Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Serological Assay
2.3. Host Range Determination
2.4. Seed Transmission
2.5. Total RNA Extraction and RT-PCR
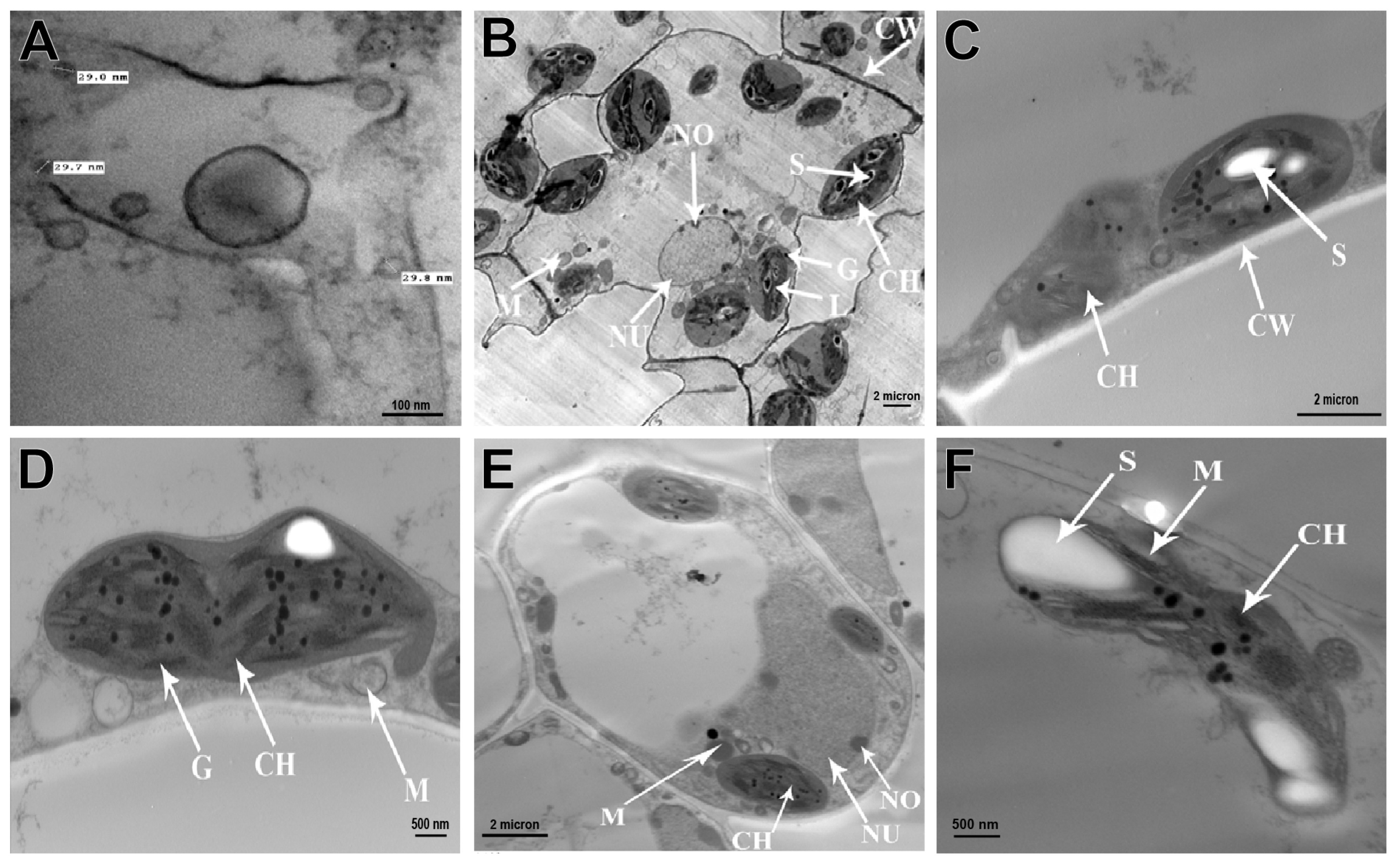
2.6. Anatomical and Cytological Studies
2.6.1. Electron Microscopy
2.6.2. Immunogold Labeling
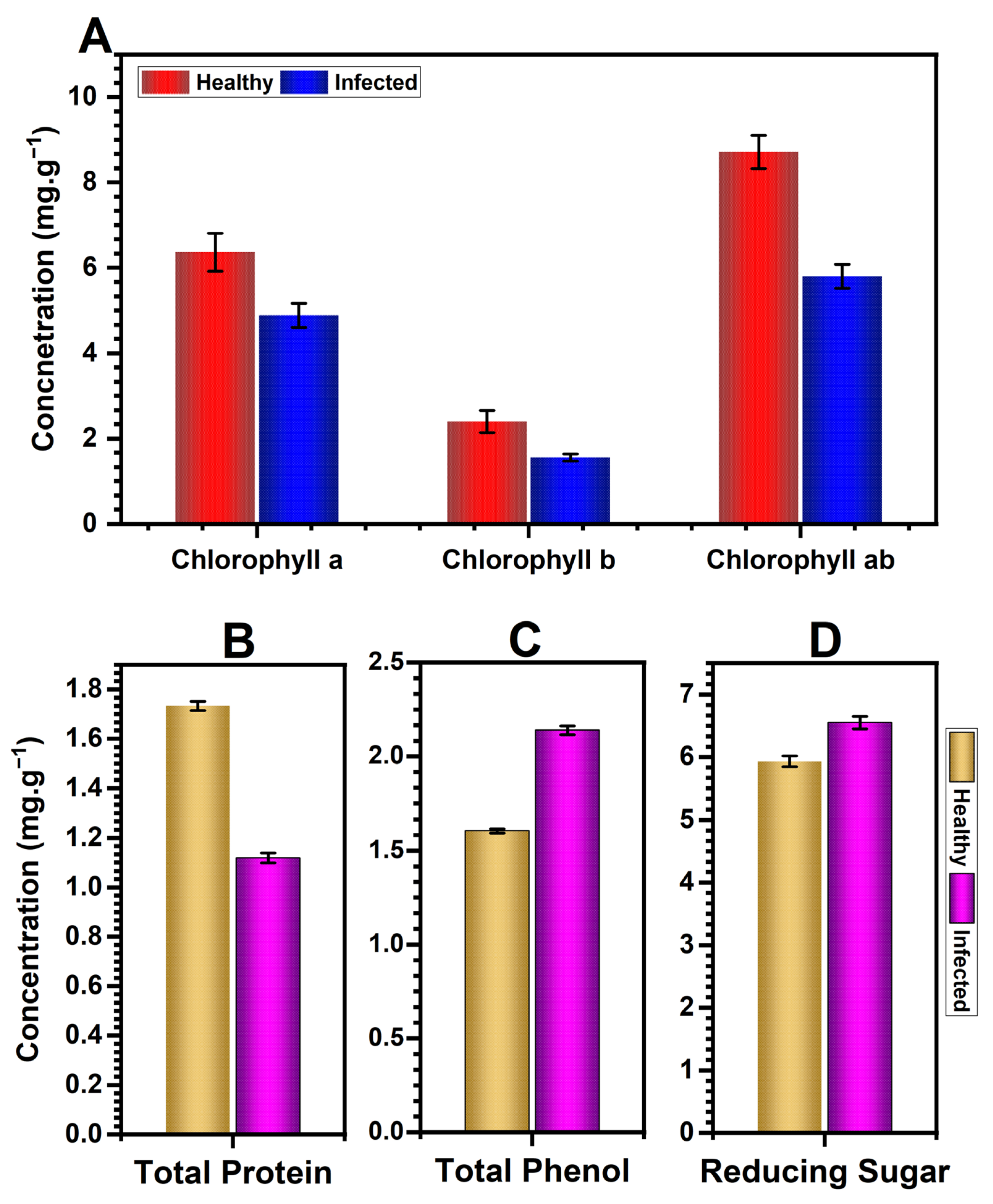
2.6.3. Determination of Photosynthetic Pigments
2.6.4. Measurement of Total Protein
2.6.5. Determination of Phenolic Content
2.6.6. Determination of Reducing Sugars
2.7. Assessment of Morphological and Horticultural Attributes
3. Results
3.1. Phenotype and Serological Assays
3.2. Host Range Studies
3.3. Seed Transmission
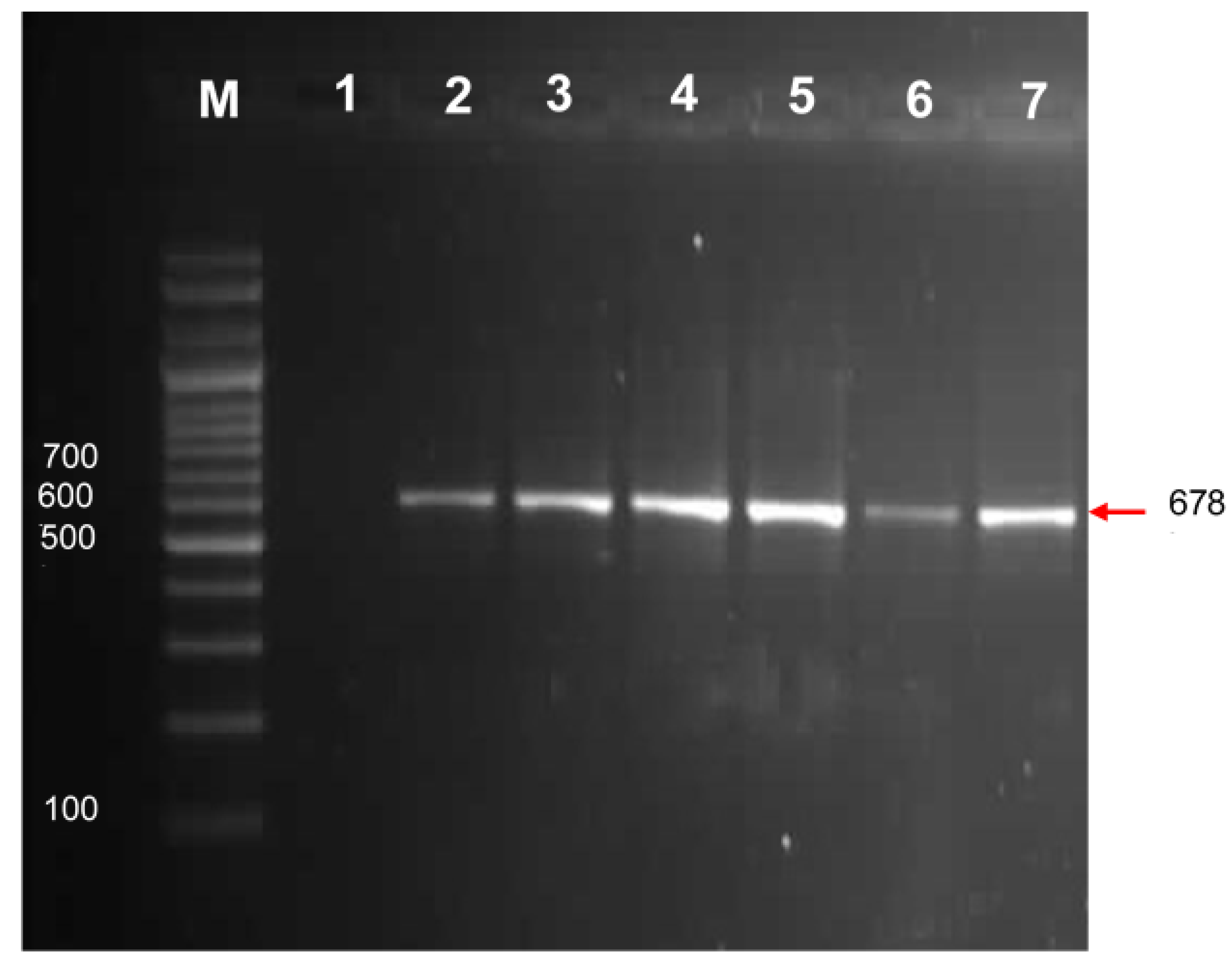
3.4. One-Step RT-PCR
3.5. Histological and Cytological Studies
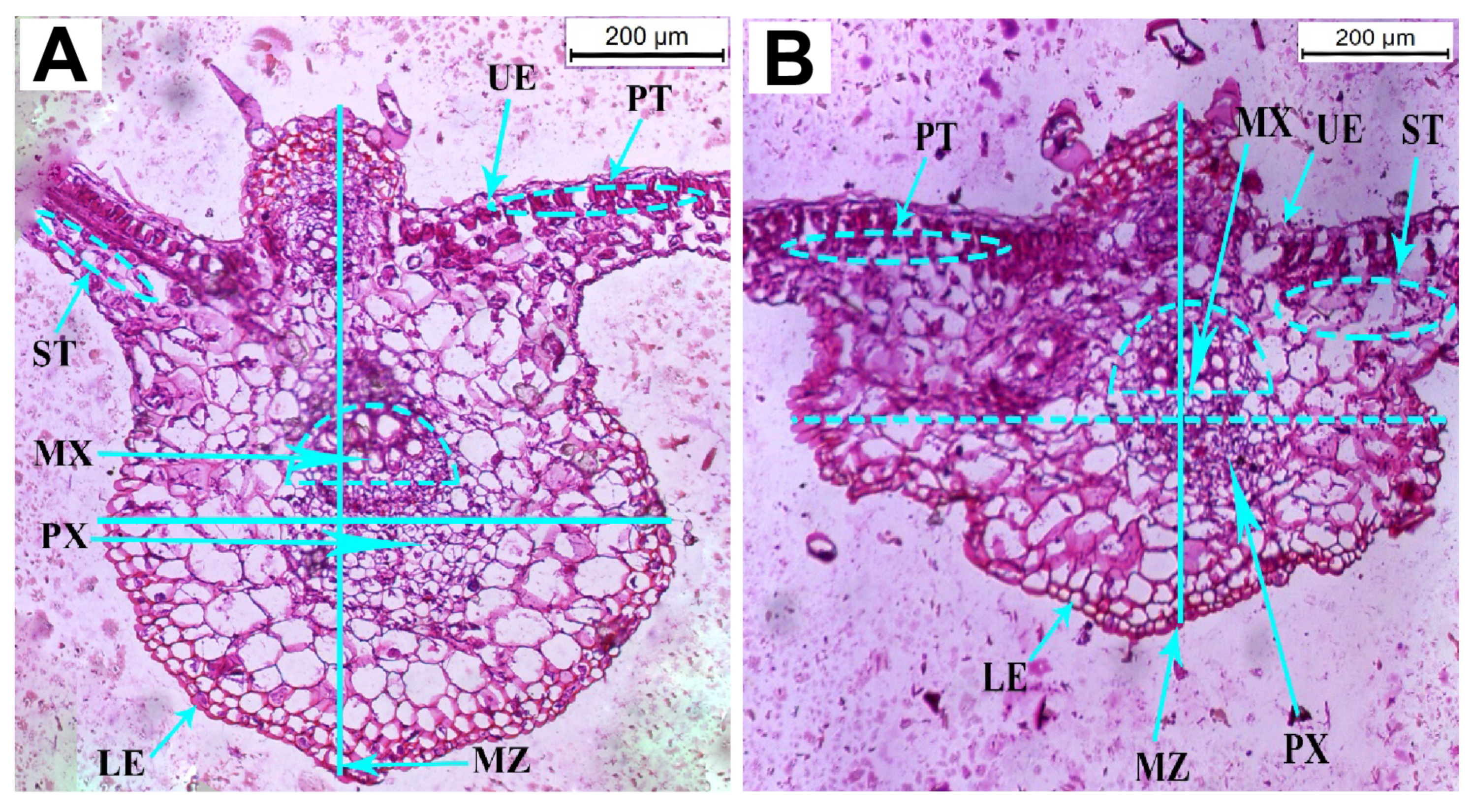
3.5.1. Histological Studies
3.5.2. Cytological Studies
3.6. Effect of CMV Infection on Biochemical Attributes
3.7. Effects of CMV Infection on Morphological and Horticultural Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOStat. Food and Agriculture Organization of the United Nations Statistics Division. Available online: http://faostat3.fao.org/home/E (accessed on 25 January 2023).
- Usha, R.; Lakshmi, M.; Ranjani, M. Nutritional, sensory and physical analysis of pumpkin flour incorporated into weaning mix. Malays. J. Nutr. 2010, 16, 379–387. [Google Scholar]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Aslam, J. Utilization of pumpkin, pumpkin powders, extracts, isolates, purified bioactives and pumpkin based functional food products; a key strategy to improve health in current post COVID 19 period—An updated review. Appl. Food Res. 2022, 2, 100241. [Google Scholar] [CrossRef]
- Radouane, N.; Ezrari, S.; Belabess, Z.; Tahiri, A.; Tahzima, R.; Massart, S.; Jijakli, H.; Benjelloun, M.; Lahlali, R. Viruses of cucurbit crops: Current status in the Mediterranean Region. Phytopathol. Mediterr. 2021, 60, 493–519. [Google Scholar] [CrossRef]
- Lecoq, H. Cucurbits. In Virus and Virus-like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 665–688. [Google Scholar] [CrossRef]
- Desbiez, C. The never-ending story of cucurbits and viruses. Acta Hortic. 2020, 1294, 173–192. [Google Scholar] [CrossRef]
- Lecoq, H.; Desbiez, C. Viruses of cucurbit crops in the Mediterranean region: An ever-changing picture. Adv. Virus Res. 2012, 84, 67–126. [Google Scholar]
