Progress in the Preparation of Calcium Carbonate by Indirect Mineralization of Industrial By-Product Gypsum
Abstract
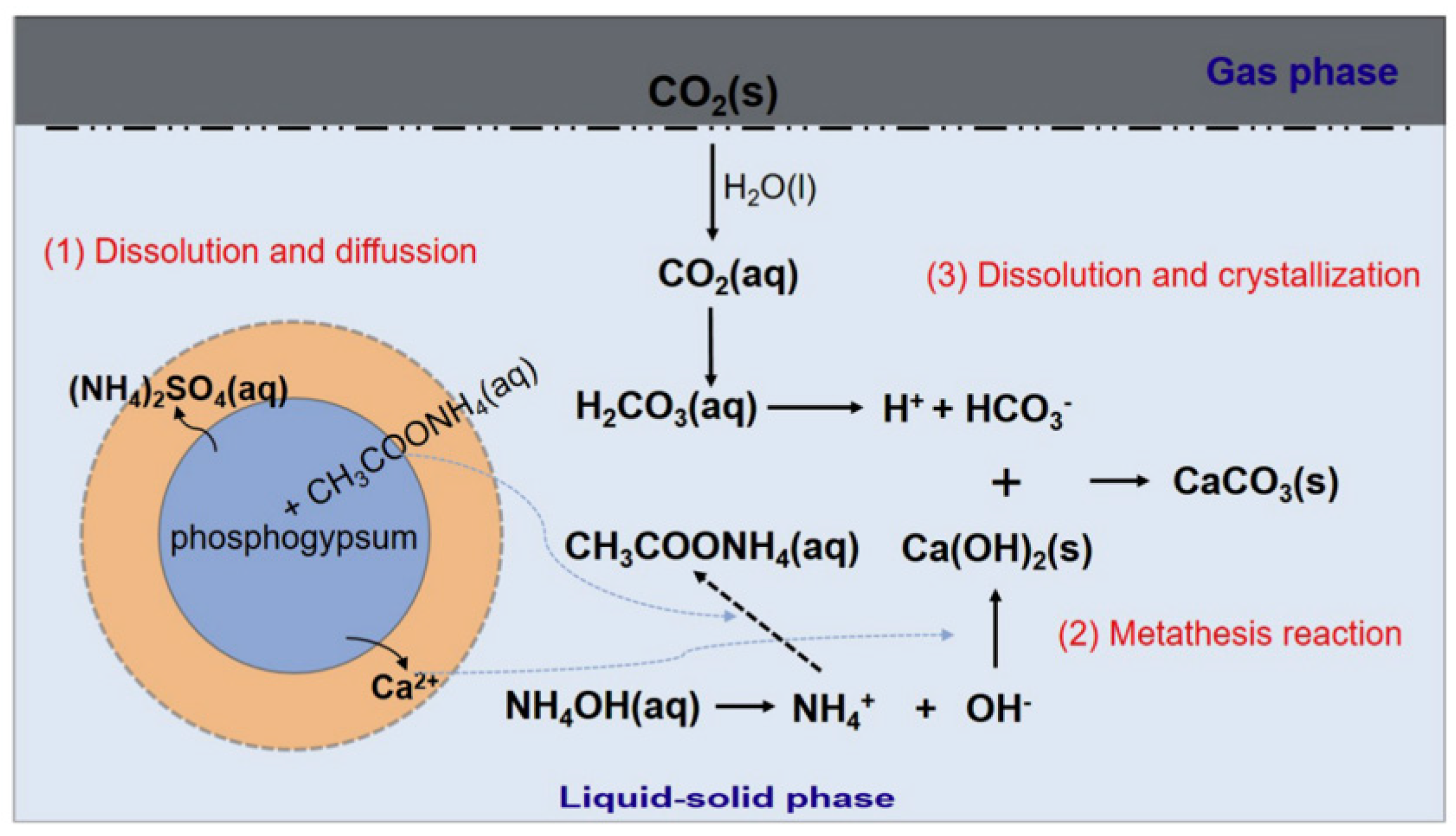
:1. Introduction
2. Chemical Composition of Industrial By-Product Gypsum and Principles of Indirect Mineralization
| FGDG [40] | FGDG [41] | FGDG [42] | PG [43] | PG [36] | PG [44] | RG [45] | RG [29] | RG [46] | |
|---|---|---|---|---|---|---|---|---|---|
| Origin | Spain | Turkey | China | Lithuania | Kazakhstan | Spain | Malaysia | Malaysia | Italy |
| wt.%(CaO) | 42.20 | 32.50 | 32.25 | 38.60 | 28.54 | 32.00 | 32.20 | 33.12 | 29.30 |
| wt.% (SO3) | 54.30 | 46.51 | 42.58 | 53.48 | 29.97 | 46.00 | 31.60 | 29.93 | 38.45 |
| wt.% (SiO2) | <0.1 | - | 0.78 | 0.37 | 15.40 | 2.52 | 1.90 | - | 2.31 |
| wt.% (Na2O) | - | - | - | - | 4.44 | 0.01 | - | - | 0.74 |
| wt.% (Al2O3) | 0.60 | 0.56 | 0.12 | 0.13 | 1.13 | 0.20 | 0.70 | - | 1.25 |
| wt.% (Fe2O3) | 0.30 | 0.43 | 0.32 | 0.03 | 0.85 | - | 28.99 | 29.23 | 3.97 |
| wt.% (MgO) | 0.17 | - | 0.52 | 0.04 | 0.66 | - | - | - | 2.46 |
| wt.% (P2O5) | - | - | - | 0.82 | 2.52 | 0.65 | - | - | - |
| wt.% (F) | - | - | - | 0.14 | 7.90 | - | - | - | 0.03 |
| wt.% (TiO2) | - | 0.02 | - | - | 0.16 | - | 5.64 | 6.21 | 1.29 |
| wt.% (MnO) | - | 0.03 | - | - | 0.15 | - | 0.41 | 0.58 | - |
| wt.% (Eu2O3) | - | - | - | - | - | - | 0.26 | 0.34 | - |
| wt.% (ZnO) | - | - | - | - | - | - | 0.24 | 0.19 | - |
| wt.% (V2O5) | - | - | - | - | - | - | 0.22 | 0.17 | 0.18 |
| wt.% (Crystalline water) | 23.00 | 19.70 | 18.34 | 6.40 | - | 18.40 | - | - | 19.05 |
3. Main Technical Routes and Research Progress of Indirect Mineralization of By-Product Gypsum
3.1. Main Technical Routes for Indirect Mineralization of By-Product Gypsum
3.1.1. Alkali Leaching
3.1.2. pH Swing
3.1.3. Salt Leaching
3.1.4. Complexation
| Craft Classification | Ingredients | Leaching | Leaching Rate (%) | Mineralization | Mineralization Efficiency (%) | Calcium Carbonate | Purity (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaching Agent | Conditions | Additives | Conditions | Crystalline | Appearance | ||||||
| Alkali leaching | PG | NaOH | RT; ATP; PG/H2O mass ratio = 4; OH−/Ca2+ molar ratio = 2;3 h | 97 | - | RT; ATP 20 cm3/s; 20; 15 min; Aging 24 h | 95 | Calcite | Fine-grained | >90 | [88] |
| PG | NaOH | OH−/Ca molar ratio =2; RT; 3 h | - | - | RT; ATP 20 cm3/s; 20; 15 min | 80 | Calcite | - | 90 | [51] | |
| FGDG | NaOH | S/L = 1:13, 300 rpm; 20 °C; 1 h | - | - | RT; 1 L/min; 32.32 m/s Venturi reactor | - | Calcite | Rhombus | 99.63 | [60] | |
| PG | NaOH | 3 h, 25.0 mL Liquid waste; 12.5 g PG, pH 12.0 | 90 | - | RT; ATP 20 cm3/s; 20; 90 min; pH = 6.7 | 80 | Calcite | - | - | [58] | |
