Characterization of a New Powdered, Milk-Based Medicinal Plant (Alcea rosea) Drink Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Process Yield
2.2.2. Proximate Analysis
2.2.3. Total Phenolic Content
2.2.4. Antioxidants Capacity
2.2.5. Measurement of Color Properties
2.2.6. Viscosity Measurement
2.2.7. FTIR
2.2.8. Sensory Evaluation
2.2.9. Mineral Contents
2.2.10. Powder Characterization
2.2.11. Solubility
2.2.12. Particle Size Distribution (PSD)
2.2.13. Scanning Electron Microscopy (SEM) Analysis
2.2.14. Statistical Analysis
3. Results and Discussion
3.1. Process Yield
3.2. Proximate Analysis
3.3. Total Phenolic Content (TPC)
3.4. Antioxidant Capacity
3.5. Color Measurements
3.6. Viscosity
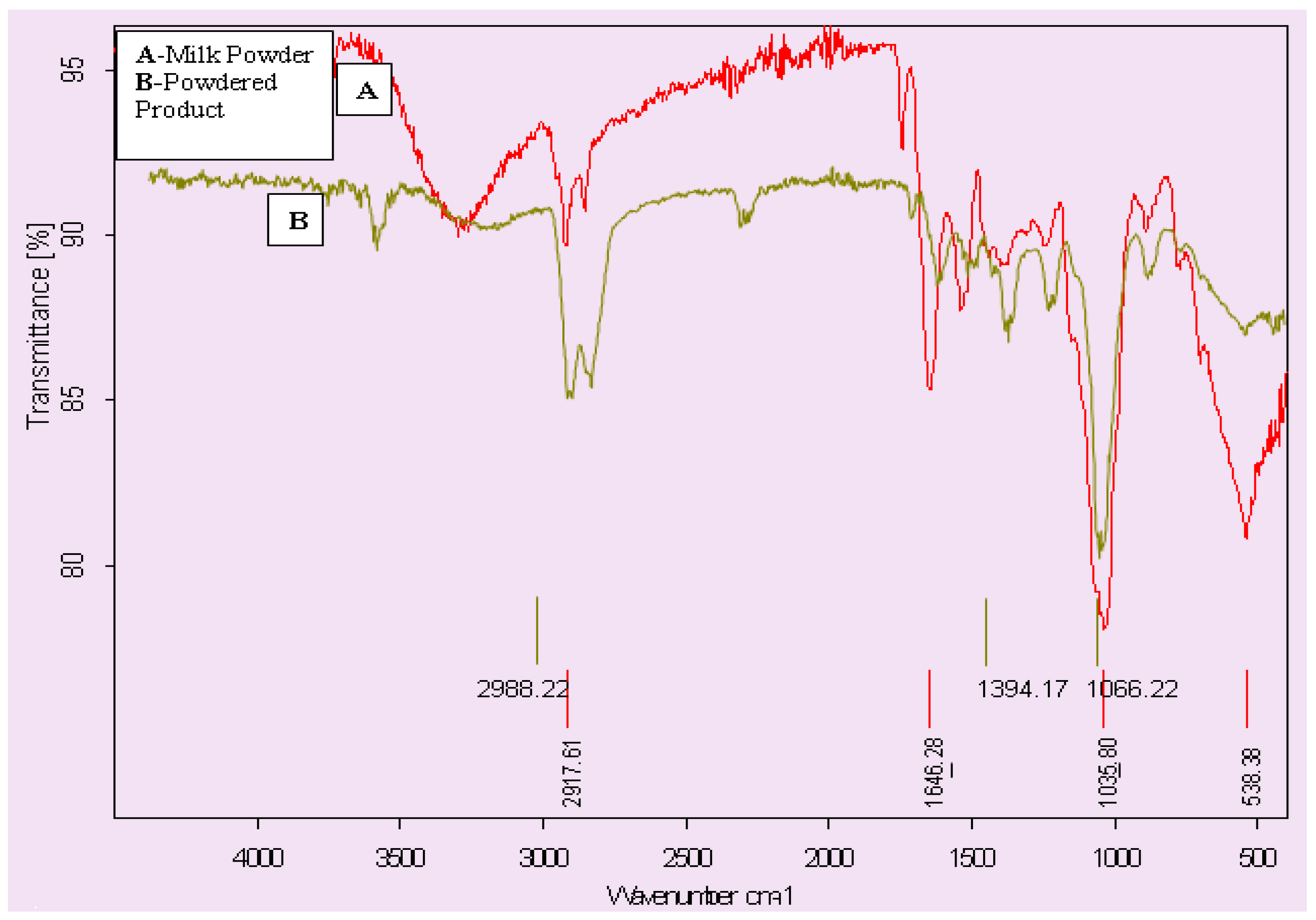
3.7. FTIR
3.8. Sensory Evaluation
3.9. Mineral Contents
3.10. Powder Characterization and Particle Size Distribution
3.11. Particle Appearance
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Dudek, M.; Matławska, I.; Szkudlarek, M. Phenolic acids in the flowers of Althaea rosea var. nigra. Acta Pol. Pharm. 2006, 63, 207–211. [Google Scholar] [PubMed]
- Hussain, L.; Akash, M.S.H.; Tahir, M.; Rehman, K.; Ahmed, K.Z. Hepatoprotective effects of methanolic extract of Alcea rosea against acetaminophen-induced hepatotoxicity in mice. Bangladesh J. Pharmacol. 2014, 9, 322–327. [Google Scholar] [CrossRef]
- Ekpo, K.E.; Onigbinde, A.O.; Asia, I.O. Pharmaceutical potentials of the oils of some popular insects consumed in southern Nigeria. Afr. J. Pharm. Pharmacol. 2009, 3, 51–57. [Google Scholar]
- Wang, D.F.; Shang, J.Y.; Yu, Q.H. Analgesic and anti-inflammatory effects of the flower of Althaea rosea (L.) Cav. China J. Chin. Mater. Med. 1989, 4, 46–48. [Google Scholar]
- Lone, P.; Bhardwaj, A.; Bahar, F. Study of indigenous/traditional medicinal plant knowledge-An endeavour towards new drug discovery. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Ammar, N.; El-Hakeem, R.A.; El-Kashoury, E.-S.; El-Kassem, L.A. Evaluation of the phenolic content and antioxidant potential of Althaea rosea cultivated in Egypt. J. Arab. Soc. Med. Res. 2013, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Fahamiya, N.; Shiffa, M.; Mohd, A. A Comprehensive Review on Althaea rosea Linn. Indo Am. J. Pharm. Res. 2016, 6, 6888–6894. [Google Scholar]
- Kim, M.S.; Chathuranga, K.; Kim, H.; Lee, J.-S.; Kim, C.-J. Anti-influenza properties of herbal extract of Althaea rosea in mice. Korean J. Veter Res. 2018, 58, 153–158. [Google Scholar] [CrossRef]
- Shehzad, M.R.; Hanif, M.A.; Rehman, R.; Bhatti, I.A.; Hanif, A. Hollyhock. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–391. [Google Scholar]