- Muniyappa, V.; Maruthi, M.; Babitha, C.; Colvin, J.; Briddon, R.; Rangaswamy, K. Characterisation of pumpkin yellow vein mosaic virus from India. Ann. Appl. Biol. 2003, 142, 323–331. [Google Scholar] [CrossRef]
- Zechmann, B.; Müller, M.; Zellnig, G. Cytological modifications in zucchini yellow mosaic virus (ZYMV)-infected Styrian pumpkin plants. Arch. Virol. 2003, 148, 1119–1133. [Google Scholar] [CrossRef]
- Mohammed, H.; Manglli, A.; Zicca, S.; El Hussein, A.; Mohamed, M.; Tomassoli, L. First report of Papaya ringspot virus in pumpkin in Sudan. New Dis. Rep. 2012, 26, 26–33. [Google Scholar] [CrossRef]
- Ito, T.; Ogawa, T.; Samretwanich, K.; Sharma, P.; Ikegami, M. Yellow leaf curl disease of pumpkin in Thailand is associated with Squash leaf curl China virus. Plant Pathol. 2008, 57, 766. [Google Scholar] [CrossRef]
- Hamza, E.S.; Al-Naggar, A.; El-Shabrawi, H.; Tolba, I. Characteristics of cucumber mosaic virus isolates infecting cucurbits in Egypt. Al-Azhar J. Agric. Res. 2022, 47, 172–184. [Google Scholar] [CrossRef]
- Sivakumaran, K.; Bao, Y.; Roossinck, M.J.; Kao, C.C. Recognition of the core RNA promoter for minus-strand RNA synthesis by the replicases of brome mosaic virus and cucumber mosaic virus. J. Virol. 2000, 74, 10323–10331. [Google Scholar] [CrossRef]
- Sivakumaran, K.; Chen, M.-H.; Roossinck, M.J.; Kao, C.C. Core promoter for initiation of Cucumber mosaic virus subgenomic RNA4A. Mol. Plant Pathol. 2002, 3, 43–52. [Google Scholar] [CrossRef]
- Palukaitis, P.; Roossinck, M.J.; Dietzgen, R.G.; Francki, R.I. Cucumber mosaic virus. Adv. Virus Res. 1992, 41, 281–348. [Google Scholar]
- García-Arenal, F.; Escriu, F.; Aranda, M.A.; Alonso-Prados, J.L.; Malpica, J.M.; Fraile, A. Molecular epidemiology of Cucumber mosaic virus and its satellite RNA. Virus Res. 2000, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Cucumber mosaic virus, a model for RNA virus evolution. Mol. Plant Pathol. 2001, 2, 59–63. [Google Scholar] [CrossRef]
- Palukaitis, P.; García-Arenal, F. Cucumoviruses. Adv. Virus Res. 2003, 62, 241–323. [Google Scholar] [PubMed]
- Gibbs, A.J.; Harrison, B.D. Cucumber mosaic virus. In Descriptions of Plant Viruses: No. 1. Set 1; Gibbs, A.J., Harrison, B.D., Murant, A.F., Eds.; Commonwealth Mycological Institute: Kew Surrey, UK, 1970. [Google Scholar]
- Abdullahi, I.; Ikotun, T.; Winter, S.; Thottappilly, G.; Atiri, G. Investigation on seed transmission of cucumber mosaic virus in cowpea. Afr. Crop Sci. J. 2001, 9, 677–684. [Google Scholar] [CrossRef]
