Minimising Coal Mining’s Impact on Biodiversity: Artificial Soils for Post-Mining Land Reclamation
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal By-Product and Waste Materials
2.2. Formulation of Soil Substitute Covers
2.3. Study Area
2.4. Analytical Procedures
2.5. Seed Mixture of Meadow Habitats
2.6. Assessment of Developed Vegetation and Inventory of Plant Species
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Soil Substitute Covers
3.2. Assessment of Developed Method for Reclamation of the Study Area
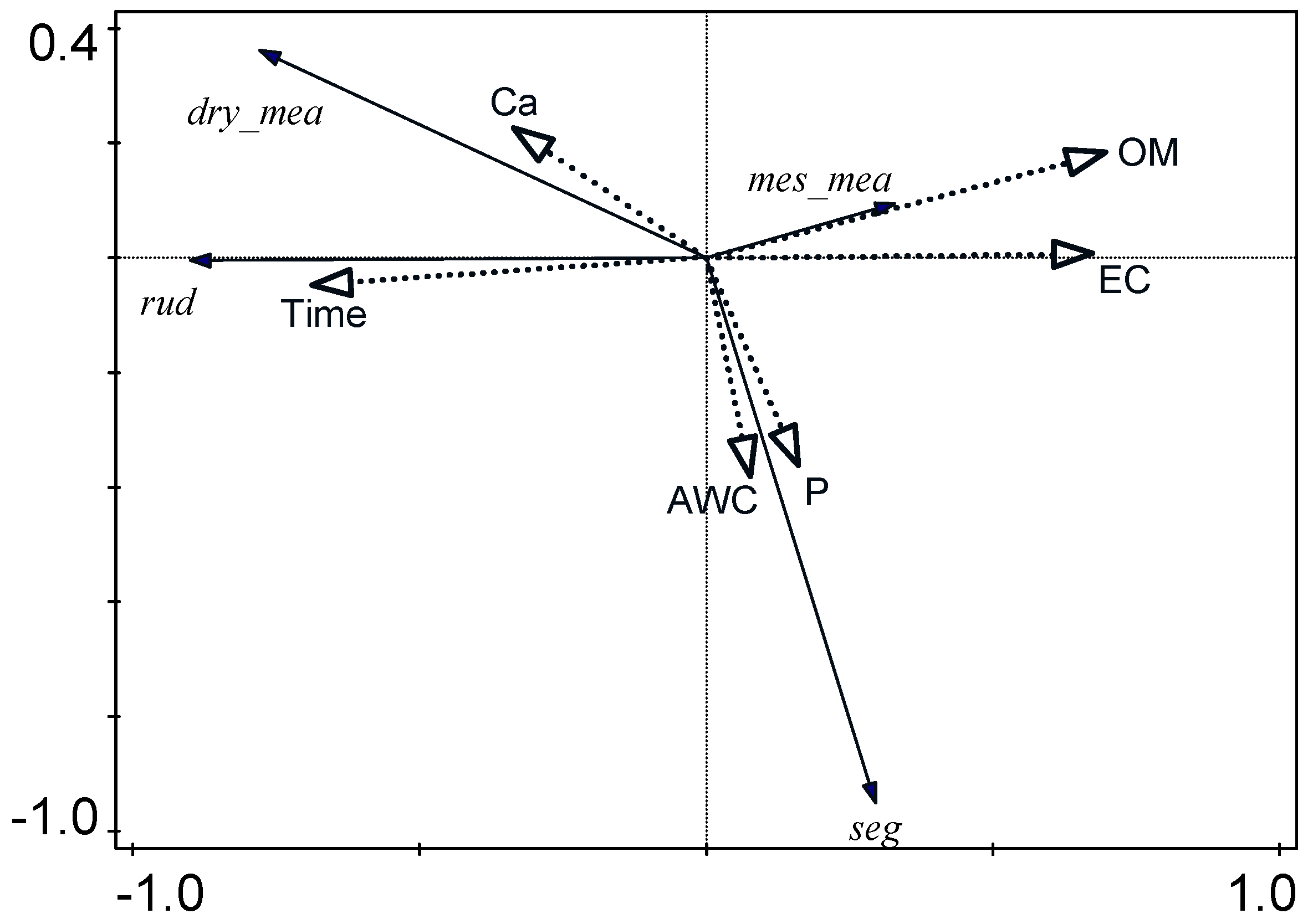
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Statistics—Eurostat. Energy, Transport and Environment Statistics; Eurostat: Luxembourg, 2020. [Google Scholar]
- Statistics of Poland Environment; Central Statistic Office in Poland: Warsaw, Poland, 2021. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2021,1,22.html (accessed on 6 June 2023).
- Klojzy-Kaczmarczyk, B.; Mazurek, J.; Staszczak, J. Analysis of the quality of waste from coal mining in relation to the requirements for inter mining waste. Miner. Resour. Manag. 2016, 95, 227–242. [Google Scholar]
- BRGM. Management of Mining, Quarrying and Ore-Processing Waste in the European Union: Orléans, France. 2021. Available online: https://ec.europa.eu/environment/pdf/waste/studies/mining/0204finalreportbrgm.pdf (accessed on 6 June 2023).
