Improving CO2 Absorption Using Artificial Intelligence and Modern Optimization for a Sustainable Environment
Abstract
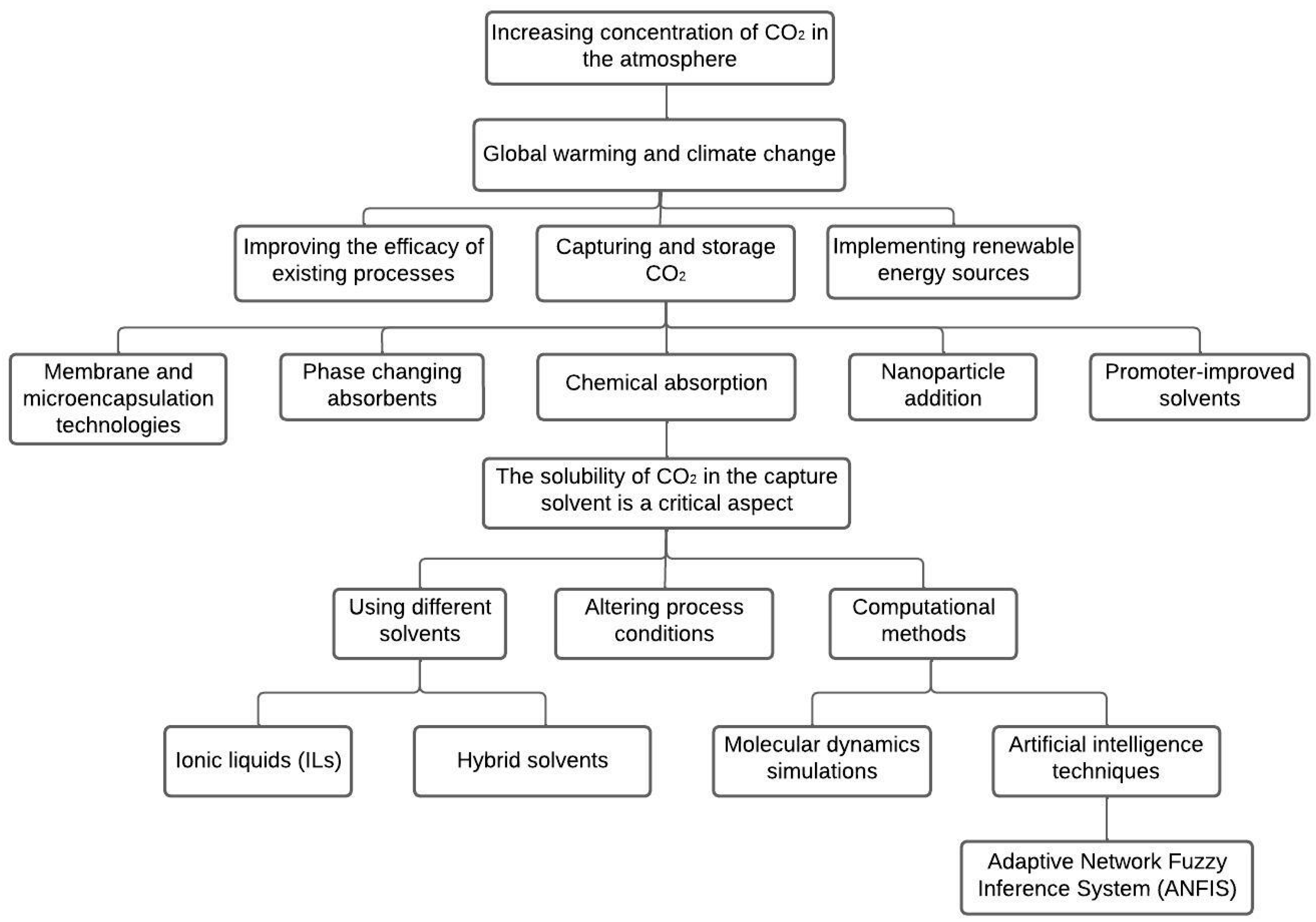
1. Introduction
- A new application of improved grey wolf optimizer is proposed to improve CO2 absorption;
- The optimal values of concentration of [TBP][MeSO3], temperature, and pressure of CO2 are determined;
- The value of mol fraction is increased.
2. Dataset
3. Methodology
3.1. ANFIS-Model
3.2. Improved Grey Wolf Optimizer
3.2.1. Encircling Phase
3.2.2. Hunting Phase
3.2.3. Attacking Phase
4. Assessing Societal Benefits: Key Determinants Evaluation
- Reducing CO2 emissions: The study focuses on developing efficient and ecologically friendly energy conversion technologies, carbon capture and storage (CCS), and advanced CO2 absorption solutions to reduce CO2 emissions from various sectors that consume high levels of energy;
- Environmental sustainability: The use of sustainable energy sources and carbon capture and storage techniques has minimal consequences and promotes environmental sustainability;
- Solvent optimization: This study focuses on optimizing the solvent composition and concentration using advanced computational methods such as molecular dynamics simulations and artificial intelligence techniques, resulting in improved CO2 solubility and selectivity;
- Lower costs: The literature provides insights on blended solvents and guidelines for a high-pressure CO2 absorption process for cost-effective blue H2 production;
- Improved technology: The previous studies discussed the use of ionic liquids (ILs) and hybrid solvents, resulting in high CO2 solubility and selectivity, and exceptional properties, such as low volatility, high thermal stability, non-flammability, and tunability. However, this study contributes to the development of new and advanced CO2 absorption technologies.