- Doolittle, S.P. A new infectious mosaic disease of cucumber. Phytopathology 1916, 6, 145–147. [Google Scholar]
- Green, S.K.; Kim, J. Characteristics and Control of Viruses Infecting Peppers: A Literature Review; Asian Vegetable Research and Development Center Shanhua: Tainan, Taiwan, 1991; Volume 18. [Google Scholar]
- Zitter, T.; Murphy, J. Cucumber mosaic. Plant Health Instr. 2009, 10, 2009–051801. [Google Scholar] [CrossRef]
- Mahjabeen; Akhtar, K.P.; Sarwar, N.; Saleem, M.Y.; Asghar, M.; Iqbal, Q.; Jamil, F.F. Effect of cucumber mosaic virus infection on morphology, yield and phenolic contents of tomato. Arch. Phytopathol. Plant Prot. 2012, 45, 766–782. [Google Scholar] [CrossRef]
- Shalaby, A.A. Molecular detection of an Egyptian isolate of cucumber mosaic virus (CMV) from infected banana using RT-PCR and nucleic acid prob and partial sequence identification. Egypt. J. Genet. Cytol. 2002, 31, 183–189. [Google Scholar]
- Makkouk, K.; Rizkallah, L.; Madkour, M.; El-Sherbeeny, M.; Kumari, S.; Amriti, A.; Solh, M. Survey of faba bean (Vicia faba L.) for viruses in Egypt. Phytopathol. Mediterr. 1994, 33, 207–211. [Google Scholar]
- Abd El-Aziz, M. Studies on Some Viruses Infecting Cowpea Plants; Alexandria University: Alexandria, Egypt, 2019. [Google Scholar]
- Wagih, E.E.; Zalat, M.M.; Kawanna, M.A. Cytological, histological and molecular characterization of two isolates of Cucumber Mosaic Virus (CMV) in Egypt. Int. J. Phytopathol. 2021, 10, 9–18. [Google Scholar] [CrossRef]
- El-Kady, M.A.S.; Gamal El Din, A.S.; Nakhla, M.K.; Abdel Salam, A.M. A strain of cucumber mosaic virus isolated sugar beet in Egypt. In Proceedings of the 1st National Conference of Pests & Field Crops in Egypt, Ismailia, Egypt, 21–23 October 1985; pp. 617–626. [Google Scholar]
- Mokbel, S.; Ahmed, E.; El-Kammar, H.; Kheder, A. Molecular characterization of cucumber mosaic virus and structural changes of infected sugar beet plants. J. Virol. Sci. 2020, 8, 12–27. [Google Scholar]
- Yu, C.; Wu, J.; Zhou, X. Detection and subgrouping of Cucumber mosaic virus isolates by TAS-ELISA and immunocapture RT-PCR. J. Virol. Methods 2005, 123, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, H.; Wang, X.; Chen, J.; Zhu, W. Multiplex RT-PCR detection of Cucumber mosaic virus subgroups and Tobamoviruses infecting Tomato using 18S rRNA as an internal control. Acta Biochim. Biophys. Sin. 2011, 43, 465–471. [Google Scholar] [CrossRef]