- Yekta, R.; Mirmoghtadaie, L.; Hosseini, H.; Norouzbeigi, S.; Hosseini, S.M.; Shojaee-Aliabadi, S. Development and characterization of a novel edible film based on Althaea rosea flower gum: Investigating the reinforcing effects of bacterial nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 158, 327–337. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2020. [Google Scholar]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2020, 96, 254–260. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using In Vitro Models. J. Agric. Food Chem. 2020, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, H.; Sakyi-Dawson, E.; Tano-Debrah, K.; Saalia, F. Development and characterization of dehydrated peanut–cowpea milk powder for use as a dairy milk substitute in chocolate manufacture. Food Res. Int. 2010, 43, 79–85. [Google Scholar] [CrossRef]
- Schokker, E.P.; Church, J.S.; Mata, J.P.; Gilbert, E.P.; Puvanenthiran, A.; Udabage, P. Reconstitution properties of micellar casein powder: Effects of composition and storage. Int. Dairy J. 2011, 21, 877–886. [Google Scholar] [CrossRef]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Leite, T.S.; Augusto, P.E.; Cristianini, M. The use of high pressure homogenization (HPH) to reduce consistency of concentrated orange juice (COJ). Innov. Food Sci. Emerg. Technol. 2014, 26, 124–133. [Google Scholar] [CrossRef]
- Gallo, L.; Ramírez-Rigo, M.V.; Piña, J.; Bucalá, V. A comparative study of spray-dried medicinal plant aqueous extracts. Drying performance and product quality. Chem. Eng. Res. Des. 2015, 104, 681–694. [Google Scholar] [CrossRef]
- Walton, D.E. The morphology of spray-dried particles a qualitative view. Dry. Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.; Calderas, F.; Laredo, R.G.; Sánchez-Olivares, G.; Gallegos-Infante, J.; Rocha-Guzmán, N.; Rodríguez-Ramírez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Pacheco, V.M.M.; Porras, A.O.O.; Velasco, E.; Morales-Valencia, E.M.; Navarro, A. Effect of the milk-whey relation over physicochemical and rheological properties on a fermented milky drink. Ing. Compet. 2017, 19, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.T.; Meena, G.S.; Upadhyay, N.; Khetra, Y.; Borad, S.; Singh, A.K. Production and characterization of milk protein concentrates 60 (MPC60) from buffalo milk. LWT 2018, 91, 368–374. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Song, F.-L.; Gan, R.-Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.L.; Deshpande, S.S.; Salunkhe, D.K. Food Antioxidants: Technological, Toxicological, Health Perspective, 1st ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–32. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.M.; Ren, Y.R.; Wang, L. Pharmacology and Clinical Application of Traditional Chinese Medicines, 1st ed.; Huaxia Press: Beijing, China, 1999; pp. 348–387. [Google Scholar]