5. Results and Discussion
5.1. Modeling Phase
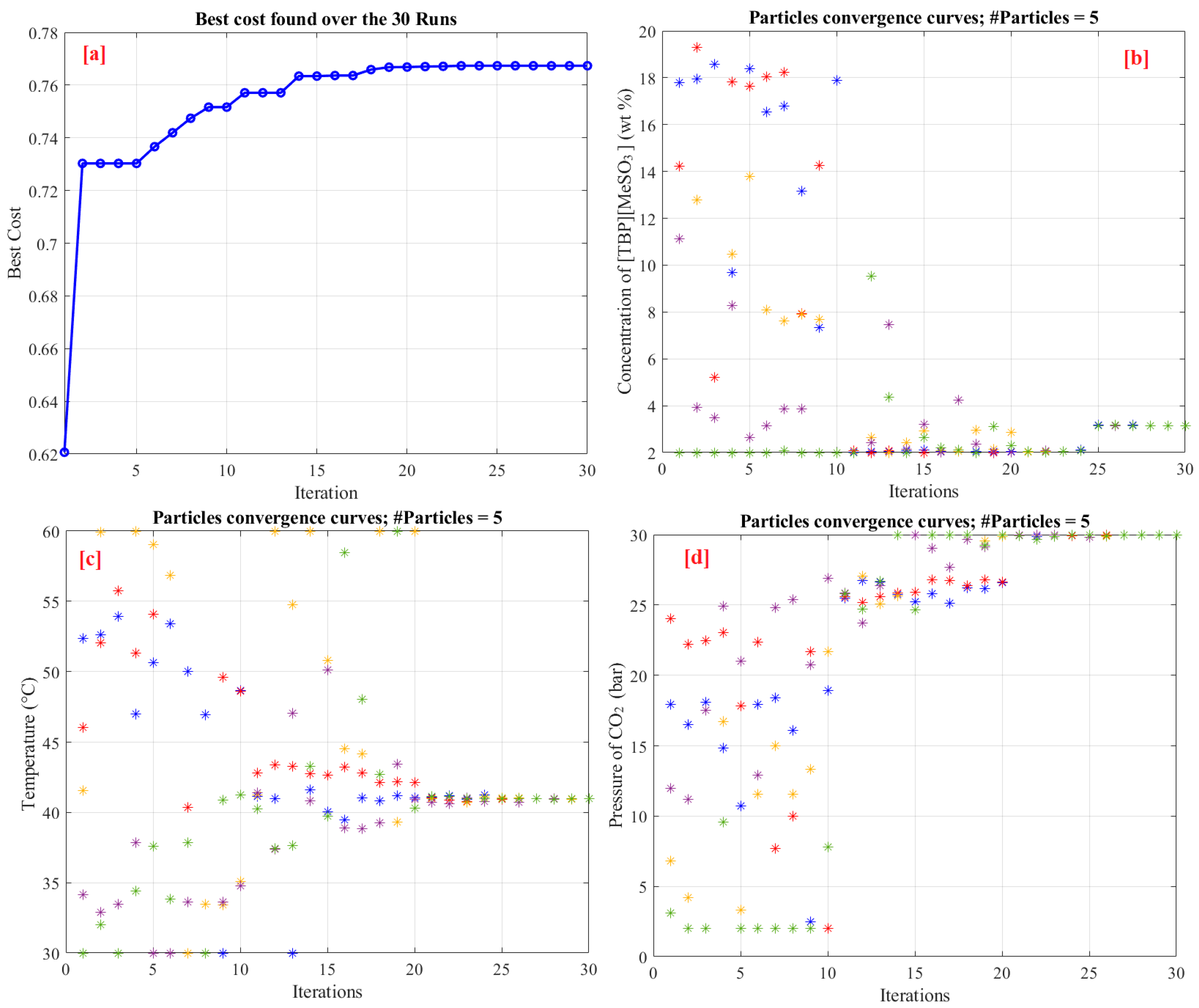
5.2. Optimization Phase
6. Recommendations for Policy Implementation
7. Study Limitations and Future Works
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| List of Abbreviations | |
| AI | artificial intelligence |
| ANFIS | adaptive neuro-fuzzy inference system |
| ANN | artificial neural network |
| ANOVA | analysis of variance |
| CCS | carbon capture and storage |
| CO2 | carbon dioxide |
| df | degrees of freedom |
| DIPA | diisopropanolamine |
| DLH | learning-based hunting |
| FL | fuzzy logic |
| GD | gradient descent |
| GWO | grey wolf optimizer |
| HHO | Harris Hawks optimization |
| HSD | honestly significant difference |
| IGWO | intelligent grey wolf optimizer |
| ILs | ionic liquids |
| LSE | least squares estimate |
| MDEAs | methyl diethanolamines |
| MEAs | monoethanolamines |
| MS | mean square |
| PSO | particle swarm optimization |
| PZ | piperazine |
| RMSE | root-mean-square deviation |
| SC | subtractive clustering |
| SMA | slime mould algorithm |
| SMR | steam methane reforming |
| SS | sum of squares |
| TEAs | triethanolamines |
| [TBP][MeSO3] | concentration of tetrabutylphosphonium methanesulfonate |
| List of symbols | |
| A, B | MFs of a and b |
| c | final crisp output, output |
| C | parameter |
| scalar variable | |
| d | dimension |
| Euclidean distance between the solutions and | |
| f(.) | cost function |
| output | |
| j | neighboring solution |
| k | iteration |
| Ld | lower limit |
| n | total number of rules |
| absorbed CO2 molecules | |
| initial moles of the CO2 | |
| Peq | pressure at equilibrium |
| R | gas constant, radius |
| r | random generator in the range [0 1] |
| R2 | coefficient of determination |
| r2, r3 | integer number |
| random numbers associated to the ith solution at an iteration k | |
| random generated variable | |
| T | temperature of the reservoir |
| Ud | upper limit |
| Vres | volume of the reservoir |
| Vs | volume of the aqueous MEA-[TBP][MeSO3] |
| Wavg | weighted average |
| weight | |
| x | three controlling parameters |
| updated solutions using DLH | |
| updated solutions using GWO | |
| the ith solution update at an iteration k | |
| initial solution | |
| ith solution (wolf) | |
| current and updated positions of the ith solution at iteration k | |