| pH swing | RG | H2SO4 NH3·H2O | 25 °C; ATP; 600 rpm; 2.5 h; NH3·H2O regulating pH | 5200 ppm | 5 wt% MEA | RT; ATP 20 vol% CO2; 1.4 M; 4 h; 600 rpm | 98.9 | Calcite | Rhombus | - | [29] |
| RG | H2SO4 NH3·H2O | 25 °C; ATP; 600 rpm; 2.5 h; NH3·H2O regulating pH | - | - | 25–150 °C; 1–30% Split pressure CO2; 400 rpm; 3 h | 98.8 | Calcite | Rhombus | - | [68] | |
| RG | H2SO4 NH3·H2O | 25 °C; 10 bar; 1000 rpm; 0.5 h; NH3·H2O regulating pH | 100 | - | 25 °C 8 bar; 50 mL; 400 rpm; 30 min | 100 | Calcite | Cube | 98 | [70] | |
| RG | H2SO4 NH3·H2O | 2 M H2SO4; 70 °C; 1000 rpm; 1 h; NH3·H2O regulating pH | 100 | NH4HCO3 | 30 min; 75 °C; ATP | 98 | Calcite | Rhombus | 92.57 | [71] | |
| RG | H2SO4 NH3·H2O | 70 °C; 1.5 M H2SO4; 2 h; 1 bar; NH3·H2O regulating pH | 95.8 | - | RT; ATP; 8 mL/min CO2; 50 min | 93 | Calcite | - | - | [59] | |
| PG | NaOH, HCl | 2 mol/L NaOH; HCl 3 mol/L; 60 °C;10 m L/g; 1 h | 99.6 | NH3·H2O | 30 °C; ATP; 20 mL; 0.1 L/min; 1h | 98.57 | Spherulite | Sphere | 99.4 | [72] | |
| Salting leaching | PG | NaCl | 30 °C, ATP; L/S = 50; 60 min | 49.42 | NH3·H2O | 30 °C; ATP; CO2: 80 mL/min; 60 min | 96.31 | Calcite | Rhombus | - | [54] |
| PG | NH4Cl | 2 mol/L NH4Cl; 10:1 mL/g; 60 min; 60 °C | 18.7 g/L | NH3·H2O | 30 °C; ATP; CO2: 50 mL/min; 1h | 98.22 | Calcite | Rhombus | 99.8 | [76] | |
| PG | CH3COONH4 | 6 mol/L; 80 °C; 10 mL/g; 60 min | 98.1 | NH3·H2O | 30 °C; ATP; 100 mL/min; 90 min | 98.32 | Spherulite | Sphere | 99.6 | [75] | |
| FGDG | NH4Cl | 4 mol/L NH4Cl; 80:1 mL/g; 60 min; 50 °C | 95.6 | NH3·H2O | 40 °C; ATP; CO2:80 mL/min; 1 h | 98.54 | Calcite | Rhombus | - | [77] | |
| Complexation | FGDG | Asp | 30 °C; Asp/BG = 2.5; ATP; L/S = 30; 60 min | 48.9 | - | 30 °C; ATP; 0.5 L/min; 50 min | 46.5 | Spherulite | Sphere | - | [55] |
| PG | Sodium gluconate | SG (nSG/nPGCa2+ = 1:2.5); 100 mL H2O; 9.88 g PG; 20 min | 84 | sodium triphosphate | 15 °C; ATP; (nNaOH/nadditive/nCa) = 2:0.3:1; 300 rpm; 0.4 L/min; Aging 45 min | 96 | Spherulite | Sphere | 96.87 | [53] | |
3.2. Application of Other Technologies in the Indirect Mineralization of Industrial By-Product Gypsum
4. Conclusions and Prospects
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Li, C.; Liang, C.; Chen, Z.; Di, Y.; Zheng, S.; Wei, S.; Sun, Z. Surface modification of calcium carbonate: A review of theories, methods and applications. J. Cent. South Univ. 2021, 28, 2589–2611. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, G.; Lyu, J.; Cao, Y.; Xu, W.; Ma, K.; Song, L.; Yue, H.; Liang, B. CO2 mineralization of carbide slag for the production of light calcium carbonates. Chin. J. Chem. Eng. 2022, 43, 86–98. [Google Scholar] [CrossRef]
