1. Introduction
The development and selection of new amphiploid lines of cereals are crucial to identify the most performing ones in terms of crop production and survival in harsh environmental conditions [
1,
2]. Nowadays, one of the main factors reducing plant productivity is the poor seedling establishment in dry and semi-arid areas due to different abiotic stresses, such as rare annual precipitation, high evaporation, water scarcity, and soil salinity [
3,
4]. Primary Trans Chromosomal Tritipyrum (PTCT) lines, derived from the natural hybridization of
Triticum aestivum L. and
Thinopyrum bessarabicum, are recombinant chromosomal lines of hexaploid Tritipyrum, a new salt-tolerant species of cereal [
5,
6]. It was demonstrated that Tritipyrum lines could tolerate a salt concentration of about 250 mmol NaCl [
7,
8]. Thus, these novel amphiploid lines provide a new opportunity for producing grain and forage in saline soils and brackish waters in arid and semi-arid areas.
The first step toward reaching high production yields is the effective establishment of a crop. Seed germination dynamics play a crucial role in this process [
9]. The uniformity of development, yield, and quality of the harvested products are all greatly influenced by the quality of the seeds and how they are stored [
10]. The seed’s nature and its physical, morphological, and nutrient storage conditions are crucial to enable the proper seedling establishment and the least amount of mechanical and biological losses [
11,
12]. Understanding the intricate factors affecting seed longevity is extremely important from an ecological, agronomical, and economic perspective [
13]. It is recognized that the aging rate of the seeds is strongly influenced by environmental and genetic factors, such as storage temperature, seed moisture content, and seed quality [
14]. These factors could affect all the seed properties that determine the successful plant establishment. Seed vigor is the sum of the seed properties which determine the potential for high germination abilities, rapid, uniform emergence, and development of normal seedlings under a wide range of field conditions, as defined by the Association of Official Seed Analysts (AOSA) [
15,
16].
The uniformity of emergence and seedling establishment, with consequently farmers’ income, could be reduced by seed deterioration. Almost every year, 25% of the harvested seeds lose their quality due to deterioration [
17]. Seed deterioration may occur as a natural phenomenon, starting with a chain of biochemical events, such as membrane damage and disruption of biochemical processes [
18]. Thereby, most of the vital properties of seeds are diminished, starting with a decline in germination and emergence [
19], leading to poor seedling establishment [
20]. During seed deterioration, chromosomal aberration and permanent chemical and structural changes occur at the cellular level that leads to a decreased viability of seeds [
21].
In natural conditions, seed deterioration induces decreased germination percentages, poor seedling production, lowered vigor, declined viability, and seed death [
22]. Furthermore, deteriorated seeds have a more non-uniform establishment than healthy seeds [
23]. These issues lead to low seedling emergence, and few plants per hectare occur in the form of spots in the field [
23]. The viability of damaged seeds can be influenced by a decrease in total carbohydrates and an increase in lipid peroxidation during storage [
24]. Seed quality and viability decrease are further triggered by adverse environmental conditions, leading to a decay rate variation among varieties of the same species [
10,
25,
26]. Under high salt concentrations, for example, seed decay induces chromosome abnormalities during cell division (e.g., chromosome stickiness, laggard chromosome, disturbed and irregular anaphase, and anaphase bridge formation) [
27,
28]. Katabale and colleagues [
29] observed high rates of chromosomal abnormalities after exposing onion (
Allium cepa L.) seedlings to aqueous extracts of neem (
Azadirachta indica) leaf. Another study by Pavlova [
30] evaluated nickel’s toxic effects on root-meristem cell division in the seedlings of
Plantago lanceolata L., observing anaphase bridges, chromosome adhesion, retardation, and extrusion of nuclear material into the cytoplasm of these cells. The percentage of chromosome alterations usually increases in a concentration and time-dependent manner.