| random selected neighbor solution | |
| prey positions at an iteration k | |
| solution selected randomly from the solutions’ pool | |
| ZCO2 | compressibility of the CO2 |
| Greek symbols | |
| 𝛼 | alpha (wolf position) |
| 𝛽 | beta (wolf position) |
| 𝛿 | delta (wolf position) |
| ω | omega (remaining set of wolves) |
| scalar parameter | |
References
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large Scale Application of Carbon Capture to Process Industries–A Review. J. Clean. Prod. 2022, 362, 132300. [Google Scholar] [CrossRef]
- Jouhara, H.; Olabi, A.G. Industrial Waste Heat Recovery. Energy 2018, 160, 1–2. [Google Scholar] [CrossRef]
- Zacharczuk, W.; Andruszkiewicz, A.; Tatarek, A.; Alahmer, A.; Alsaqoor, S. Effect of Ca-Based Additives on the Capture of SO2 during Combustion of Pulverized Lignite. Energy 2021, 231, 120988. [Google Scholar] [CrossRef]
- Adaileh, W.; Alahmer, A. Reduction of the Spark Ignition Engine Emissions Using Limestone Filter. Can. J. Pure Appl. Sci. 2014, 8, 2761–2767. [Google Scholar]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste Heat Recovery Technologies and Applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Alsaqoor, S.; Alahmer, A.; Aljabarin, N.; Gougazeh, M.; Czajczynska, D.; Krzyzynska, R. Effects of Utilization of Solid and Semi-Solid Organic Waste Using Pyrolysis Techniques. In Proceedings of the 2017 8th International Renewable Energy Congress (IREC), Amman, Jordan, 21–23 March 2017; pp. 1–5. [Google Scholar]
- Aladayleh, W.; Alahmer, A. Recovery of Exhaust Waste Heat for ICE Using the Beta Type Stirling Engine. J. Energy 2015, 2015, 495418. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The Aluminium Industry: A Review on State-of-the-Art Technologies, Environmental Impacts and Possibilities for Waste Heat Recovery. Int. J. Thermofluids 2020, 1, 100007. [Google Scholar] [CrossRef]
- Fierro, J.J.; Escudero-Atehortua, A.; Nieto-Londoño, C.; Giraldo, M.; Jouhara, H.; Wrobel, L.C. Evaluation of Waste Heat Recovery Technologies for the Cement Industry. Int. J. Thermofluids 2020, 7, 100040. [Google Scholar] [CrossRef]
- Beguedou, E.; Narra, S.; Afrakoma Armoo, E.; Agboka, K.; Damgou, M.K. Alternative Fuels Substitution in Cement Industries for Improved Energy Efficiency and Sustainability. Energies 2023, 16, 3533. [Google Scholar] [CrossRef]
