar] [CrossRef]
- Abo-Naf, S.; El-Amiry, M.; Abdel-Khalek, A. FT-IR and UV-Vis optical absorption spectra of γ-irradiated calcium phosphate glasses doped with Cr2O3, V2O5 and Fe2O3. Opt. Mater. 2008, 30, 900–909. [Google Scholar] [CrossRef]
- ElBatal, F.H.; Ouis, M.A.; Morsi, R.M.M.; Marzouk, S.Y. Interaction of gamma rays with some sodium phosphate glasses containing cobalt. J. Non-Cryst. Solids 2010, 356, 46–55. [Google Scholar] [CrossRef]
- Abid, M.; Et-Tabirou, M.; Hafid, M. Glass forming region, ionic conductivity and infrared spectroscopy of vitreous sodium lead mixed phosphates. Mater. Res. Bull. 2001, 36, 407–421. [Google Scholar] [CrossRef]
- Fayad, A.M.; Ouis, M.A.; ElBatal, F.H.; ElBatal, H.A. Shielding Behavior of Gamma-Irradiated MoO3 or WO3-Doped Lead Phosphate Glasses Assessed by Optical and FT Infrared Absorption Spectral Measurements. Silicon 2017, 10, 1873–1879. [Google Scholar] [CrossRef]
- Maity, A.; Jana, S.; Ghosh, S.; Sharma, S. Spectroscopic investigation on Europium (Eu3+) doped strontium zinc lead phosphate glasses with varied ZnO and PbO compositions. J. Non-Cryst. Solids 2020, 550, 120322. [Google Scholar] [CrossRef]
- Monisha, M.; Sayyed, M.I.; Mazumder, N.; Arayro, J.; Kamath, S.D. Color tuneability behaviour and energy transfer analysis on Dy3+-Eu3+ co-doped glasses for NUV-WLEDs application. J. Mater. Sci. Mater. Electron. 2023, 34, 487. [Google Scholar] [CrossRef]
- Abul-Magd, A.A.; Abu-Khadra, A.S.; Taha, A.M.; Basry, A. Influence of La3+ ions on the structural, optical and dielectric properties and ligand field parameters of Fe3+ hybrid borate glasses. J. Non-Cryst. Solids 2023, 599, 121981. [Google Scholar] [CrossRef]
- Salem, S.M.; Abou-Elnasr, T.Z.; El-Gammal, W.A.; Mahmoud, A.S.; Saudi, H.A.; Mostafa, A.G. Optical Parameters and Electrical Transport Properties of Some Barium-Sodium-Borate Glasses Doped Bismuth Oxide. Am. J. Aerosp. Eng. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- El-Samrah, M.; El-Mohandes, A.; El-Khayatt, A.; Chidiac, S. MRCsC: A user-friendly software for predicting shielding effectiveness against fast neutrons. Radiat. Phys. Chem. 2021, 182, 109356. [Google Scholar] [CrossRef]
- El-Khayatt, A. NXcom—A program for calculating attenuation coefficients of fast neutrons and gamma-rays. Ann. Nucl. Energy 2011, 38, 128–132. [Google Scholar] [CrossRef]
- Abdo, A.E.-S. Calculation of the cross-sections for fast neutrons and gamma-rays in concrete shields. Ann. Nucl. Energy 2002, 29, 1977–1988. [Google Scholar] [CrossRef]










| Constituent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mole% | wt.% | mole% | wt.% | mole% | wt.% | mole% | wt.% | mole% | wt.% | |
| PbO | 19.20 | 34.46 | 19.43 | 34.46 | 19.67 | 34.46 | 19.91 | 34.46 | 20.16 | 34.46 |
| CaO | 22.36 | 10.08 | 22.63 | 10.08 | 22.90 | 10.08 | 23.18 | 10.08 | 23.47 | 10.08 |
| P2O5 | 35.33 | 40.33 | 35.76 | 40.33 | 36.19 | 40.33 | 36.64 | 40.33 | 37.09 | 40.33 |
| ZnO | 23.10 | 15.12 | 21.82 | 14.12 | 20.51 | 13.11 | 19.16 | 12.10 | 17.78 | 11.09 |
| Eu2O3 | 0.36 | 1.01 | 0.73 | 2.02 | 1.11 | 3.02 | 1.50 | 4.03 | ||
| Glass | ρ a | M | VM | VO | OPD |
|---|---|---|---|---|---|
| g/cm3 | g/mole | cm3/mole | mole/cm3 | ||
| S | 5.04 | 152.14 | 63.04 | 26.12 | 79.95 |
| S1 | 5.058 | 154.86 | 63.53 | 26.07 | 79.61 |
| S2 | 5.076 | 157.59 | 63.54 | 25.62 | 79.89 |
| S3 | 5.094 | 160.32 | 63.73 | 25.34 | 79.93 |
| S4 | 5.112 | 163.05 | 63.90 | 25.04 | 80.00 |
| Sample | ˂A˃ | ˂Z˃ | ˂N˃ × 1026 | [34] | [35] | [36] | [37] | [38] | [40] |
|---|---|---|---|---|---|---|---|---|---|