- Luo, J.; Kong, F.; Ma, X. Role of aspartic acid in the synthesis of spherical vaterite by the Ca(OH)2–CO2 reaction. Cryst. Growth Des. 2016, 16, 728–736. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Svenskaya, Y.I.; Fattah, H.; Inozemtseva, O.A.; Ivanova, A.G.; Shtykov, S.N.; Gorin, D.A.; Parakhonskiy, B.V. Key parameters for size- and shape-controlled synthesis of vaterite particles. Cryst. Growth Des. 2018, 18, 331–337. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Yan, Z.L.; Jiao, M.R.; Xu, D.D.; Jiang, J.X. A novel method for preparing calcium carbonate particles: Thermal decomposition from calcium hydrogen carbonate solution. Key Eng. Mater. 2016, 697, 113–118. [Google Scholar]
- Yoo, Y.; Kim, I.; Lee, D.; Yong Choi, W.; Choi, J.; Jang, K.; Park, J.; Kang, D. Review of contemporary research on inorganic CO2 utilization via CO2 conversion into metal carbonate-based materials. J. Ind. Eng. Chem. 2022, 116, 60–74. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, K.H.; Ha, H.Y.; Jung, K.-D.; Lee, J.H. Techno-economic and environmental evaluation of nano calcium carbonate production utilizing the steel slag. J. CO2 Util. 2020, 37, 113–121. [Google Scholar] [CrossRef]
- Luo, X.; Wei, C.; Li, X.; Deng, Z.; Li, M.; Fan, G. A green approach to prepare polymorph CaCO3 for clean utilization of salt gypsum residue and CO2 mineralization. Fuel 2023, 333, 126305. [Google Scholar] [CrossRef]
- Altiner, M.; Top, S.; Kaymakoğlu, B. Ultrasonic-assisted production of precipitated calcium carbonate particles from desulfurization gypsum. Ultrason Sonochem 2021, 72, 105421. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Cheng, H.; Zhang, Z.; Cheng, F. A review of carbon dioxide sequestration by mineral Carbonation of Industrial Byproduct Gypsum. J. Clean. Prod. 2021, 302, 126930. [Google Scholar] [CrossRef]
- Owais, M.; Järvinen, M.; Taskinen, P.; Said, A. Experimental study on the extraction of calcium, magnesium, vanadium and silicon from steelmaking slags for improved mineral carbonation of CO2. J. CO2 Util. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Feng, Y.; Li, H.; Xu, C. Resource utilization of strongly acidic wastewater and red gypsum by a harmless self-treatment process. Process Saf. Environ. 2023, 172, 594–603. [Google Scholar] [CrossRef]
- Guan, Q.; Sui, Y.; Yu, W.; Bu, Y.; Zeng, C.; Liu, C.; Zhang, Z.; Gao, Z.; Chi, R. Deep removal of phosphorus and synchronous preparation of high-strength gypsum from phosphogypsum by crystal modification in NaCl-HCl solutions. Sep. Purif. Technol. 2022, 298, 121592. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, H.; Chen, Q.; Huang, Z.; Zhang, C.; Yang, T. Preparation of waterproof block by silicate clinker modified FGD gypsum. Constr. Build. Mater. 2019, 214, 318–325. [Google Scholar] [CrossRef]