- Bauerek, A.; Diatta, J.; Pierzchała, Ł.; Więckol-Ryk, A.; Krzemień, A. Development of Soil Substitutes for the Sustainable Land Reclamation of Coal Mine-Affected Areas. Sustainability 2022, 14, 4604. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Plant Nutrient Functions and Deficiency and Toxicity Symptoms. Nutr. Manag. Module 2011, 9, 1–16. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Polish Scientific Publishers PWN: Warsaw, Poland, 1999. [Google Scholar]
- Hooda, P.S. Trace Elements in Soils; John Wiley and Sons: Chichester, UK, 2010. [Google Scholar]
- Krull, E.S.; Skjemstad, J.O.; Baldock, J.A. Functions of Soil Organic Matter and the Effect on Soil Properties; Cooperative Research Centre for Greenhouse Accounting: Canberra, Australia, 2004. [Google Scholar]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Cheng, C.M.; Amaya, M.; Butalia, T.; Baker, R.; Walker, H.W.; Massey-Norton, J.; Wolfe, W. Short-term influence of coal mine reclamation using coal combustion residues on groundwater quality. Sci. Total Environ. 2016, 571, 834–854. [Google Scholar] [CrossRef]
- Sajwan, K.S.; Twardowska, I.; Punshon, T.; Alva, A.K. Coal Combustion Byproducts and Environmental Issues; Springer Science + Business, Media, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Siddique, R. Utilization of coal combustion by-products in sustainable construction materials. Resour. Conserv. Recycl. 2010, 54, 1060–1066. [Google Scholar] [CrossRef]
- Skousen, J.; Yang, J.E.; Lee, J.S.; Ziemkiewicz, P. Review of fly ash as a soil amendment. Geosystem Eng. 2013, 16, 249–256. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Gitari, W.M.; Petrik, L.F.; Etchebers, O.; Key, D.L.; Iwuoha, E.; Okujeni, C. Passive neutralisation of acid mine drainage by fly ash and its derivatives: A column leaching study. Fuel 2008, 87, 1637–1650. [Google Scholar] [CrossRef]
- Rudisell, M.T.; Stuart, B.J.; Novak, G.; Payne, H.; Togni, C.S. Use of flue gas desulfurization by-product for mine sealing and abatement of acid mine drainage. Fuel 2001, 80, 837–843. [Google Scholar] [CrossRef]
- Xenidis, A.; Mylona, E.; Paspaliaris, I. Potential use of lignite fly ash for the control of acid generation from sulphidic wastes. Waste Manag. 2002, 22, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Ingelmo, F.; Canet, R.; Ibañez, M.A.; Pomares, F.; García, J. Use of MSW compost, dried sewage sludge and other wastes as partial substitutes for peat and soil. Bioresour. Technol. 1998, 63, 123–129. [Google Scholar] [CrossRef]
- Pérez-Gimeno, A.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Gómez, I.; Zorpas, A.A. The use of wastes (organic and inorganic) in land restoration in relation to their characteristics and cost. Waste Manag. Res. 2019, 37, 502–507. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Uzun, I. Use of Spent Mushroom Compost in Sustainable Fruit Production. J. Fruit Ornam. Plant Res. 2004, 12, 157–165. [Google Scholar]
- Jonczy, I.; Gawor, Ł. Coal mining and post-metallurgic dumping grounds and their connections with exploitation of raw materials in the region of Ruda Śląska. Arch. Min. Sci. 2017, 62, 301–311. [Google Scholar] [CrossRef]
- Larondelle, N.; Haase, D. Valuing post-mining landscapes using an ecosystem services approach—An example from Germany. Ecol. Indic. 2012, 18, 567–574. [Google Scholar] [CrossRef]
- Bauerek, A.; Bebek, M.; Fraczek, R.; Paw, K.; Kasperkiewicz, W. Variability of chemical composition of acidic runoff waters from an active spoil heap of mining wastes representing sediments of the cracow sandstone series of the upper silesian coal basin. Prz. Geol. 2017, 65, 450–458. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data; University of South Bohemia in Ceske Budejovice: Ceskie Budejovice, Czech Republic, 2000. [Google Scholar]
- Miller, R.; Donahue, R. Soils in our Environment: An Introduction to Soils and Plant Growth, 7th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1995. [Google Scholar]
- Regulation of the Minister of Environment on the Manner of Conducting Assessment of Pollution of the Ground Surface:Warsaw, Poland, 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 6 July 2023).