| S | 32.31 | 14.89 | 2.78 | 57.5 | 53.7 | 54.8 | 57.2 | 56.3 | 45.5 |
| S1 | 32.42 | 14.93 | 2.77 | 57.7 | 53.9 | 55.0 | 57.4 | 56.5 | 56.5 |
| S2 | 32.53 | 14.96 | 2.77 | 57.8 | 54.1 | 55.2 | 57.6 | 56.6 | 46.1 |
| S3 | 32.64 | 15.00 | 2.77 | 58.0 | 54.3 | 55.4 | 57.7 | 56.8 | 46.4 |
| S4 | 32.75 | 15.04 | 2.77 | 58.2 | 54.5 | 55.6 | 57.9 | 57.0 | 46.6 |
| E(γ) MeV | S | S1 | S2 | S3 | S4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μm | μm | μm | μm | μm | |||||||||||
| Exp. | Theo. | Div. | Exp. | Theo. | Div. | Exp. | Theo. | Div. | Exp. | Theo. | Div. | Exp. | Theo. | Div. | |
| 0.08 | 0.988 | 1.005 | 1.7 | 1.030 | 1.046 | 1.5 | 1.070 | 1.086 | 1.5 | 1.110 | 1.126 | 1.5 | 1.150 | 1.167 | 1.5 |
| 0.238 | 0.294 | 0.298 | 1.4 | 0.296 | 0.300 | 1.4 | 0.298 | 0.302 | 1.4 | 0.300 | 0.304 | 1.4 | 0.302 | 0.306 | 1.4 |
| 0.356 | 0.160 | 0.161 | 0.6 | 0.160 | 0.162 | 1.1 | 0.161 | 0.162 | 0.9 | 0.162 | 0.163 | 0.7 | 0.162 | 0.164 | 1.1 |
| 0.662 | 0.087 | 0.087 | 0.1 | 0.087 | 0.087 | 0.3 | 0.087 | 0.087 | 0.3 | 0.087 | 0.087 | 0.3 | 0.087 | 0.088 | 0.3 |
| 1.173 | 0.059 | 0.059 | −0.4 | 0.059 | 0.059 | −0.2 | 0.059 | 0.059 | −0.2 | 0.059 | 0.059 | −0.2 | 0.059 | 0.059 | −0.2 |
| 1.325 | 0.055 | 0.055 | 0.5 | 0.055 | 0.055 | 0.3 | 0.055 | 0.055 | 0.4 | 0.055 | 0.055 | 0.5 | 0.055 | 0.055 | 0.5 |
| 2.614 | 0.040 | 0.040 | 0.2 | 0.040 | 0.040 | 0.0 | 0.040 | 0.040 | 0.1 | 0.040 | 0.040 | 0.1 | 0.040 | 0.040 | 0.1 |
| Energy | S | S1 | S2 | S3 | S4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theo. | Exp. | Theo. | Exp. | Theo. | Theo. | Exp. | Theo. | Exp. | Theo. | |
| 0.080 | 9.9 | 9.7 | 10.4 | 10.2 | 10.9 | 10.7 | 11.4 | 11.2 | 11.9 | 11.7 |
| 0.238 | 7.7 | 7.6 | 7.8 | 7.7 | 7.9 | 7.7 | 7.9 | 7.8 | 8.0 | 7.9 |
| 0.356 | 5.1 | 5.1 | 5.1 | 5.1 | 5.2 | 5.1 | 5.2 | 5.2 | 5.2 | 5.2 |
| 0.662 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 |
| 1.173 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| 1.325 | 5.9 | 5.8 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 |
| 2.614 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 | 9.7 |
| Elem. | S | S1 | S2 | S3 | S4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD | ΣR | ΣR | ΣR | ΣR | ΣR | |||||
| O | 1.603 | 0.0649 | 1.606 | 0.0650 | 1.609 | 0.0651 | 1.611 | 0.0653 | 1.614 | 0.0654 |
| Si | 0.832 | 0.0245 | 0.835 | 0.0246 | 0.838 | 0.0247 | 0.841 | 0.0248 | 0.844 | 0.0249 |
| Ca | 0.360 | 0.0088 | 0.361 | 0.0088 | 0.363 | 0.0088 | 0.364 | 0.0088 | 0.365 | 0.0089 |
| Zn | 0.607 | 0.0111 | 0.569 | 0.0104 | 0.530 | 0.0097 | 0.491 | 0.0090 | 0.452 | 0.0083 |
| Eu | 0.044 | 0.0005 | 0.088 | 0.0011 | 0.132 | 0.0016 | 0.177 | 0.0021 | ||
| Pb | 1.638 | 0.0170 | 1.643 | 0.0171 | 1.649 | 0.0172 | 1.655 | 0.0172 | 1.661 | 0.0173 |
| ΣR | 0.1264 | 0.1265 | 0.1266 | 0.1267 | 0.1268 | |||||
| ΣR [56] | DS | 0.1011 | BB | 0.1253 | ML | 0.1260 | II | 0.1280 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Khayatt, A.M.; Saudi, H.A.; AlRowis, N.H. Synthesis and Characterization of Zinc-Lead-Phosphate Glasses Doped with Europium for Radiation Shielding. Sustainability 2023, 15, 9245. https://doi.org/10.3390/su15129245
El-Khayatt AM, Saudi HA, AlRowis NH. Synthesis and Characterization of Zinc-Lead-Phosphate Glasses Doped with Europium for Radiation Shielding. Sustainability. 2023; 15(12):9245. https://doi.org/10.3390/su15129245
Chicago/Turabian StyleEl-Khayatt, Ahmed M., Heba A. Saudi, and Norah H. AlRowis. 2023. "Synthesis and Characterization of Zinc-Lead-Phosphate Glasses Doped with Europium for Radiation Shielding" Sustainability 15, no. 12: 9245. https://doi.org/10.3390/su15129245