- Brooks, M.W.; Lynn, S. Recovery of calcium carbonate and hydrogen sulfide from waste calcium sulfide. Ind. Eng. Chem. Res. 1997, 36, 4236–4242. [Google Scholar] [CrossRef]
- de Beer, M.; Doucet, F.J.; Maree, J.P.; Liebenberg, L. Synthesis of high-purity precipitated calcium carbonate during the process of recovery of elemental sulphur from gypsum waste. Waste Manag. 2015, 46, 619–627. [Google Scholar] [CrossRef]
- Song, W.; Zhou, J.; Wang, B.; Li, S.; Han, J. New insight into investigation of reduction of desulfurization ash by pyrite for clean generation SO2. J. Clean. Prod. 2020, 253, 120026. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jin, B.; Liu, X.; Yang, L. Crystal structure formation of hemihydrate calcium sulfate whiskers (HH-CSWs) prepared using FGD gypsum. Polyhedron 2019, 173, 114140. [Google Scholar] [CrossRef]
- Liao, R.; Yu, H.; Lin, H.; Yang, P. A Quantitative study on three-dimensional pore parameters and physical properties of sodic soils restored by FGD gypsum and leaching water. J. Environ. Manag. 2019, 248, 109303. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, S.; Zhang, Y.; Fang, Z.Z.; Zhang, M.; Sun, P.; Li, Q.; Zhang, Y.; Li, P.; Jin, W. Potentially more ecofriendly chemical pathway for production of high-purity TiO2 from titanium slag. Acs Sustain. Chem. Eng. 2019, 7, 4821–4830. [Google Scholar] [CrossRef]
- Pyagai, I.; Zubkova, O.; Babykin, R.; Toropchina, M.; Fediuk, R. Influence of impurities on the process of obtaining calcium carbonate during the processing of phosphogypsum. Materials 2022, 15, 4335. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Ma, K.; Liu, C.; Tang, S.; Yan, Z.; Yue, H.; Liang, B. an integrated absorption–mineralization process for CO2 capture and sequestration: Reaction mechanism, recycling stability, and energy evaluation. ACS Sustain. Chem. Eng. 2021, 9, 16577–16587. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; le Saché, E.; Hurd Price, C.A.; Reina, T.R.; Navarrete, B. From biogas upgrading to CO2 utilization and waste recycling: A novel circular economy approach. J. CO2 Util. 2021, 47, 101496. [Google Scholar] [CrossRef]
- Rahmani, O. An experimental study of accelerated mineral carbonation of industrial waste red gypsum for CO2 sequestration. J. CO2 Util. 2020, 35, 265–271. [Google Scholar] [CrossRef]
- Xie, H.; Yue, H.; Zhu, J.; Liang, B.; Li, C.; Wang, Y.; Xie, L.; Zhou, X. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals. Engineering 2015, 1, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Liao, W.; Zhang, G. Emerging CO2-mineralization technologies for co-utilization of industrial solid waste and carbon resources in China. Minerals 2021, 11, 274. [Google Scholar] [CrossRef]