- Egilegor, B.; Jouhara, H.; Zuazua, J.; Al-Mansour, F.; Plesnik, K.; Montorsi, L.; Manzini, L. ETEKINA: Analysis of the Potential for Waste Heat Recovery in Three Sectors: Aluminium Low Pressure Die Casting, Steel Sector and Ceramic Tiles Manufacturing Sector. Int. J. Thermofluids 2020, 1, 100002. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Bahaa, A.; Eisa, T.; Alawadhi, H.; Al-Asheh, S.; Chae, K.-J.; Olabi, A.G. Synthesis and Performance Evaluation of Various Metal Chalcogenides as Active Anodes for Direct Urea Fuel Cells. Renew. Sustain. Energy Rev. 2021, 150, 111470. [Google Scholar] [CrossRef]
- Tanveer, W.H.; Abdelkareem, M.A.; Kolosz, B.W.; Rezk, H.; Andresen, J.; Cha, S.W.; Sayed, E.T. The Role of Vacuum Based Technologies in Solid Oxide Fuel Cell Development to Utilize Industrial Waste Carbon for Power Production. Renew. Sustain. Energy Rev. 2021, 142, 110803. [Google Scholar] [CrossRef]
- Alrbai, M.; Hayajneh, H.S.; Al-Dahidi, S.; Alahmer, A. Thermodynamics Analysis of a Lab Scale Humidification-Dehumidification Desalination System Employing Solar Energy and Fogging Approach. Sol. Energy 2022, 247, 397–407. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Shehata, N.; Alami, A.H.; Maghrabie, H.M.; Abdelkareem, M.A. Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy. Energies 2022, 15, 8639. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Lootah, M.A.; Sayed, E.T.; Wilberforce, T.; Alawadhi, H.; Yousef, B.A.A.; Olabi, A.G. Fuel Cells for Carbon Capture Applications. Sci. Total Environ. 2021, 769, 144243. [Google Scholar] [CrossRef] [PubMed]
- Silveira, B.H.M.; Costa, H.K.M.; Santos, E.M. Bioenergy with Carbon Capture and Storage (BECCS) in Brazil: A Review. Energies 2023, 16, 2021. [Google Scholar] [CrossRef]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Podder, J.; Patra, B.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K. A Review of Carbon Capture and Valorization Technologies. Energies 2023, 16, 2589. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Da Costa, J.C.D.; May, E.F. The Removal of CO2 and N2 from Natural Gas: A Review of Conventional and Emerging Process Technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid Amine Sorbents for CO2 Capture by Chemical Adsorption: A Review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Geng, M.; Peng, X.; Feng, J. Effects of Liquid Density on the Gas-Liquid Interaction of the Ionic Liquid Compressor for Hydrogen Storage. Energies 2023, 16, 3193. [Google Scholar] [CrossRef]