- Hamdy Abd El-Aziz, M.; Aly Younes, H. Detection of Cucumber mosaic cucumovirus in infected cowpea plants (Vigna unguiculata L.) from northern Egypt. Nov. Res. Microbiol. J. 2019, 3, 326–340. [Google Scholar] [CrossRef]
- Waigmann, E.; Ueki, S.; Trutnyeva, K.; Citovsky, V. The ins and outs of nondestructive cell-to-cell and systemic movement of plant viruses. Crit. Rev. Plant Sci. 2004, 23, 195–250. [Google Scholar] [CrossRef]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Yan, S.L.; Lehrer, A.T.; Hajirezaei, M.R.; Springer, A.; Komor, E. Modulation of carbohydrate metabolism and chloroplast structure in sugarcane leaves which were infected by Sugarcane Yellow Leaf Virus (SCYLV). Physiol. Mol. Plant Pathol. 2008, 73, 78–87. [Google Scholar] [CrossRef]
- Ayres, P.G.; Press, M.C.; Spencer-Phillips, P.T. Effects of pathogens and parasitic plants on source-sink relationships. Photoassimilate Distrib. Plants Crops 1996, 8, 479–499. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.K. Interactions between beet necrotic yellow vein virus and beet soilborne virus in different sugar beet cultivars. Andalou J. Agric. Sci. 2010, 25, 68–74. [Google Scholar]
- Lin, H.-X.; Rubio, L.; Smythe, A.B.; Falk, B.W. Molecular population genetics of Cucumber mosaic virus in California: Evidence for founder effects and reassortment. J. Virol. 2004, 78, 6666–6675. [Google Scholar] [CrossRef] [PubMed]
- Sass, J.E. Botanical Microtechnique, 3rd ed.; The Iowa State University Press: Ames, IA, USA, 1958. [Google Scholar]
- Osmont, K.; Freeling, M. Characterization of extended auricle (eta) a developmental mutant that affects the blade/sheath boundary in maize. In Proceedings of the Poster from the 43rd Maize Genetics Conference, Lake Geneva, WI, USA, 10–13 March 2005. [Google Scholar]
- Kim, K.S.; Fulton, R.W. Ultrastructure of Datura stramonium infected with an Euphorbia virus suggestive of a whitefly-transmitted geminivirus. Phytopathology 1984, 74, 236–241. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N, N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ammar, E.-D.; Tsai, C.-W.; Whitfield, A.E.; Redinbaugh, M.G.; Hogenhout, S.A. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Ann. Rev. Entomol. 2009, 54, 447–468. [Google Scholar] [CrossRef]