- Li, Y.; Ni, W.; Duan, P.; Zhang, S.; Wang, J. Experimental study and mechanism analysis of preparation of alpha-calcium sulfate hemihydrate from FGD gypsum with dynamic method. Materials 2022, 15, 3382. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Ma, B.; Wang, X.; Zhang, W.; Chen, Y.; Wang, C. Nitric acid pressure leaching of limonitic laterite ores: Regeneration of HNO3 and Simultaneous Synthesis of Fibrous CaSO4·2H2O by-products. J. Cent. South Univ. 2020, 27, 3249–3258. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Ding, H.; Qiu, J.; Guo, Z. Recycling flue gas desulfurisation gypsum and phosphogypsum for cemented paste backfill and its acid resistance. Constr. Build Mater. 2021, 275, 122170. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Du, Z.; Cheng, H.; Cheng, F. Effect of impure components in flue gas desulfurization (FGD) gypsum on the generation of polymorph CaCO3 during carbonation reaction. J. Hazard. Mater. 2019, 369, 236–243. [Google Scholar] [CrossRef]
- Tokpayev, R.; Khavaza, T.; Ibraimov, Z.; Kishibayev, K.; Atchabarova, A.; Abdimomyn, S.; Abduakhytova, D.; Nauryzbayev, M. Phosphogypsum conversion under conditions of SC-CO2. J. CO2 Util. 2022, 63, 102120. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, J.; Liu, Q.; Hu, Q.; Sun, X.; Li, J.; Liu, W.; Lin, Z. Efficient removal of iron from red gypsum via synergistic regulation of gypsum phase transformation and iron speciation. Sci. Total Environ. 2021, 791, 148319. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, C.; Qu, G.; Liu, S.; Ren, Y.; Chen, B.; Li, J.; Liu, L. A critical review of the typical by-product clean ecology links in the Chinese phosphorus chemical industry in China: Production technologies, Fates and Future Directions. J. Environ. Chem. Eng. 2022, 10, 106685. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A review of the production cycle of titanium dioxide pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Leiva, C.; García Arenas, C.; Vilches, L.F.; Vale, J.; Gimenez, A.; Ballesteros, J.C.; Fernández-Pereira, C. Use of FGD gypsum in fire resistant panels. Waste Manag. 2010, 30, 1123–1129. [Google Scholar] [CrossRef]
- Altiner, M. Effect of alkali types on the production of calcium carbonate particles from gypsum waste for fixation of CO2 by mineral carbonation. Int. J. Coal Prep. Util. 2019, 39, 113–131. [Google Scholar] [CrossRef]
- Ma, Y.; Nie, Q.; Xiao, R.; Hu, W.; Han, B.; Polaczyk, P.A.; Huang, B. Experimental investigation of utilizing waste flue gas desulfurized gypsum as backfill materials. Constr. Build Mater. 2020, 245, 118393. [Google Scholar] [CrossRef]