- Shahid, S.A.; Zaman, M.; Heng, L. Introduction to Soil Salinity, Sodicity and Diagnostics Techniques. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 1–42. [Google Scholar] [CrossRef]
- Sørensen, M.V.; Strimbeck, R.; Nystuen, K.O.; Kapas, R.E.; Enquist, B.J.; Graae, B.J. Draining the Pool? Carbon Storage and Fluxes in Three Alpine Plant Communities. Ecosystems 2018, 21, 316–330. [Google Scholar] [CrossRef]
- Dudek, T.; Wolański, P.; Rogut, K. Accumulation of organic carbon in soil under various types of highland temperate meadows. J. Elem. 2020, 25, 85–96. [Google Scholar] [CrossRef]
- Krishnaveni, A.; Chinnasamy, S.; Elumalai, J.; Muthaiyan, P. Sugar Industry Wastes as Wealth of Organic Carbon for Soil. In Environmental Factors Affecting Human Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Asik, B.B.; Turan, M.A.; Celik, H.; Katkat, A.V. Effects of Humic Substances on Plant Growth and Mineral Nutrients Uptake of Wheat (Triticum durum cv. Salihli) Under Conditions of Salinity. Asian J. Crop Sci. 2009, 1, 87–95. [Google Scholar] [CrossRef]
- Çelik, H.; Katkat, A.V.; Aşik, B.B.; Turan, M.A. Effects of humus on growth and nutrient uptake of maize under saline and calcareous soil conditions. Zemdirb. Agric. 2010, 97, 15–22. [Google Scholar]
- Kang, J.; Hesterberg, D.; Osmond, D.L. Soil Organic Matter Effects on Phosphorus Sorption: A Path Analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Antybiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Neitzke, M. Changes in energy fixation and efficiency of energy capture in above-ground biomass along an environmental gradient in calcareous grasslands. Flora 2002, 197, 103–117. [Google Scholar] [CrossRef]