- Herbers, K.; Tacke, E.; Hazirezaei, M.; Krause, K.-P.; Melzer, M.; Rohde, W.; Sonnewald, U. Expression of a luteoviral movement protein in transgenic plants leads to carbohydrate accumulation and reduced photosynthetic capacity in source leaves. Plant J. 1997, 12, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, R.; Moya, J.H.; Xoconostle-Cázares, B.; Lucas, W.J. Influence of cucumber mosaic virus infection on the mRNA population present in the phloem translocation stream of pumpkin plants. Funct. Plant Biol. 2007, 34, 292–301. [Google Scholar] [CrossRef]
- Zitikaitė, I.; Staniulis, J.; Urbanavičienė, L.; Žižytė, M. Cucumber mosaic virus identification in pumpkin plants. Zemdirb.-Agric. 2011, 98, 421–426. [Google Scholar]
- Jossey, S.; Babadoost, M. Occurrence and distribution of pumpkin and squash viruses in Illinois. Plant Dis. 2008, 92, 61–68. [Google Scholar] [CrossRef]
- Svoboda, J.; Leisova-Svobodova, L. First report of squash mosaic virus in ornamental pumpkin in the Czech Republic. Plant Dis. 2011, 95, 1321. [Google Scholar] [CrossRef] [PubMed]
- Ashwathappa, K.V.; Krishna Reddy, M.; Venkataravanappa, V.; Madhavi Reddy, K.; Hemachandra Reddy, P.; Lakshminarayana Reddy, C.N. Genome characterization and host range studies of Cucumber mosaic virus belonging to the Subgroup IB infecting chilli in India and screening of chilli genotypes for identification of resistance. Virus Dis. 2021, 32, 535–547. [Google Scholar] [CrossRef]
- Vitti, A.; Pagán, I.; Bochicchio, B.; De Stradis, A.; Piazzolla, P.; Scopa, A.; Nuzzaci, M. Cucumber mosaic virus is unable to self-assemble in tobacco plants when transmitted by seed. Plants 2022, 11, 3217. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.S.; Anderson, E.J. Seed transmission of cucumber mosaic virus in spinach. Phytopathology 1997, 87, 924–931. [Google Scholar] [CrossRef]
- Ali, A.; Kobayashi, M. Seed transmission of Cucumber mosaic virus in pepper. J. Virol. Methods 2010, 163, 234–237. [Google Scholar] [CrossRef]
- Arogundade, O.; Balogun, O.S.; Kumar, P.L. Seed transmissibility of Cucumber mosaic virus in Capsicum species. Int. J. Veg. Sci. 2019, 25, 146–153. [Google Scholar] [CrossRef]
- Francki, R.; Mossop, D.; Hatta, T. Cucumber mosaic virus. No. 213. In Descriptions of Plant Viruses; Commonwealth Mycology Institute and Association of Applied Biology: Kew Surren, UK, 1979. [Google Scholar]