- Nizevičienė, D.; Vaičiukynienė, D.; Michalik, B.; Bonczyk, M.; Vaitkevičius, V.; Jusas, V. The treatment of phosphogypsum with zeolite to use it in binding material. Constr. Build Mater. 2018, 180, 134–142. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I.; Borrero-López, A.M.; Flores-Alés, V.; Alejandre, F.J.; Franco, J.M.; Santos, A.; Esquivias, L. Characterization and analysis of the carbonation process of a lime mortar obtained from phosphogypsum waste. Int. J. Environ. Res. Public Health 2021, 18, 6664. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, O. Siderite precipitation using by-product red gypsum for CO2 sequestration. J. CO2 Util. 2018, 24, 321–327. [Google Scholar] [CrossRef]
- Marian, N.M.; Perotti, M.; Indelicato, C.; Magrini, C.; Giorgetti, G.; Capitani, G.; Viti, C. From high-volume industrial waste to new ceramic material: The case of red gypsum muds in the TiO2 industry. Ceram. Int. 2023, 49, 15034–15043. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, H.; Feng, X.; Chen, M.; Shi, Q.; Chen, M.; Zhang, Q. Efficient removal of lead impurity for the purification and recycling of nickel from secondary sources based on ball-milling activated CaCO3. J. Environ. Chem. Eng. 2021, 9, 106737. [Google Scholar] [CrossRef]
- Lian, F.; Gao, S.; Fu, Q.; Wu, Y.; Wang, J.; Huang, Q.; Hu, S. A comprehensive study of phosphorus removal and recovery with a Fe-loaded sulfoaluminate cement (FSC) adsorbent. J. Water Process Eng. 2021, 39, 101744. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Nilsen, D.N.; Gerdemann, S.J.; Rush, G.E.; Penner, L.R.; Walters, R.P.; Turner, P.C. Continuing Studies on Direct Aqueous Mineral Carbonation of CO2 Sequestration; USDOE Office of Fossil Energy (FE) (US): Washington, DC, USA, 2002.
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of carbon dioxide: A literature review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Cárdenas-Escudero, C.; Morales-Flórez, V.; Pérez-López, R.; Santos, A.; Esquivias, L. Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J. Hazard. Mater. 2011, 196, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Baojun, Y.; Mengmeng, Y.; Bainian, W.; Xiaoyu, F.; Qiang, W. A new route to synthesize calcium carbonate microspheres from phosphogypsum. Mater. Res. Express 2019, 6, 045042. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T.; Ma, G. Indirect mineral carbonation of phosphogypsum for CO2 sequestration. Energy 2020, 206, 118148. [Google Scholar] [CrossRef]
- Gong, Y.; Zhu, X.; Yang, Z.; Zhang, X.; Li, C. Indirect aqueous carbonation of CaSO4·2H2O with aspartic acid as a recyclable additive. RSC Adv. 2022, 12, 26556–26564. [Google Scholar] [CrossRef] [PubMed]
- Blencoe, J.G.; Palmer, D.A.; Anovitz, L.M.; Beard, J.S. Carbonation of metal silicates for long-term CO2 sequestration 2020. Available online: https://www.freepatentsonline.com/10632418.html (accessed on 12 April 2023).