Seed storability is one of the key factors that assure plant propagation and crop production [
31]. This important characteristic has not been described for PTCT lines developed in our previous work [
11]. Currently, conventional methods to evaluate seed storability include seed vigor accelerated aging (AA), followed by germination and seedling growth tests [
31,
32]. Additionally, after AA, deterioration tests and electrical conductivity (EC), pH level, and cellular leakage of solutes during seeds’ water uptake can be measured [
33]. Biochemical processes augment cell membrane permeability in seeds during storage. As a result, some cellular solutes (sugars, amino acids, fatty acids, proteins, enzymes, and inorganic ions like K
+, Ca
+2, Mg
+2, Na
+, and Mn
+2) are released to the external environment during water uptake for germination [
31]. The amount of solute leakage can be used as an indicator for screening the storability of cultivars [
34]. The frequencies of chromosomal abnormalities arising from chromosomal fusions formed through errors in re-joining DNA double-strand breaks (DSBs) by the cell’s recombination pathways can occur in AA tests [
35].
One of the strategies used to improve plant growth in semi-arid and salinity-affected environments is the priming of seeds. Many works highlighted the use of seed priming to increase germination rate and crop development uniformity for overcoming poor germination and erratic crop stand [
36]. This treatment is provided before planting, in the early germination stage, when water is absorbed but prevents roots from coming out. In this process, seeds are soaked in water or various osmotic solutions and then dried until the initial moisture is achieved [
37]. Some biochemical processes are required during priming to initiate germination (e.g., water absorption, hydrolysis or metabolism of inhibitory materials, and enzymatic activities) [
38]. The induced effects remain even after redrying the seeds. Materials of high molecular weights are usually used for priming. Polyethylene glycol (PEG6000-8000) is the most used for seed priming. PEG is a non-toxic substance with a high molecular weight, osmotic pressure, and water solubility, which does not penetrate plant tissues while serving as a suitable compound for inducing drought stress [
39,
40]. Salicylic Acid (SA) is a phenolic growth-regulating compound that is present in low quantities (mg of fresh weight or less) in growing plants and applied for seed priming [
41]. It was demonstrated that rice seed priming by SA increases seed vitality [
42]. Salts are frequently utilized for seed priming [
43,
44]. Pretreatment with mineral salts (halo-priming) is an easy, low-cost, and low-risk technique effective in seed priming for plantations under salinity stress conditions [
45]. Priming is a simple, low-cost, low-risk approach to increase the vigor of deteriorated seeds.