- Megahed, A.; El-Dougdoug, K.A.; Othman, B.; Lashin, S.; Hassanin, M.; Ibrahim, M.; Sofy, A. Molecular identification and analysis of coat protein gene of Cucumber mosaic cucumovirus sugar beet Egyptian isolate. Int. J. Plant Pathol. 2014, 5, 70–83. [Google Scholar] [CrossRef]
- Kumari, R.; Bhardwaj, P.; Singh, L.; Zaidi, A.A.; Hallan, V. Biological and molecular characterization of cucumber mosaic virus subgroup II isolate causing severe mosaic in cucumber. Indian J. Virol. 2013, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- El-Dougdoug, K.; Sofy, A.; Hameed, G.; Dawood, R. Intraspecific diversity of Cucumber mosaic Cucumoviridae in Egypt. Int. J. Virol. 2014, 10, 94–102. [Google Scholar] [CrossRef]
- Pavithra, B.S.; Govin, K.; Renuka, H.M.; Krishnareddy, M.; Jalali, S.; Samuel, D.K.; Himabindu, K. Characterization of cucumber mosaic virus infecting coleus (Plectranthus barbatus) in Karnataka. Virus Dis. 2019, 30, 403–412. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Nakkeeran, S. Molecular characterization of Cucumber mosaic virus infection in chilli (Capsicum annuum L.) and its phylogenetic analysis. Int. J. Chem. Stud. 2020, 8, 2967–2970. [Google Scholar]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Afreen, B.; Baghel, G.; Fatma, M.; Usman, M.; Naqvi, Q. Studies on molecular detection of Cucumber mosaic virus and its anatomical and biochemical changes in Daucus carota L. Int. J. Gen. Eng. Biotechnol. 2010, 1, 187–192. [Google Scholar]
- Janjić, N.; Erić, Ž. Morphometric and anatomical-histometric characteristics of two varieties of the species Solanum lycopersicum L. infected by Cucumber mosaic virus. Agro. Knowl. J. 2012, 13, 591–602. [Google Scholar] [CrossRef]
- Kunkalikar, S.; Byadgi, A.; Kulkarni, V.; Krishnareddy, M.; Prabhakar, A. Histopathology and histochemistry of papaya ringspot disease in papaya. Indian J. Virol. 2007, 18, 33–35. [Google Scholar]
- El-Attar, A.K.; Mokbel, S.A.; El-Banna, O.-H.M. Molecular characterization of alfalfa mosaic virus and its effect on basil (Ocimum basilicum) tissues in Egypt. J. Virol. Sci. 2019, 5, 97–113. [Google Scholar]
- Wan, J.; Cabanillas, D.G.; Zheng, H.; Laliberté, J.-F. Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol. 2015, 167, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, U.; Berger, P. Association of potato virus Y gene products with chloroplasts in tobacco. Mol. Plant. Microbe Interact. 1991, 4, 452–457. [Google Scholar] [CrossRef]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar] [CrossRef] [PubMed]