- Pérez-Moreno, S.M.; Gázquez, M.J.; Bolívar, J.P. CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments. Chem. Eng. J. 2015, 262, 737–746. [Google Scholar] [CrossRef]
- Romero-Hermida, I.; Santos, A.; Pérez-López, R.; García-Tenorio, R.; Esquivias, L.; Morales-Flórez, V. New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J. CO2 Util. 2017, 18, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of industrial waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Altiner, M.; Top, S.; Kaymakoğlu, B.; Seçkin, İ.Y.; Vapur, H. Production of precipitated calcium carbonate particles from gypsum waste using venturi tubes as a carbonation zone. J. CO2 Util. 2019, 29, 117–125. [Google Scholar] [CrossRef]
- Xue, S.; Li, M.; Jiang, J.; Millar, G.J.; Li, C.; Kong, X. Phosphogypsum stabilization of bauxite residue: Conversion of its alkali characteristics. J. Environ. Sci. 2019, 77, 1–10. [Google Scholar] [CrossRef]
- Biyoune, M.G.; Bouargane, B.; Idboufrade, A.; Marrouche, A.; Atbir, A.; Boukbir, L.; Mançour-billah, S. New procedure for water-salinity reduction using phosphogypsum waste and carbon dioxide resulting in useful compounds formation. Nanotechnol. Environ. Eng. 2021, 6, 33. [Google Scholar] [CrossRef]
- Kelly, K.E.; Silcox, G.D.; Sarofim, A.F.; Pershing, D.W. An evaluation of ex situ, industrial-scale, aqueous CO2 mineralization. Int. J. Greenh Gas. Con. 2011, 5, 1587–1595. [Google Scholar] [CrossRef]
- Kim, G.; Kim, S.; Kim, M.-J. Effect of sucrose on CO2 storage, vaterite content, and caco3 particle size in indirect carbonation using seawater. J. CO2 Util. 2022, 57, 101894. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Rim, G.; Roy, N.; Zhao, D.; Kawashima, S.; Stallworth, P.; Greenbaum, S.G.; Park, A.-H.A. CO2 utilization in built environment via the PCO2 swing carbonation of alkali solid wastes with different mineralogy. Faraday Discuss. 2021, 230, 187–212. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Jadhav, R.; Fan, L.-S. CO2 mineral sequestration: Chemically enhanced aqueous carbonation of serpentine. Can. J. Chem. Eng. 2003, 81, 885–890. [Google Scholar] [CrossRef]
- Rahmani, O.; Tyrer, M.; Junin, R. Calcite precipitation from by-product red gypsum in aqueous carbonation process. RSC Adv. 2014, 4, 45548–45557. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Mohammadian, E.; Hamidi, H.; Daud, A.R.M.; Manan, M. Extraction of calcium from red gypsum for calcium carbonate production. Fuel Process. Technol. 2015, 130, 12–19. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Daud, A.R.M. Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Azdarpour, A.; Karaei, M.A.; Hamidi, H.; Mohammadian, E.; Barati, M.; Honarvar, B. CO2 sequestration using red gypsum via pH-swing process: Effect of carbonation temperature and NH4HCO3 on the process efficiency. Int. J. Miner. Process. 2017, 169, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Chen, Q.; Sun, H.; Peng, T. Modified phosphogypsum sequestrating CO2 and characteristics of the carbonation product. Energy 2019, 182, 224–235. [Google Scholar] [CrossRef]
- Azdarpour, A.; Afkhami Karaei, M.; Hamidi, H.; Mohammadian, E.; Honarvar, B. CO2 sequestration through direct aqueous mineral carbonation of red gypsum. Petroleum 2018, 4, 398–407. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Manan, M.; Hamidi, H.; Mohammadian, E. Direct carbonation of red gypsum to produce solid carbonates. Fuel Process. Technol. 2014, 126, 429–434. [Google Scholar] [CrossRef]
- Wenjin, D.; Chen, Q.; Hongjuan, S.; Tongjiang, P.; Peng, T. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T.; Ma, G. Utilization of phosphogypsum to prepare high-purity CaCO3 in the NH4Cl–NH4OH–CO2 system. Acs Sustain. Chem. Eng. 2020, 8, 11649–11657. [Google Scholar] [CrossRef]
- Ding, W.; Qiao, J.; Zeng, L.; Sun, H.; Peng, T. Desulfurization gypsum carbonation for CO2 sequestration by using recyclable ammonium salt. Int. J. Greenh Gas Con. 2023, 123, 103843. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. The influence of chelating agents on the kinetics of calcite dissolution. J. Colloid Interface Sci. 1998, 204, 187–197. [Google Scholar] [CrossRef]
- Nowack, B. Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 2002, 36, 4009–4016. [Google Scholar] [CrossRef]