- Venterink, H.O.; Güsewell, S. Competitive interactions between two meadow grasses under nitrogen and phosphorus limitation. Funct. Ecol. 2010, 24, 877–886. [Google Scholar] [CrossRef]
- John, H.; Dullau, S.; Baasch, A.; Tischew, S. Re-introduction of target species into degraded lowland hay meadows: How to manage the crucial first year? Ecol. Eng. 2016, 86, 223–230. [Google Scholar] [CrossRef]
- Weigelt, A.; Bol, R.; Bardgett, R.D. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia 2005, 142, 627–635. [Google Scholar] [CrossRef]
- Van Dobben, H.F.; Wamelink, G.W.W.; Slim, P.A.; Kamiński, J.; Piórkowski, H. Species-rich grassland can persist under nitrogen-rich but phosphorus-limited conditions. Plant Soil 2017, 411, 451–466. [Google Scholar] [CrossRef]
- Kamiński, J.; Chrzanowski, S. Floristic differentiation and natural values of meadows in comparision with phosphorus abundance in peat-muck soils. Woda Sr. Obsz. Wiej. 2009, 9, 77–88. [Google Scholar]
- Mafongoya, P.L.; Sileshi, G.W. Indices to identify and quantify ecosystem services in sustainable food systems. In The Role of Ecosystem Services in Sustainable Food Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 43–71. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 409–472. [Google Scholar]
- Boroń, M.; Ociepińska, M.; Żeber-Dzikowska, I.; Gworek, B.; Kondzielski, I.; Chmielewski, J. Xerothermic pavements–a meadow biodiversity richness. Jaworzno case study. Environ. Prot. Nat. Resour. 2019, 30, 29–34. [Google Scholar] [CrossRef]