- Radwan, D.E.M.; Lu, G.; Fayez, K.A.; Mahmoud, S.Y. Protective action of salicylic acid against bean yellow mosaic virus infection in Vicia faba leaves. J. Plant Physiol. 2008, 165, 845–857. [Google Scholar] [CrossRef]
- Ahmed, N.; Thakur, M.; Bajaj, K. Nature of resistance and effect of yellow vein mosaic virus on moisture, chlorophyll, chlorophyllase and carbohydrate contents of okra. Veg. Sci. 1986, 13, 339–353. [Google Scholar]
- Singh, M.; Singh, J.; Cheema, S. Effect of Cucumber mosaic virus on chlorophyll content and mineral elements in chilli. Plant Dis. Res. 1998, 13, 125–128. [Google Scholar]
- Shakeel, M.; Amer, M.; Al-Saleh, M.; Ashfaq, M.; Haq, M. Changes in chlorophyll, phenols, sugars and mineral contents of cucumber plants infected with cucumber mosaic virus. J. Phytopathol. Pest Manag. 2016, 3, 1–11. [Google Scholar]
- Shalitin, D.; Wolf, S. Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol. 2000, 123, 597–604. [Google Scholar] [CrossRef]
- Arias, M.C.; Lenardon, S.; Taleisnik, E. Carbon metabolism alterations in sunflower plants infected with the Sunflower chlorotic mottle virus. J. Phytopathol. 2003, 151, 267–273. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H.; Wobus, U.; Weber, H. Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. J. Exp. Bot. 2003, 54, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Blasing, O.E.; Gibon, Y.; Gunther, M.; Hohne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Usadel, B.R.; Scheible, W.-R.D.; Stitt, M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Hu, H.; Larkin, P.; Meinke, H.; Shabala, S.; Ahmed, I.; Zhou, M. Agronomical, biochemical and histological response of resistant and susceptible wheat and barley under BYDV stress. PeerJ 2018, 6, e4833. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Vega, J.; Oliveira, J.G.; Gomes, M. Sugarcane yellow leaf virus infection leads to alterations in photosynthetic efficiency and carbohydrate accumulation in sugarcane leaves. Fitopatol. Bras. 2005, 30, 10–16. [Google Scholar] [CrossRef]
- Shalitin, D.; Wang, Y.; Omid, A.; Gal-On, A.; Wolf, S. Cucumber mosaic virus movement protein affects sugar metabolism and transport in tobacco and melon plants. Plant Cell Environ. 2002, 25, 989–997. [Google Scholar] [CrossRef]
- Kofalvi, S.; Nassuth, A. Influence of wheat streak mosaic virus infection on phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol. Mol. Plant Pathol. 1995, 47, 365–377. [Google Scholar] [CrossRef]