- Ghoorah, M.; Dlugogorski, B.Z.; Balucan, R.D.; Kennedy, E.M. Selection of acid for weak acid processing of wollastonite for mineralisation of CO2. Fuel 2014, 122, 277–286. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, J. Effects of Ca-ligand stability constant and chelating agent concentration on the CO2 storage using paper sludge ash and chelating agent. J. CO2 Util. 2020, 40, 101202. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, X.; Gao, S.; Wang, Y.; Yang, L.; Zhang, Q.; Chen, Y.; Wang, B.; Yang, B. Preparation of calcium carbonate microrods from the gypsum scale layer of evaporation equipment. RSC Adv. 2022, 12, 10584–10591. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Q.; Chi, W.; Cai, M.; Wang, R.; Fu, Z.; Xie, J.; Zou, Z. Multiple crystallization pathways of amorphous calcium carbonate in the presence of poly(aspartic acid) with a chain length of 30. CrystEngComm 2022, 24, 4809–4818. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Zhang, Z.; Pan, Z.; Cheng, H.; Cheng, F. Glycine-induced synthesis of vaterite by direct aqueous mineral carbonation of desulfurization gypsum. Environ. Chem. Lett. 2022, 20, 2261–2269. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Wei, Y.; Li, K.; Yu, H.; Wang, X.; Ji, L.; Yan, S. Glycine-mediated leaching-mineralization cycle for CO2 sequestration and CaCO3 production from coal fly ash: Dual functions of glycine as a proton donor and receptor. Chem. Eng. J. 2022, 440, 135900. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Feng, L.; He, Q.; Ji, L.; Yan, S. Insights into dual functions of amino acid salts as CO2 Carriers and CaCO3 regulators for integrated CO2 absorption and mineralisation. J. CO2 Util. 2021, 48, 101531. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Wang, Y.; Wang, Y.; Ji, L.; Yan, S. Regenerable glycine induces selective preparation of vaterite CaCO3 by calcium leaching and CO2 mineralization from coal fly ash. Chem. Eng. J. 2023, 459, 141536. [Google Scholar] [CrossRef]
- Contreras, M.; Pérez-López, R.; Gázquez, M.J.; Morales-Flórez, V.; Santos, A.; Esquivias, L.; Bolívar, J.P. Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO₂ sequestration. Waste Manag. 2015, 45, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- van Linden, N.; Bandinu, G.L.; Vermaas, D.A.; Spanjers, H.; van Lier, J.B. Bipolar membrane electrodialysis for energetically competitive ammonium removal and dissolved ammonia production. J. Clean. Prod. 2020, 259, 120788. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E.; Ojumu, T. Circular indirect carbonation of coal fly ash for carbon dioxide capture and utilization. J. Environ. Chem. Eng. 2022, 10, 108269. [Google Scholar] [CrossRef]
- Kuldeep; Badenhorst, W.D.; Kauranen, P.; Pajari, H.; Ruismäki, R.; Mannela, P.; Murtomäki, L. Bipolar membrane electrodialysis for sulfate recycling in the metallurgical industries. Membranes 2021, 11, 718. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Shen, J.; Van der Bruggen, B. Application of bipolar membrane electrodialysis in environmental protection and resource recovery: A review. Membranes 2022, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Donata Konopacka-Łyskawa; Barbara Kościelska; Marcin łapiński precipitation of spherical vaterite particles via carbonation route in the bubble column and the gas-lift reactor. Jom-Us 2019, 71, 1041–1048. [CrossRef] [Green Version]
- Xie, H.; Wang, J.; Hou, Z.; Wang, Y.; Liu, T.; Tang, L.; Jiang, W. CO2 sequestration through mineral carbonation of waste phosphogypsum using the technique of membrane electrolysis. Environ. Earth Sci 2016, 75, 1216. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Wang, H.; Li, C.; Gong, Y.; Wang, Y. Progress in the Preparation of Calcium Carbonate by Indirect Mineralization of Industrial By-Product Gypsum. Sustainability 2023, 15, 9629. https://doi.org/10.3390/su15129629
Wu B, Wang H, Li C, Gong Y, Wang Y. Progress in the Preparation of Calcium Carbonate by Indirect Mineralization of Industrial By-Product Gypsum. Sustainability. 2023; 15(12):9629. https://doi.org/10.3390/su15129629
Chicago/Turabian StyleWu, Baizhi, Haibin Wang, Chunlei Li, Yuan Gong, and Yi Wang. 2023. "Progress in the Preparation of Calcium Carbonate by Indirect Mineralization of Industrial By-Product Gypsum" Sustainability 15, no. 12: 9629. https://doi.org/10.3390/su15129629
APA StyleWu, B., Wang, H., Li, C., Gong, Y., & Wang, Y. (2023). Progress in the Preparation of Calcium Carbonate by Indirect Mineralization of Industrial By-Product Gypsum. Sustainability, 15(12), 9629. https://doi.org/10.3390/su15129629