- Wong, C.-C.; Li, H.-B.; Cheng, K.-W.; Chen, F. A systematic survey of antioxidant activity of Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Kalušević, A.; Levic, S.; Čalija, B.; Pantić, M.; Belović, M.; Pavlović, V.; Bugarski, B.; Milić, J.; Žilić, S.; Nedović, V. Microencapsulation of anthocyanin-rich black soybean coat extract by spray drying using maltodextrin, gum Arabic and skimmed milk powder. J. Microencapsul. 2017, 34, 475–487. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process. Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Levic, S.; Kalusevic, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedovic, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley and Sons Ltd.: Chichester, UK, 2004; ISBN 0470854278. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Barzegar, Y.; Ghasemi, Y.; Berenjian, A. Green synthesis and characterization of silver nanoparticles using Alcea rosea flower extract as a new generation of antimicrobials. Chem. Ind. Chem. Eng. Q. 2017, 23, 31–37. [Google Scholar] [CrossRef]
- Khoei, S.; Azarian, M.; Khoee, S. Effect of Hyperthermia and Triblock Copolymeric Nanoparticles as Quercetin Carrier on DU145 Prostate Cancer Cells. Curr. Nanosci. 2012, 8, 690–696. [Google Scholar] [CrossRef]
- Augusto, A.D.S.; Barsanelli, P.L.; Pereira, F.M.V.; Pereira-Filho, E.R. Calibration strategies for the direct determination of Ca, K, and Mg in commercial samples of powdered milk and solid dietary supplements using laser-induced breakdown spectroscopy (LIBS). Food Res. Int. 2017, 94, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuck, P.; Ouest, A. Milk powder: Physical and functional properties of milk powders. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 117–124. Available online: https://agris.fao.org/agris-search/search.do?recordID=FR20210181426 (accessed on 4 February 2023).
- Fitzpatrick, J.; Iqbal, T.; Delaney, C.; Twomey, T.; Keogh, M. Effect of powder properties and storage conditions on the flowability of milk powders with different fat contents. J. Food Eng. 2004, 64, 435–444. [Google Scholar] [CrossRef]
- Murphy, E.G. Infant Milk Formula Manufacture: Process and Compositional Interactions in High Dry Matter Wet-Mixes. Ph.D. Thesis, University College Cork, 2015. Available online: https://core.ac.uk/download/pdf/61579252.pdf (accessed on 27 January 2023).