- Tokarczyk, N. Forest encroachment on temperate mountain meadows-Scale, drivers, and current research directions. Geogr. Pol. 2017, 90, 463–480. [Google Scholar] [CrossRef]
- Lamarque, P.; Tappeiner, U.; Turner, C.; Steinbacher, M.; Bardgett, R.D.; Szukics, U.; Schermer, M.; Lavorel, S. Stakeholder perceptions of grassland ecosystem services in relation to knowledge on soil fertility and biodiversity. Reg. Environ. Chang. 2011, 11, 791–804. [Google Scholar] [CrossRef]
- Villoslada Peciña, M.; Ward, R.D.; Bunce, R.G.H.; Sepp, K.; Kuusemets, V.; Luuk, O. Country-scale mapping of ecosystem services provided by semi-natural grasslands. Sci. Total Environ. 2019, 661, 212–225. [Google Scholar] [CrossRef] [PubMed]
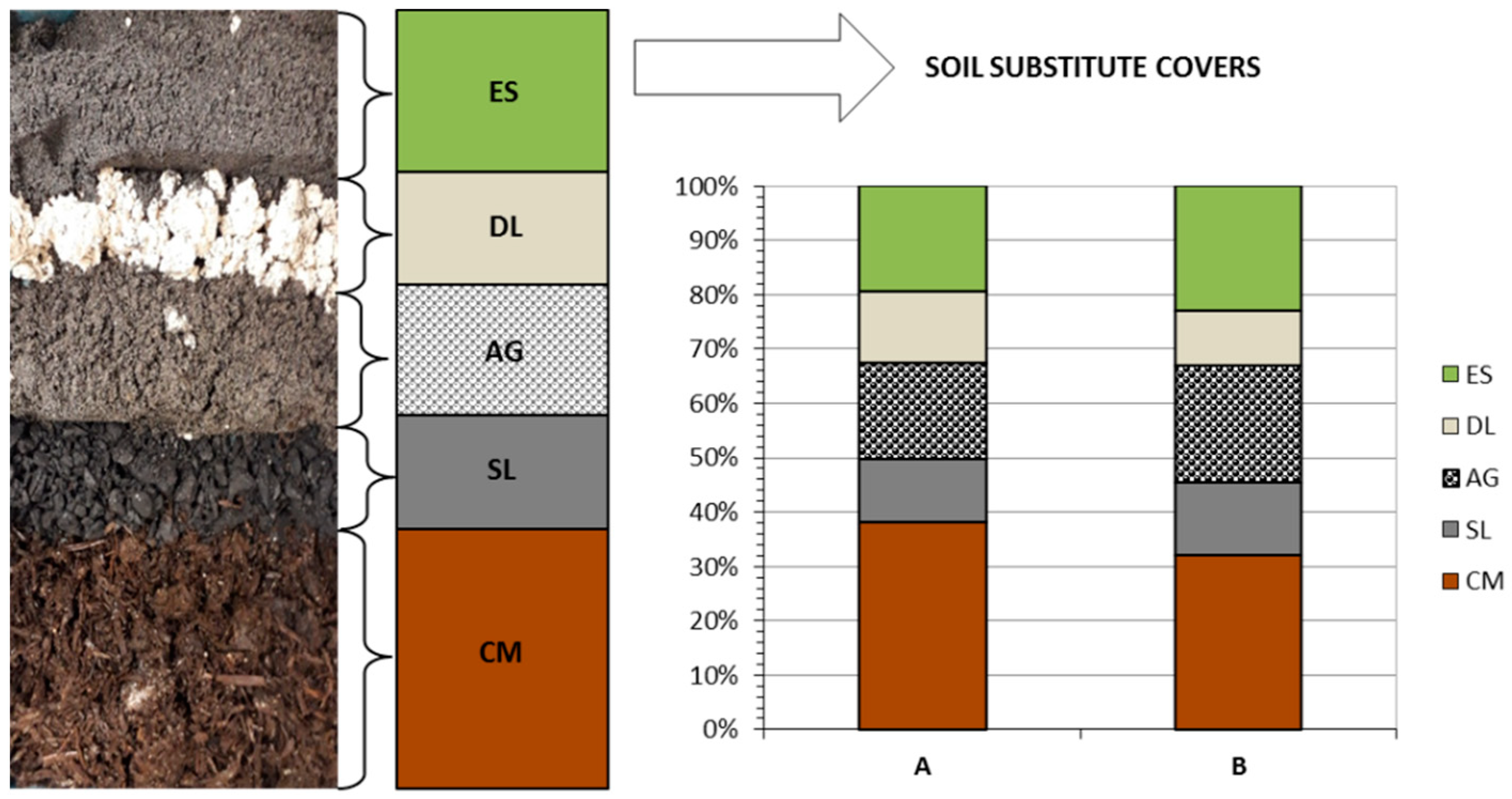
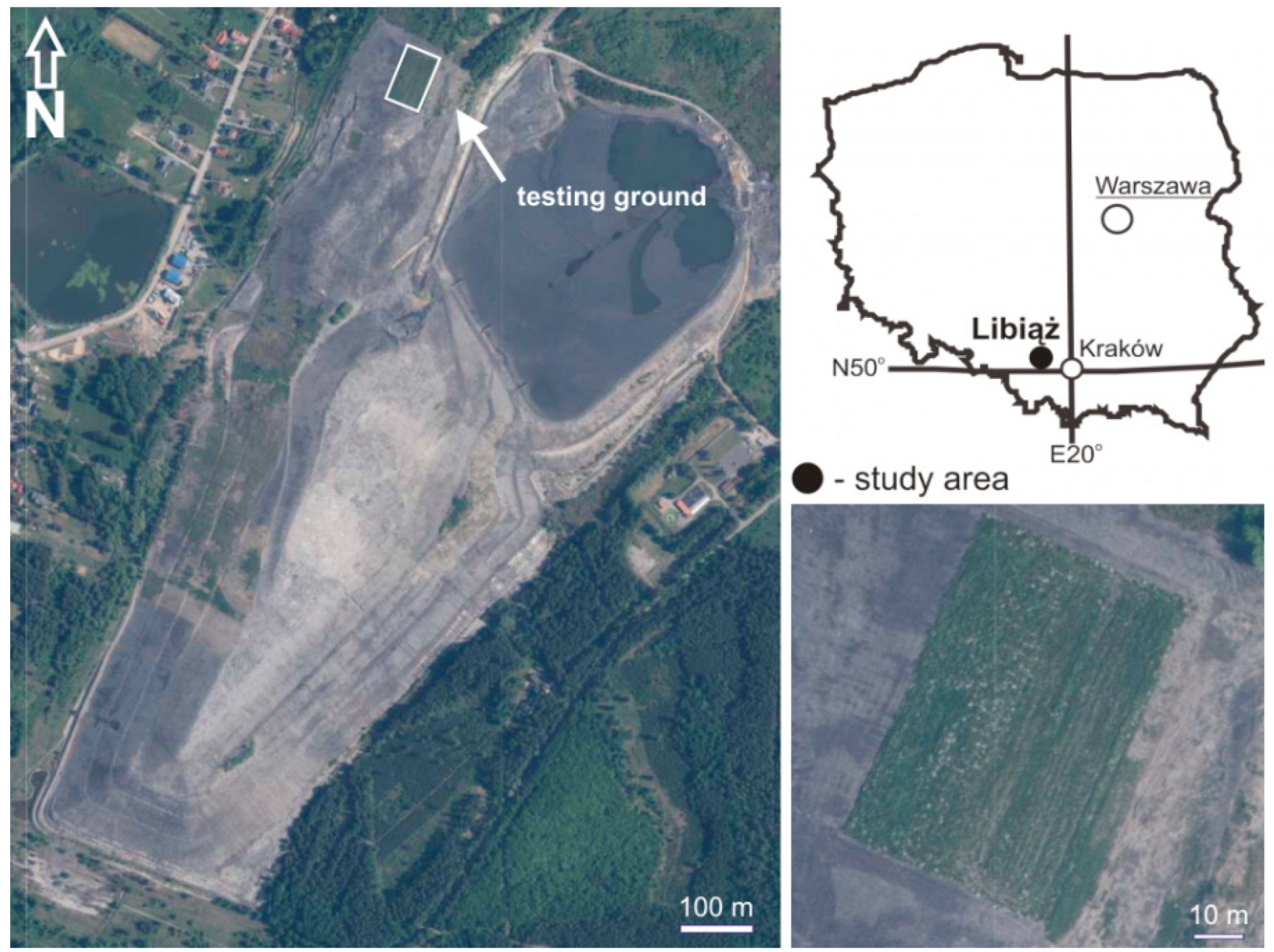

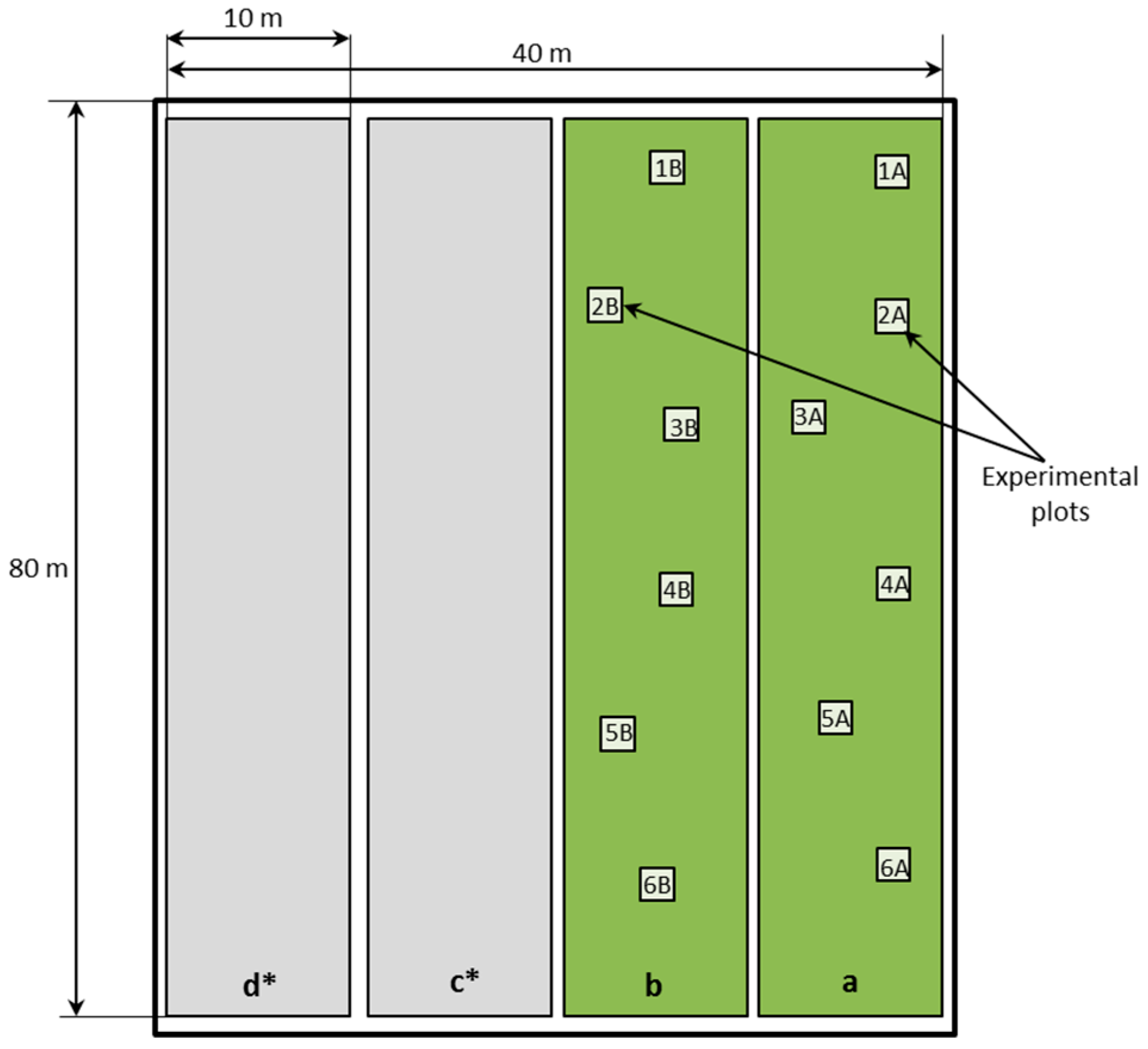
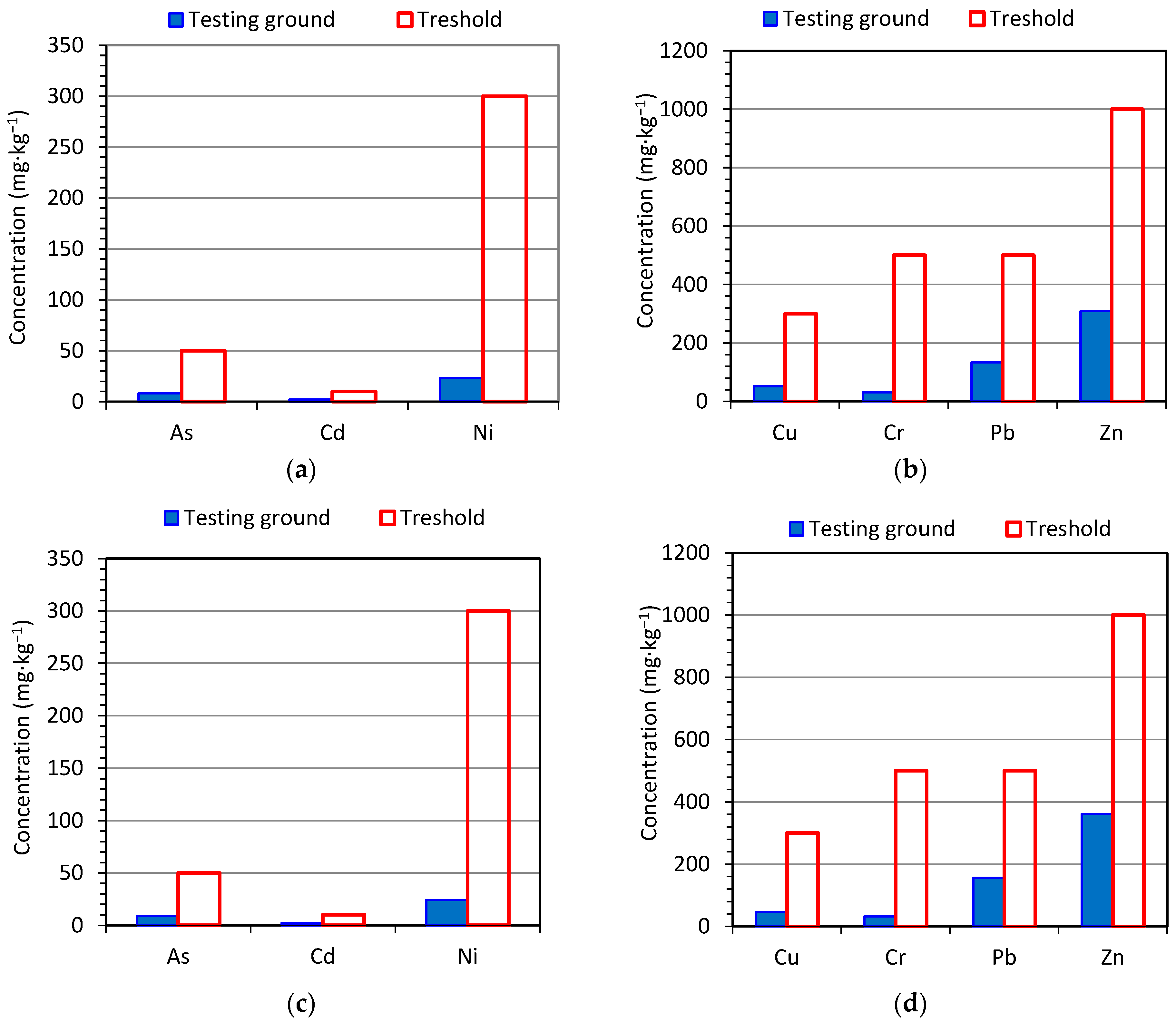
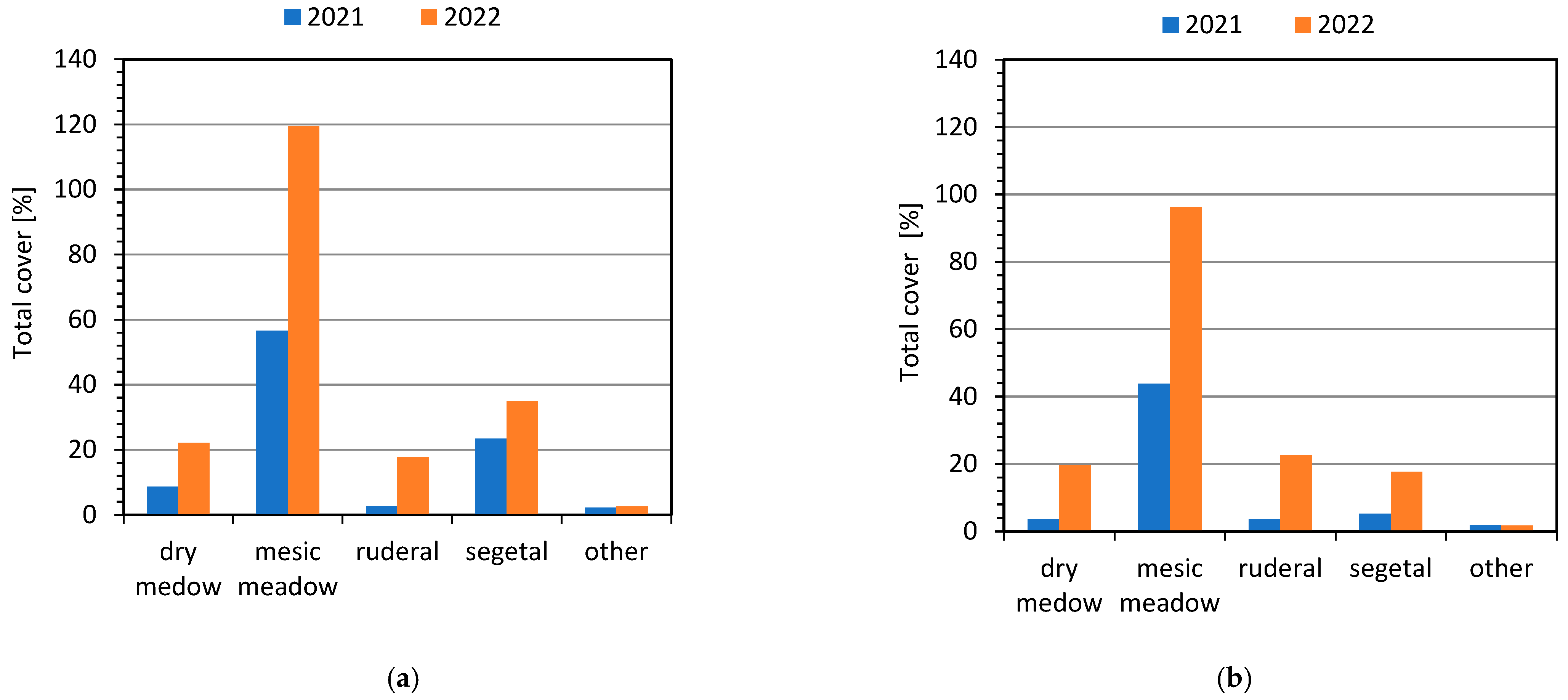

| Parameter | CCBs | Wastes [5] | |||
|---|---|---|---|---|---|
| ES | DL [5] | AG | SL | CM | |
| pH | 9.8 | 9.6 | 7.50 | 8.0 | 7.1 |
| EC (mS·cm−1) | 0.35 | 1.57 | 0.50 | 0.90 | 7.76 |
| OM (%) | 4.38 | 7.02 | 15.9 | 35.6 | 60.4 |
| Ca (%) | 2.74 | 32.0 | 0.43 | 0.34 | 8.22 |
| N (%) | <0.15 | 0.32 | 0.18 | 0.40 | 2.36 |
| K (%) | 2.14 | 0.04 | 2.32 | 1.69 | 1.03 |
| Mg (%) | 1.69 | 5.44 | 0.24 | 0.57 | 0.42 |
| P (%) | 0.11 | 0.01 | 0.02 | 0.03 | 0.78 |
| Na (%) | 0.32 | 0.01 | 0.09 | 0.08 | 0.13 |
| S (%) | 0.32 | 0.24 | 3.95 | 0.63 | 1.97 |
| Cd (mg·kg−1) | 1 | <1 | 4 | <1 | <1 |