- Ali, S.A.; Mulk, W.U.; Ullah, Z.; Khan, H.; Zahid, A.; Shah, M.U.H.; Shah, S.N. Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review. Energies 2022, 15, 9098. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Mezzetta, A.; Perillo, V.; Guazzelli, L.; Chiappe, C. Thermal Behavior Analysis as a Valuable Tool for Comparing Ionic Liquids of Different Classes. J. Therm. Anal. Calorim. 2019, 138, 3335–3345. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, L.; Bai, Y.; Li, F.; Dong, H.; Wang, H.; Zhang, X.; Zeng, S. Superbase Ionic Liquid-Based Deep Eutectic Solvents for Improving CO2 Absorption. ACS Sustain. Chem. Eng. 2020, 8, 2523–2530. [Google Scholar] [CrossRef]
- Zainul Anuar, M.a.b.U.; Taha, M.F.; Md Yunus, N.M.; Mat Ghani, S.M.; Idris, A. An Optimization Study of Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and Tetrabutylphosphonium Methanesulfonate Hybrid Solvent Using RSM-CCD Methodology. Processes 2021, 9, 1186. [Google Scholar] [CrossRef]
- Rezk, H.; Mohammed, R.H.; Rashad, E.; Nassef, A.M. ANFIS-Based Accurate Modeling of Silica Gel Adsorption Cooling Cycle. Sustain. Energy Technol. Assessments 2022, 50, 101793. [Google Scholar] [CrossRef]
- Nassef, A.M.; Fathy, A.; Abdelkareem, M.A.; Olabi, A.G. Increasing Bio-Hydrogen Production-Based Steam Reforming ANFIS Based Model and Metaheuristics. Eng. Anal. Bound. Elem. 2022, 138, 202–210. [Google Scholar] [CrossRef]
- Alahmer, A.; Rezk, H.; Aladayleh, W.; Mostafa, A.O.; Abu-Zaid, M.; Alahmer, H.; Gomaa, M.R.; Alhussan, A.A.; Ghoniem, R.M. Modeling and Optimization of a Compression Ignition Engine Fueled with Biodiesel Blends for Performance Improvement. Mathematics 2022, 10, 420. [Google Scholar] [CrossRef]
- Mun, J.-H.; Shin, B.-J.; Kim, S.-M.; You, J.K.; Park, Y.C.; Chun, D.-H.; Lee, J.-S.; Min, B.-M.; Lee, U.; Kim, K.-M. Optimal MEA/DIPA/Water Blending Ratio for Minimizing Regeneration Energy in Absorption-Based Carbon Capture Process: Experimental CO2 Solubility and Thermodynamic Modeling. Chem. Eng. J. 2022, 444, 136523. [Google Scholar] [CrossRef]
- Kum, J.; Oh, H.-T.; Park, J.; Kang, J.-H.; Lee, C.-H. Techno-Economic Analysis and Optimization of a CO2 Absorption Process with a Solvent Looping System at the Absorber Using an MDEA/PZ Blended Solvent for Steam Methane Reforming. Chem. Eng. J. 2023, 455, 140685. [Google Scholar] [CrossRef]