The storage-tolerant lines development is one of the key characteristics of the ongoing plant breeding program. However, this method of introducing current lines has been little investigated. In this work, we hypothesized that 13 germplasms of Tritipyrum could be valid tolerant storage lines.
To test this hypothesis, we analyzed 13 germplasms of Tritipyrum to determine seed vigor by assessing germination and emergence traits, cell leakage, and cytogenetic abnormalities. Three different seed priming treatments were performed on the artificially aged seeds, and germination and emergence traits were measured to evaluate the potential increase in germination vitality of aged seeds.
4. Discussion
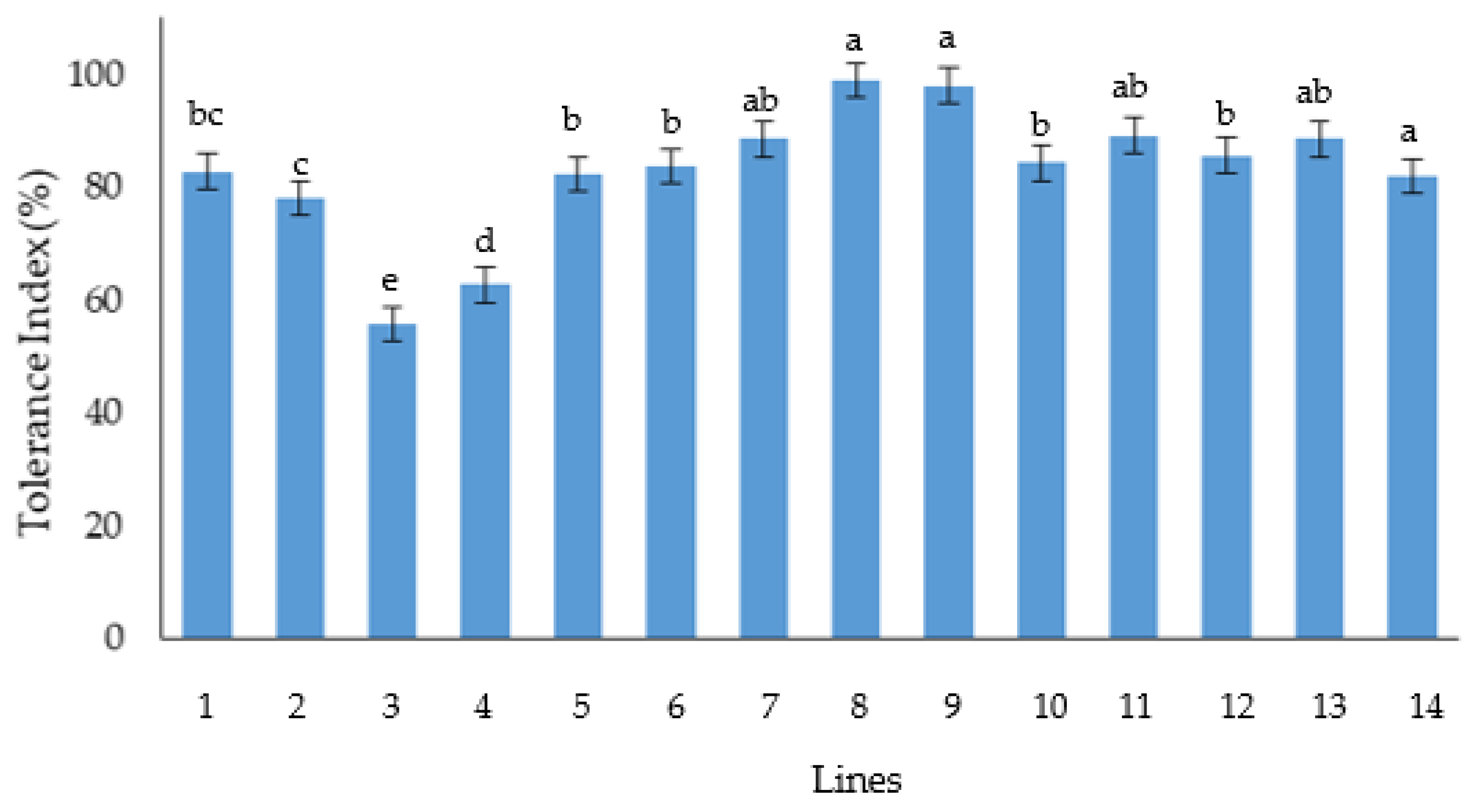
The reduction percentage and the Tolerance Index of germination and emergence traits of the aged seed showed that (Ma/b)(Cr/b)F4, (St/b)(Cr/b)F4 PTCT lines are the most tolerant lines to seed deterioration. Instead, the more sensitive lines were (Ka/b)(Cr/b)F2 and La(4B,4D)/b. The results obtained from seed aging about (St/b)(Cr/b)F3 line is promising, also considering its potential in salinity tolerance, as demonstrated by Pirsalami et al. (2021) [
59]. Furthermore, seed aging decreased cell membrane stability and increased the electrolyte, protein, and potassium leakages. This physiological parameter was inversely related to seed quality [
60]. Differences in the EC values indicated that the membrane’s nature and extent of protection were not the same in the studied seed lines. Seed deterioration is associated with increased permeability of the seed membrane, leading to higher electrolyte leakage during imbibition [
61]. During storage, the seeds gradually get old and lose their strength through coagulation and the breakdown of proteins [
62]. The more sensitive the storage condition, the faster the process develops as the temperature of the seed mass increases [
63]. We noticed that artificial aging causes cytological damage in seeds and chromosomal abnormalities. It is known that chromosomal aberrations increase during seed aging [
64]. We observed that, at mitosis, the enhancing seed age led to the percentage reduction of the dividing cells. With increasing temperature, the number of cells under division decreases while chromosomal abnormalities and injuries increase, leading, for example, to the formation of anaphase and telophase bridges [
65,
66,
67]. The chromosomal bridge may be caused by chromosomal adhesion and the subsequent inability of chromosomes to initiate a normal anaphase separation. It may be attributed to unequal transmission or the origin of chromosomal fragments [
68]. Hence, cell division phases are considered helpful for detecting the seed deterioration process. This study’s cell numbers at the interphase stage ranged from 440 to 488. MI indicates the frequency of mitotic division, in which temperature increase and reduces. With increasing deterioration time, a decrease in cell division was observed.