| Cr (mg·kg−1) | 53 | 1 | 22 | 76 | 7 |
| Cu (mg·kg−1) | 46 | 3 | 85 | 31 | 29 |
| Ni (mg·kg−1) | 47 | 9 | 26 | 33 | 7 |
| Pb (mg·kg−1) | 3 | 4 | 213 | 53 | 2 |
| Zn (mg·kg−1) | 22 | 36 | 1281 | 141 | 183 |
| Type of Meadow Habitats | Producer | Species Composition |
|---|---|---|
| Dry meadow | Apitec, Niedzwiada, Poland | Anthemis arvensis, Anthemis tinctoria, Betonica officinalis, Bromus erectus, Centaurea cyanus, Centaurea scabiosa, Cichorium intybus, Echium vulgare, Hypochoeris radicata, Knautia arnvensis, Lotus corniculatus, Salvia pratensis, Securigera varia, Verbascum nigrum, Vicia grandiflora, Dactylis glomerata |
| Mesic meadow | Kado s.c., Pszczyna, Poland | Achillea millefolium, Alopecurus pratensis, Chamomilla suaveolens, Crepis biennis, Daucus carota, Festuca arundinacea, Festuca rubra Leucanthemum vulgare, Lolium perenne, Lotus corniculatus, Melandrium album, Plantago lanceolate, Plantago media, Poa pratensis, Sanguisorba minor, Tragopogon pratensis, Trisetum flavescens |
| Parameter | Unit | Soil Substitutes Cover A | Soil Substitutes Cover B | ||||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2020 | 2021 | 2022 | ||
| pH | 8.1 | 7.67 | 7.50 | 8.0 | 7.70 | 7.40 | |
| EC | mS·cm−1 | 6.94 | 2.92 | 0.70 | 6.6 | 2.60 | 1.03 |
| OM | % | 23.05 | 23.54 | 20.14 | 22.95 | 25.34 | 20.48 |
| Ca | % | 6.33 | 3.73 | 3.50 | 5.88 | 3.77 | 4.17 |
| K | % | 1.86 | 1.82 | 1.72 | 1.86 | 1.73 | 1.72 |
| Mg | % | 1.11 | 0.65 | 0.75 | 1.08 | 0.61 | 0.61 |
| Na | % | 0.22 | 0.16 | 0.09 | 0.19 | 0.13 | 0.08 |