- Nassef, A.M.; Rezk, H.; Alahmer, A.; Abdelkareem, M.A. Maximization of CO2 Capture Capacity Using Recent RUNge Kutta Optimizer and Fuzzy Model. Atmosphere 2023, 14, 295. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon Dioxide Capture Using Liquid Absorption Methods: A Review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Khan, S.N.; Hailegiorgis, S.M.; Man, Z.; Shariff, A.M.; Garg, S. Thermophysical Properties of Concentrated Aqueous Solution of N-Methyldiethanolamine (MDEA), Piperazine (PZ), and Ionic Liquids Hybrid Solvent for CO2 Capture. J. Mol. Liq. 2017, 229, 221–229. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Chen, Y.; Yang, Z.; Lu, X. Supported Ionic Liquid Sorbents for CO2 Capture from Simulated Flue-Gas. Chinese J. Chem. Eng. 2018, 26, 2377–2384. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Brinzer, T.; Roth, E.A.; Kusuma, V.A.; Watkins, J.D.; Zhou, X.; Luebke, D.; Hopkinson, D.; Washburn, N.R.; Garrett-Roe, S. Eutectic Ionic Liquid Mixtures and Their Effect on CO2 Solubility and Conductivity. RSC Adv. 2015, 5, 51407–51412. [Google Scholar] [CrossRef]
- Lv, B.; Xia, Y.; Shi, Y.; Liu, N.; Li, W.; Li, S. A Novel Hydrophilic Amino Acid Ionic Liquid [C2OHmim][Gly] as Aqueous Sorbent for CO2 Capture. Int. J. Greenh. Gas Control 2016, 46, 1–6. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Jing, G.; Lv, B. Evaluation of the Multi-Amine Functionalized Ionic Liquid for Efficient Postcombustion CO2 Capture. Energy Fuels 2016, 30, 7489–7495. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Salameh, T.; Sayed, E.T.; Olabi, A.G.; Hdaib, I.I.; Allan, Y.; Alkasrawi, M.; Abdelkareem, M.A. Adaptive Network Fuzzy Inference System and Particle Swarm Optimization of Biohydrogen Production Process. Fermentation 2022, 8, 483. [Google Scholar] [CrossRef]
- Alahmer, H.; Alahmer, A.; Alkhazaleh, R.; Alrbai, M. Exhaust Emission Reduction of a SI Engine Using Acetone–Gasoline Fuel Blends: Modeling, Prediction, and Whale Optimization Algorithm. Energy Rep. 2023, 9, 77–86. [Google Scholar] [CrossRef]
- Alahmer, H.; Alahmer, A.; Alkhazaleh, R.; Al-Amayreh, M.I. Modeling, Polynomial Regression, and Artificial Bee Colony Optimization of SI Engine Performance Improvement Powered by Acetone–Gasoline Fuel Blends. Energy Rep. 2023, 9, 55–64. [Google Scholar] [CrossRef]