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical characterization of whole and skim dried milk powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef]
- Sharma, A.; Jana, A.; Chavan, R.S. Functionality of Milk Powders and Milk-Based Powders for End Use Applications-A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, H.K.; Nanda, V. Effect of honey addition on flowability and solubility of spray-dried low fat milk powder. Int. Proc. Chem. Biol. Environ. Eng. 2015, 86, 22–27. [Google Scholar]
- Ilari, J.-L.; Mekkaoui, L. Physical properties of constitutive size classes of spray-dried skim milk powder and their mixtures. Le Lait 2010, 85, 279–294. [Google Scholar] [CrossRef]
- Zouari, A.; Mtibaa, I.; Triki, M.; Jridi, M.; Zidi, D.; Attia, H.; Ayadi, M.A. Effect of spray-drying parameters on the solubility and the bulk density of camel milk powder: A response surface methodology approach. Int. J. Dairy Technol. 2020, 73, 616–624. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Juliano, P. Physical and chemical properties of food powders. In Encapsulated and Powdered Foods; Onwulata, C., Ed.; Taylor and Francis Group, LLC: New York, NY, USA, 2005; pp. 39–71. [Google Scholar] [CrossRef]
- Teferra, T.F. Engineering Properties of Food Materials. In Handbook of Farm, Dairy and Food Machinery Engineering; Academic Press: Cambridge, MA, USA, 2019; pp. 45–89. [Google Scholar] [CrossRef]
- Ma, U.L.; Ziegler, G.; Floros, J. Effect of Sucrose on Physical Properties of Spray-Dried Whole Milk Powder. J. Food Sci. 2008, 73, E431–E438. [Google Scholar] [CrossRef]
- da Silva, D.F.; Tziouri, D.; Ahrné, L.; Bovet, N.; Larsen, F.H.; Ipsen, R.; Hougaard, A. Reconstitution behavior of cheese powders: Effects of cheese age and dairy ingredients on wettability, dispersibility and total rehydration. J. Food Eng. 2020, 270, 109763. [Google Scholar] [CrossRef]
- Lillford, P.; Fryer, P. Food Particles and the Problems of Hydration. Chem. Eng. Res. Des. 1998, 76, 797–802. [Google Scholar] [CrossRef]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Process. Process. Intensif. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Alamilla-Beltrán, L.; Chanona-Pérez, J.; Jiménez-Aparicio, A.; Gutierrez, G. Description of morphological changes of particles along spray drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]


| Flowability | Carr’s Index (%) | Hausner Ratio | Angle of Repose (°) |
|---|---|---|---|
| Excellent | 0–10 | 1.00–1.11 | 25–30 |
| Good | 11–15 | 1.12–1.18 | 31–35 |
| Fair | 16–20 | 1.19–1.25 | 36–40 |
| Passable | 21–25 | 1.26–1.34 | 41–45 |
| Poor | 26–31 | 1.35–1.45 | 46–55 |
| Very poor | 32–37 | 1.46–1.59 | 56–65 |
| Very, very poor | >38 | >1.60 | >66 |
| Analysis | Milk Powder | Dried Hollyhock Extract | |
|---|---|---|---|
| Dry Matter (%) | 98.40 ± 0.29 b | 99.42 ± 0.43 a | |
| Ash Content (%) | 4.67 ± 0.21 b | 5.73 ±0.28 a | |
| Water Activity (aw) | 0.1463 ± 0.0033 a | 0.0931 ± 0.0009 b | |
| Protein content | 29.92 ± 2.40 a | 29.12 ± 1.26 a | |
| pH | 6.693 ± 0.006 a | 6.340 ± 0.053 b | |
| Mineral Contents | Na (mg/100 g) | 429.52 ± 22.14 b | 502.71 ± 17.37 a |
| K (mg/100 g) | 1652.18 ± 41.51 a | 1346.91 ± 41.44 b | |
| Ca (mg/100 g) | 724.02 ± 13.84 a | 704.31 ± 15.92 a | |
| P (mg/100 g) | 418.96 ± 3.48 b | 747.45 ± 1.78 a | |
| Samples | Hollyhock Amount % | Viscosity | Color Values | Sensory Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Color | Consistency | Aroma | Sandiness | Overall Accep. | |||