| Nt | % | 0.60 | 0.54 | 0.42 | 0.46 | 0.46 | 0.37 |
| Pt | % | 0.21 | 0.16 | 0.16 | 0.18 | 0.14 | 0.14 |
| St | % | 3.46 | 2.41 | 2.00 | 3.41 | 2.00 | 1.92 |
| AWC | % | 88.88 | 78.22 | ||||
| Type of Meadow Habitats | Species | Experimental Plots | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 2A | 3A | 4A | 5A | 6A | 1B | 2B | 3B | 4B | 5B | 6B | ||
| Dry meadow | Anthemis arvensis | v | v | v | v | v | v | v | |||||
| Anthemis tinctoria | v | v | v | v | v | v | v | v | v | v | |||
| Betonica officinalis | |||||||||||||
| Bromus erectus | v | v | v | v | v | v | v | ||||||
| Centaurea cyanus | v | v | v | ||||||||||
| Centaurea scabiosa | v | v | v | v | v | v | v | v | |||||
| Cichorium intybus | v | v | v | v | v | v | v | v | |||||
| Echium vulgare | v | v | |||||||||||
| Hypochoeris radicata | |||||||||||||
| Knautia arnvensis | v | ||||||||||||
| Lotus corniculatus | v | v | v | v | v | v | v | ||||||
| Salvia pratensis | v | v | v | v | v | v | v | ||||||
| Securigera varia | |||||||||||||
| Verbascum nigrum | v | ||||||||||||
| Vicia grandiflora | |||||||||||||
| Dactylis glomerata | v | v | v | v | |||||||||
| Mesic meadow | Achillea millefolium | v | v | v | v | v | v | v | v | v | v | v | v |
| Alopecurus pratensis | |||||||||||||
| Chamomilla suaveolens | v | v | v | v | v | ||||||||
| Crepis biennis | v | v | v | v | v | v | |||||||
| Daucus carota | v | v | v | ||||||||||
| Festuca arundinacea | v | v | v | v | |||||||||
| Festuca rubra | v | v | v | v | v | v | v | v | |||||
| Leucanthemum vulgare | v | v | v | v | v | v | v | v | v | ||||