- Mirjalili, S.; Mirjalili, S.M.; Lewis, A. Grey Wolf Optimizer. Adv. Eng. Softw. 2014, 69, 46–61. [Google Scholar] [CrossRef]
- Alahmer, H.; Alahmer, A.; Alkhazaleh, R.; Alrbai, M.; Alamayreh, M.I. Applied Intelligent Grey Wolf Optimizer (IGWO) to Improve the Performance of CI Engine Running on Emulsion Diesel Fuel Blends. Fuels 2023, 4, 35–57. [Google Scholar] [CrossRef]
- Nadimi-Shahraki, M.H.; Taghian, S.; Mirjalili, S. An Improved Grey Wolf Optimizer for Solving Engineering Problems. Expert Syst. Appl. 2021, 166, 113917. [Google Scholar] [CrossRef]
- Alahmer, A.; Alahmer, H.; Handam, A.; Rezk, H. Environmental Assessment of a Diesel Engine Fueled with Various Biodiesel Blends: Polynomial Regression and Grey Wolf Optimization. Sustainability 2022, 14, 1367. [Google Scholar] [CrossRef]

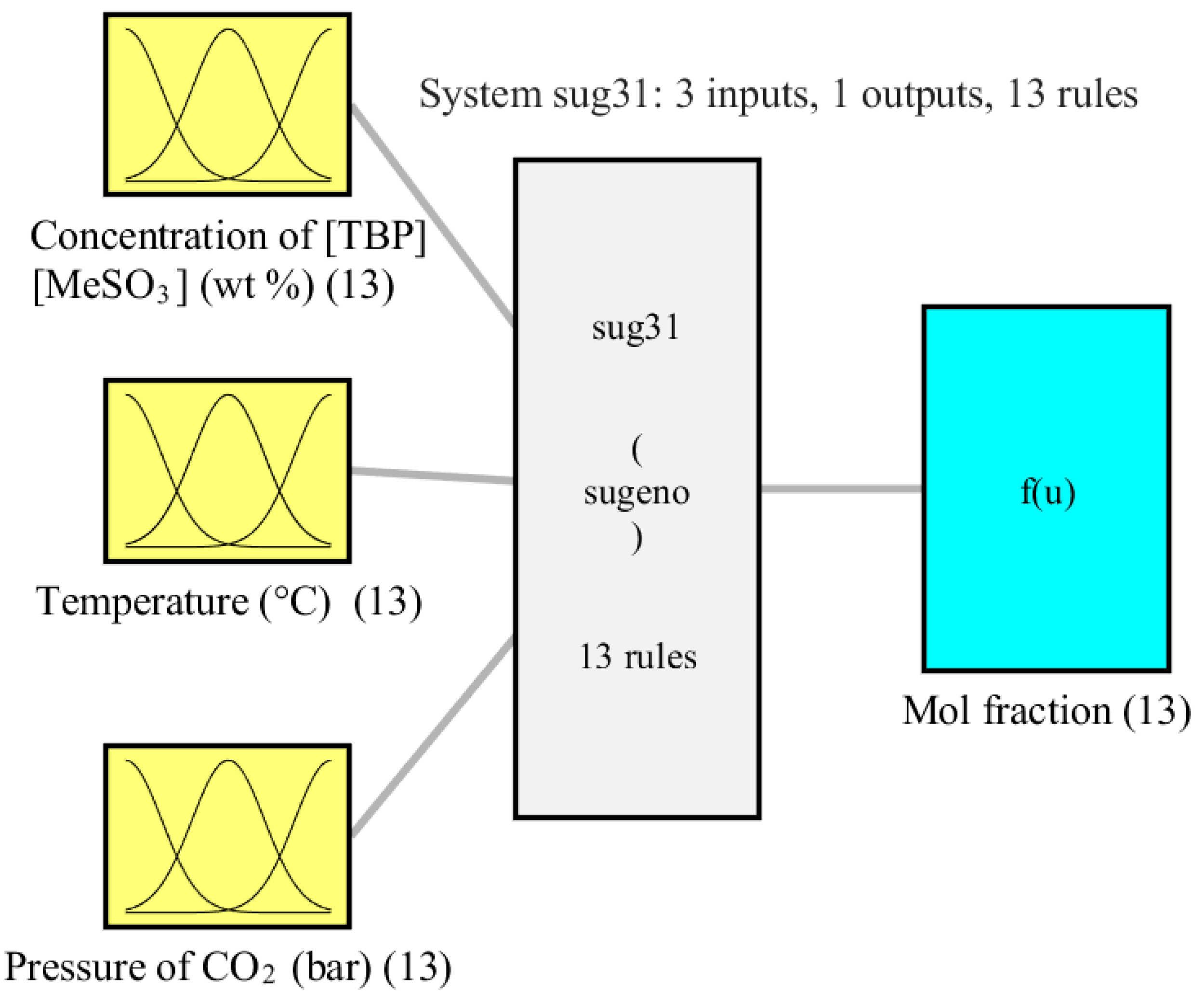

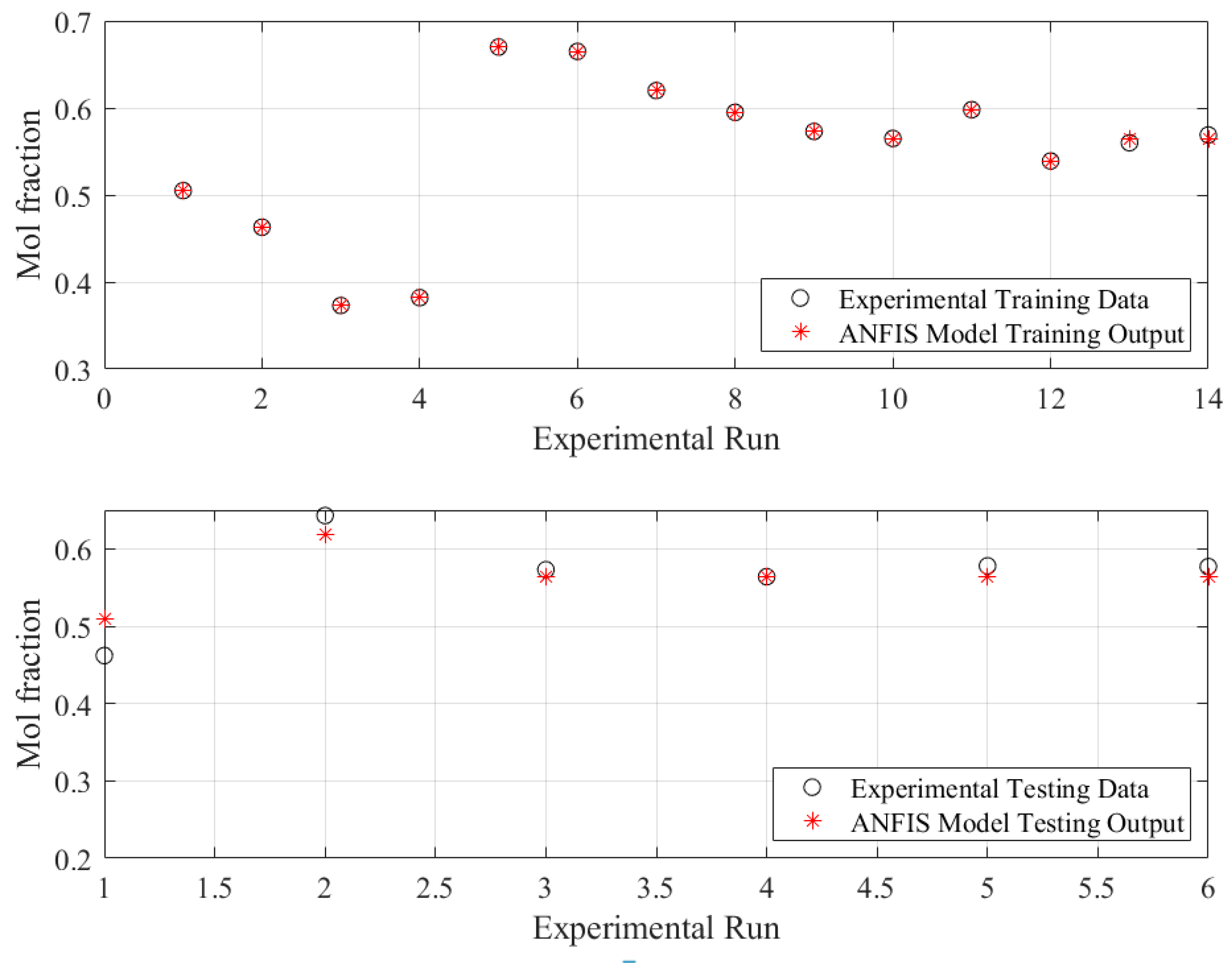
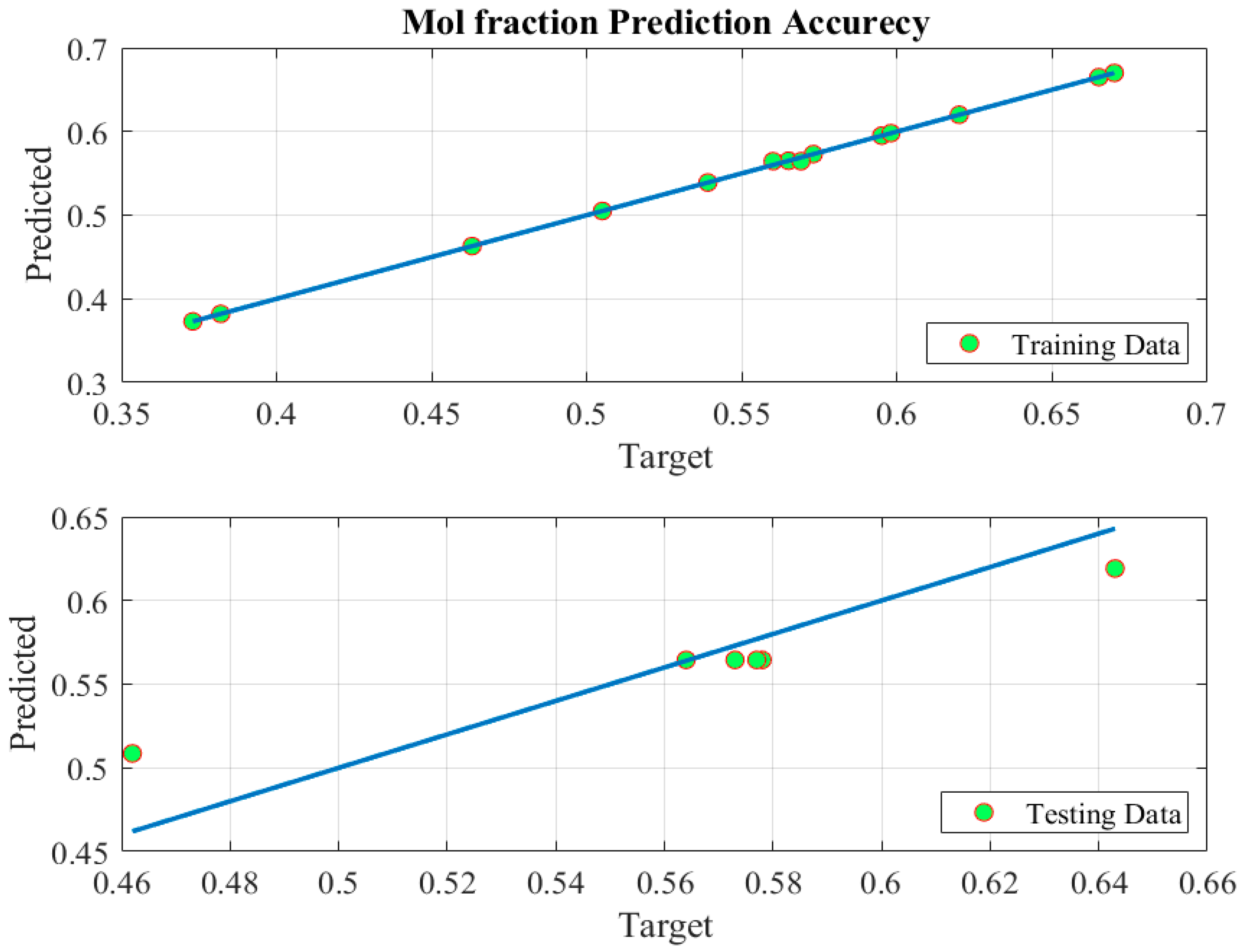


| Statistical Parameters of Different Phases | MSE | RMSE | Coefficient of Determination (R2) |
|---|---|---|---|
| Train | 2.8929 × 10−06 | 0.0017 | 0.9996 |
| Test | 0.0005 | 0.0229 | 0.9632 |
| All | 0.0002 | 0.0126 | 0.9758 |
| Strategy | Concentration of [TBP][MeSO3] (wt%) | Temperature (°C) | Pressure of CO2 (bar) | Mol Fraction |
|---|---|---|---|---|
| Experimental [28] | 2 | 30 | 30 | 0.67 |
| RSM [28] | 2.093 | 30.36 | 29.89 | 0.67 |
| IGWO and ANFIS | 3.0933 | 40.50 | 30 | 0.7674 |
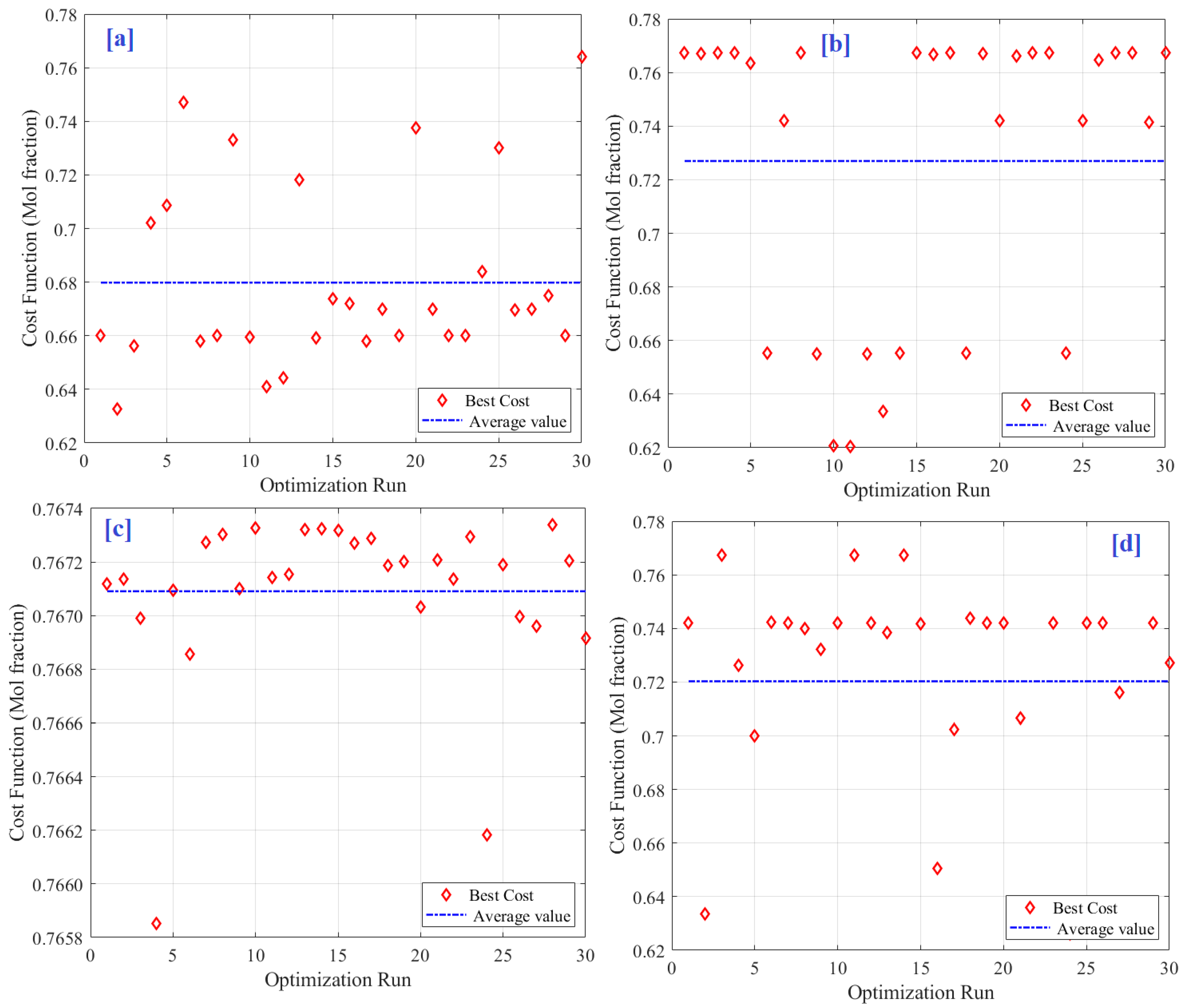
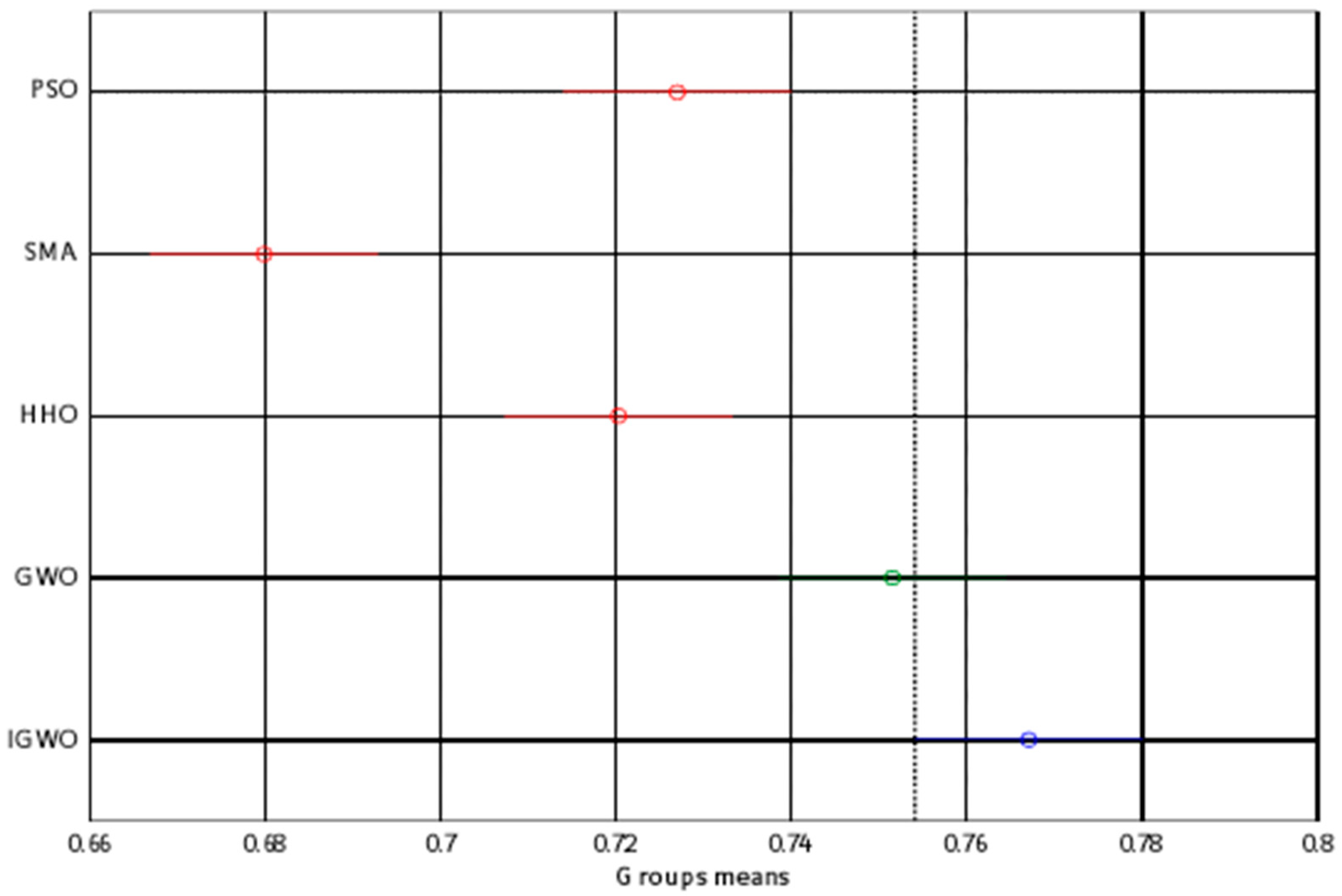
| PSO | SMA | HHO | GWO | IGWO | |
|---|---|---|---|---|---|
| Best value | 0.76739 | 0.76407 | 0.76739 | 0.76739 | 0.76734 |
| Worst value | 0.62047 | 0.63253 | 0.62624 | 0.65529 | 0.76585 |
| Average value | 0.72698 | 0.67981 | 0.72027 | 0.75151 | 0.76709 |
| STD | 0.05486 | 0.03334 | 0.04182 | 0.02738 | 0.00032 |
| Run | PSO | SMA | HHO | GWO | IGWO | Run | PSO | SMA | HHO | GWO | IGWO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.76738 | 0.66 | 0.74206 | 0.74206 | 0.76712 | 16 | 0.76671 | 0.67196 | 0.65048 | 0.74201 | 0.76727 |
| 2 | 0.76713 | 0.63253 | 0.63354 | 0.76735 | 0.76714 | 17 | 0.76738 | 0.65812 | 0.7024 | 0.76734 | 0.76729 |
| 3 | 0.76737 | 0.65613 | 0.76723 | 0.76566 | 0.76699 | 18 | 0.6552 | 0.67 | 0.74365 | 0.76729 | 0.76719 |
| 4 | 0.76739 | 0.70211 | 0.72624 | 0.75692 | 0.76585 | 19 | 0.76707 | 0.66005 | 0.74206 | 0.7636 | 0.7672 |