| Milk | 0 | 1.556 ± 0.073 efg | 81.84 ± 0.03 a | −2.80 ± 0.01 h | 5.41 ± 0.00 a | 9.22 ± 1.71 a | 9.00 ± 1.58 a | 4.66 ± 1.53 a | 9.55 ± 0.72 a | 8.88 ± 1.27 a |
| Milky hollyhock extract | 1 | 1.916 ± 0.195 ef | 75.25 ± 0.03 b | −0.33 ± 0.00 g | 4.62 ± 0.01 b | 7.11 ± 2.31 ab | 6.00 ± 2.44 a | 6.22 ± 1.43 a | 9.33 ± 1.11 a | 5.77 ± 2.05 a |
| 3 | 3.417 ± 0.282 d | 68.71 ± 0.01 c | 1.64 ± 0.03 e | 3.55 ± 0.00 c | 5.44 ± 1.08 ab | 6.44 ± 1.94 a | 6.55 ± 1.81 a | 9.11 ± 1.53 a | 5.44 ± 1.87 a | |
| 5 | 4.899 ± 0.359 c | 64.98 ± 0.01 d | 2.55 ± 0.00 c | 2.67 ± 0.01 d | 4.33 ± 1.06 b | 5.22 ± 2.68 a | 6.55 ± 1.81 a | 8.88 ± 1.45 a | 5.11 ± 1.52 a | |
| 7 | 6.641 ± 0.541 b | 62.18 ± 0.24 e | 3.09 ± 0.02 b | 2.03 ± 0.01 g | 4.45 ± 1.12 b | 6.33 ± 1.23 a | 7.11 ± 2.14 a | 9.22 ± 1.45 a | 4.33 ± 2.34 a | |
| 9 | 9.597 ± 0.741 a | 59.43 ± 0.48 f | 3.55 ± 0.03 a | 1.37 ± 0.04 h | 4.66 ± 1.80 b | 7.33 ± 1.65 a | 7.55 ± 1.74 a | 9.22 ± 1.39 a | 3.88 ± 2.47 a | |
| Reconstituted hollyhock drink | 1 | 0.848 ± 0.086 fg | 45.87 ± 0.02 h | −1.28 ± 0.01 h | −2.29 ± 0.01 j | 5.66 ± 1.57 ab | 4.55 ± 1.69 a | 5.11 ± 1.16 a | 8.88 ± 2.31 a | 3.88 ± 1.96 a |
| 3 | 1.025 ± 0.069 fg | 58.89 ± 0.02 g | −0.37 ± 0.00 g | 1.02 ± 0.01 i | 6.11 ± 1.31 ab | 5.44 ± 1.96 a | 6.22 ± 2.98 a | 9.00 ± 1.80 a | 5.00 ± 1.39 a | |
| 5 | 1.431 ± 0.036 efg | 64.50 ± 0.01 d | 1.15 ± 0.00 f | 2.21 ± 0.01 f | 4.78 ± 1.27 b | 5.33 ± 1.95 a | 6.00 ± 1.41 a | 9.22 ± 1.30 a | 5.55 ± 2.06 a | |
| 7 | 1.861 ± 0.066 ef | 64.65 ± 0.01 d | 2.21 ± 0.01 d | 2.23 ± 0.00 f | 5.11 ± 1.31 ab | 5.66 ± 2.69 a | 6.33 ± 1.93 a | 9.11 ± 1.69 a | 4.66 ± 1.54 a | |
| 9 | 2.269 ± 0.071 e | 64.91 ± 0.01 d | 2.23 ± 0.02 d | 2.55 ± 0.00 e | 4.77 ± 1.22 b | 6.22 ± 1.11 a | 6.33 ± 1.65 a | 8.66 ± 2.00 a | 5.00 ± 1.87 a | |
| Milk Powder | Dried Milky Hollyhock Extract | ||
|---|---|---|---|
| Particle Properties | Bulk Density (ρT) (kg/m3) | 0.222 ± 0.003 b | 0.315 ± 0.003 a |
| Tapped Density (ρT) (kg/m3) | 0.378 ± 0.003 b | 0.490 ± 0.001 a | |
| Carr’s Index (Cl) (%) | 40.606 ± 0.525 a | 36.932 ± 0.964 b | |
| Powder Cohesiveness Hausner Ratio (HR) | 1.684 ± 0.015 a | 1.569 ± 0.023 b | |
| Angle of Repose (AOR) (°) | 35.725 ± 2.043 b | 42.325 ± 2.489 a | |
| Apparent density | 1.481 ± 0.050 b | 3.073 ± 0.039 a | |
| Porosity (epsilon) | 64.123 ± 0.141 b | 84.360 ± 0.631 a | |
| Particle Size | D10 µm | 0.091 ± 0.001 a | 0.068 ± 0.001 b |
| D50 µm | 0.532 ± 0.005 b | 3.315 ± 0.049 a | |
| D90 µm | 249.5 ± 3.535 a | 27.35 ± 0.212 b | |
| D [4,3] µMm | 55.35 ± 1.484 a | 29.1 ± 0.84 b | |
| D [3,2] µm | 0.25 ± 0.001 a | 0.213 ± 0.008 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortas, M. Characterization of a New Powdered, Milk-Based Medicinal Plant (Alcea rosea) Drink Product. Sustainability 2023, 15, 9320. https://doi.org/10.3390/su15129320
Mortas M. Characterization of a New Powdered, Milk-Based Medicinal Plant (Alcea rosea) Drink Product. Sustainability. 2023; 15(12):9320. https://doi.org/10.3390/su15129320
Chicago/Turabian StyleMortas, Mustafa. 2023. "Characterization of a New Powdered, Milk-Based Medicinal Plant (Alcea rosea) Drink Product" Sustainability 15, no. 12: 9320. https://doi.org/10.3390/su15129320
APA StyleMortas, M. (2023). Characterization of a New Powdered, Milk-Based Medicinal Plant (Alcea rosea) Drink Product. Sustainability, 15(12), 9320. https://doi.org/10.3390/su15129320