Chromosomal abnormalities in the mitotic cells of degraded seeds are detected via micronucleosis and chromosome breakage and bridge [
69]. Chromosome fragment formation might be due to chromosomes’ stickiness followed by separation failure. Breakage and reunion of the broken ends lead to chromosome bridges, which could be observed until early telophase. Our results of cytological changes in the root-tip cells of the aged seeds confirmed the previous studies [
69,
70]. In prophase, chromosomes are already visible, preparing to enter metaphase. When they exhibit a distinct pattern of organization, any irregularities occur probably due to mitotic spindle formation and chromosome condensation triggered by already duplicated chromosomes in the interphase. Chromosome disorganization at this stage would interfere with the regularity of metaphase chromosomes due to disturbances in mitotic spindle organization. Disorganized metaphase chromosomes appear due to their diffuse arrangements in the equatorial planes of the cells. This chromosome aberration is considered a sign of spindle malformation or partial inactivation. However, little is known about the activities of the directly regulated zones [
71]. Therefore, the chromosomes organized in the equatorial plates represent regular cell divisions in the metaphase [
72]. Only low cell irregularities were detected in the control treatments in this research, while aberrations were observed in the stress treatments.
Applying priming in the accelerated seed of PTCT lines was promising (
Table 6). It was observed that primed seeds’ germination traits were greater than untreated seeds. In addition, the germination traits were enhanced by applying all three priming treatments (halo-priming, hormone-priming, and osmo-priming). Seed priming promotes efficient germination by inducing a more favorable physiological state. It regulates hydration that starts the normal metabolic process during the early stages of germination before the protrusion of the radicle [
73]. In this study, osmo-priming increased germination traits further than hydropriming and hormone-priming (
Table 6), as Mohajeri et al. (2016) observed. They reported that polyethylene glycol application for seed priming improved vetch seeds’ root dry weight and shot dry weight values [
74].
Furthermore, Chen et al. (2021) observed that PEG6000 application for seed priming improved germination percentage and germination rate [
75]. Osmo-priming can benefit abiotic stresses like drought, extreme temperature, and salinity, generating different pre-germination metabolic activities, improving the antioxidant system, and preparing the seed for radicle protrusion [
76]. In our study, priming significantly affected shoot and root length (
Table 6). The highest and lowest mean values of these traits were observed in hormonal priming with SA and the control treatments, respectively (
Table 6). These outcomes match with those attained by Tabatabaei [
77]. Many field crops have reported increasing SVI and GE values in seedlings by priming seeds [
78,
79,
80,
81]. Rosinska et al. (2023) reported the benefits of osmo-priming on germination and seedling vigor in carrots seeds. They demonstrated the potentiality of this treatment also to mitigate biotic stress [
82]. PTCT lines’ best germination percentage, germination rate, shoot length, root length, seedling vigor index, and dry seedling weight were observed in seeds treated by osmo-priming compared to the control. Possible mechanisms for improved germination traits by halo-priming, hormone-priming, and osmo-priming are activation of water-induced metabolic processes, improved repair due to enzyme activity, and production of hormones. Osmo-priming with PEG enhances some of the antioxidant enzymes, such as the activities of catalase-peroxidase in seedlings exposed to stress, thus increasing seed germination and stand establishment [
83]. In other research, Zhang and colleagues found that osmo-priming with PEG improved cell membrane stability, enhanced superoxide dismutase, peroxidase, and catalase, and decreased lipid peroxidation, compared to non-primed seeds [
84].