| 5 | 0.76337 | 0.70853 | 0.69992 | 0.76734 | 0.76709 | 20 | 0.74204 | 0.73759 | 0.74206 | 0.76728 | 0.76703 |
| 6 | 0.65522 | 0.74711 | 0.74228 | 0.74046 | 0.76686 | 21 | 0.76626 | 0.67 | 0.70643 | 0.76734 | 0.76721 |
| 7 | 0.74206 | 0.65807 | 0.74203 | 0.74202 | 0.76727 | 22 | 0.76738 | 0.66017 | 0.62842 | 0.76717 | 0.76714 |
| 8 | 0.76738 | 0.66002 | 0.73988 | 0.76725 | 0.7673 | 23 | 0.76732 | 0.66005 | 0.74206 | 0.76729 | 0.76729 |
| 9 | 0.65493 | 0.73302 | 0.7321 | 0.65952 | 0.7671 | 24 | 0.65524 | 0.68395 | 0.62624 | 0.74205 | 0.76618 |
| 10 | 0.62065 | 0.65942 | 0.74206 | 0.76303 | 0.76733 | 25 | 0.74205 | 0.73022 | 0.74206 | 0.76739 | 0.76719 |
| 11 | 0.62047 | 0.64095 | 0.76736 | 0.74202 | 0.76714 | 26 | 0.76475 | 0.66969 | 0.74203 | 0.76588 | 0.767 |
| 12 | 0.65511 | 0.64441 | 0.74206 | 0.76185 | 0.76715 | 27 | 0.76737 | 0.67 | 0.71607 | 0.74772 | 0.76696 |
| 13 | 0.63345 | 0.71819 | 0.7384 | 0.76733 | 0.76732 | 28 | 0.76733 | 0.67497 | 0.6306 | 0.76734 | 0.76734 |
| 14 | 0.65523 | 0.65924 | 0.76739 | 0.74146 | 0.76732 | 29 | 0.74145 | 0.66006 | 0.74206 | 0.65529 | 0.7672 |
| 15 | 0.76737 | 0.67367 | 0.74175 | 0.74167 | 0.76732 | 30 | 0.7673 | 0.76407 | 0.72712 | 0.76449 | 0.76692 |
| Source | Sum of Squares (SS) | Degrees of Freedom (df) | (Mean Squared) MS | F | p-Value > F |
|---|---|---|---|---|---|
| Columns | 0.13373 | 4 | 0.03343 | 24.41 | 1.8543 × 10−15 |
| Error | 0.19856 | 145 | 0.00173 | - | - |
| Total | 0.33232 | 194 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassef, A.M. Improving CO2 Absorption Using Artificial Intelligence and Modern Optimization for a Sustainable Environment. Sustainability 2023, 15, 9512. https://doi.org/10.3390/su15129512
Nassef AM. Improving CO2 Absorption Using Artificial Intelligence and Modern Optimization for a Sustainable Environment. Sustainability. 2023; 15(12):9512. https://doi.org/10.3390/su15129512
Chicago/Turabian StyleNassef, Ahmed M. 2023. "Improving CO2 Absorption Using Artificial Intelligence and Modern Optimization for a Sustainable Environment" Sustainability 15, no. 12: 9512. https://doi.org/10.3390/su15129512
APA StyleNassef, A. M. (2023). Improving CO2 Absorption Using Artificial Intelligence and Modern Optimization for a Sustainable Environment. Sustainability, 15(12), 9512. https://doi.org/10.3390/su15129512