| Lolium perenne | v | v | v | v | v | v | v | v | v | v | v | ||
| Melandrium album | v | v | v | v | |||||||||
| Plantago lanceolata | v | v | v | v | v | ||||||||
| Plantago media | |||||||||||||
| Poa pratensis | v | v | v | v | v | v | v | ||||||
| Sanguisorba minor | |||||||||||||
| Tragopogon pratensis | |||||||||||||
| Trisetum flavescens | |||||||||||||
| Parameter | EC | TIME | OM | AWC | Pt | Ca |
|---|---|---|---|---|---|---|
| Axis 1 | 0.67 | −0.69 | 0.70 | 0.08 | 0.16 | −0.34 |
| Axis 2 | 0.01 | −0.05 | 0.18 | −0.38 | −0.36 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Więckol-Ryk, A.; Pierzchała, Ł.; Bauerek, A.; Krzemień, A. Minimising Coal Mining’s Impact on Biodiversity: Artificial Soils for Post-Mining Land Reclamation. Sustainability 2023, 15, 9707. https://doi.org/10.3390/su15129707
Więckol-Ryk A, Pierzchała Ł, Bauerek A, Krzemień A. Minimising Coal Mining’s Impact on Biodiversity: Artificial Soils for Post-Mining Land Reclamation. Sustainability. 2023; 15(12):9707. https://doi.org/10.3390/su15129707
Chicago/Turabian StyleWięckol-Ryk, Angelika, Łukasz Pierzchała, Arkadiusz Bauerek, and Alicja Krzemień. 2023. "Minimising Coal Mining’s Impact on Biodiversity: Artificial Soils for Post-Mining Land Reclamation" Sustainability 15, no. 12: 9707. https://doi.org/10.3390/su15129707
APA StyleWięckol-Ryk, A., Pierzchała, Ł., Bauerek, A., & Krzemień, A. (2023). Minimising Coal Mining’s Impact on Biodiversity: Artificial Soils for Post-Mining Land Reclamation. Sustainability, 15(12), 9707. https://doi.org/10.3390/su15129707