- Srivastava, A.; Tiwari, C. Phenolic contents of cucumber as influenced by the infection of Cucumber green mottle mosaic virus (CGMMV). J. Living World 1998, 5, 1–3. [Google Scholar]
- Ofori, A.; Padi, F.K.; Ameyaw, G.A.; Dadzie, A.M.; Lowor, S. Genetic variation among cocoa (Theobroma cacao L.) families for resistance to cocoa swollen shoot virus disease in relation to total phenolic content. Plant Breed. 2015, 134, 477–484. [Google Scholar] [CrossRef]
- Freeman, B.; Beattie, G. An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 2008, 149, 1–12. [Google Scholar] [CrossRef]
- Matern, U.; Kneusel, R.E. Phenolic compounds in plant disease resistance. Phytoparasitica 1988, 16, 153–170. [Google Scholar] [CrossRef]
- Sattler, S.; Funnell-Harris, D. Modifying lignin to improve bioenergy feedstocks: Strengthening the barrier against pathogens? Front. Plant Sci. 2013, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; De Luca, V.; Brisson, N. Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 1995, 7, 1787–1799. [Google Scholar] [CrossRef]
- Wen, P.-F.; Chen, J.-Y.; Kong, W.-F.; Pan, Q.-H.; Wan, S.-B.; Huang, W.-D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Carvalho, D.d.; Ferreira, R.A.; Oliveira, L.M.d.; Oliveira, A.F.d.; Gemaque, R.C.R. Proteins and isozymes electroforesis in seeds of Copaifera Langsdorffii Desf. (Leguminosae caesalpinioideae) artificially aged. Rev. Arvore 2006, 30, 19–24. [Google Scholar] [CrossRef]
- Tornero, P.; Chao, R.A.; Luthin, W.N.; Goff, S.A.; Dangl, J.L. Large-scale structure–function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 2002, 14, 435–450. [Google Scholar] [CrossRef]
- Xu, L.; Hou, Q.; Zhao, Y.; Ni, Z.; Liang, H.; Liang, R. Biochemical responses of resistant and susceptible wheat cultivars to English grain aphid (Sitobio avenae F.) at grain-filling stage. Acad. J. Biotechnol. 2016, 4, 276–284. [Google Scholar]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhter, M.S.; Alam, M.M.; Pervin, N.; Akanda, A.M. Prevalence of Cucumber Mosaic Virus and its impact on growth and yield of different chili cultivar. Bull. Inst. Trop. Agric. Kyushu Univ. 2016, 39, 65–74. [Google Scholar] [CrossRef]
- Agrios, G.; Walker, M.; Ferro, D. Effect of cucumber mosaic virus inoculation at successive weekly intervals on growth and yield of pepper (Capsicum annuum) plants. Plant Dis. 1985, 69, 52–55. [Google Scholar] [CrossRef]
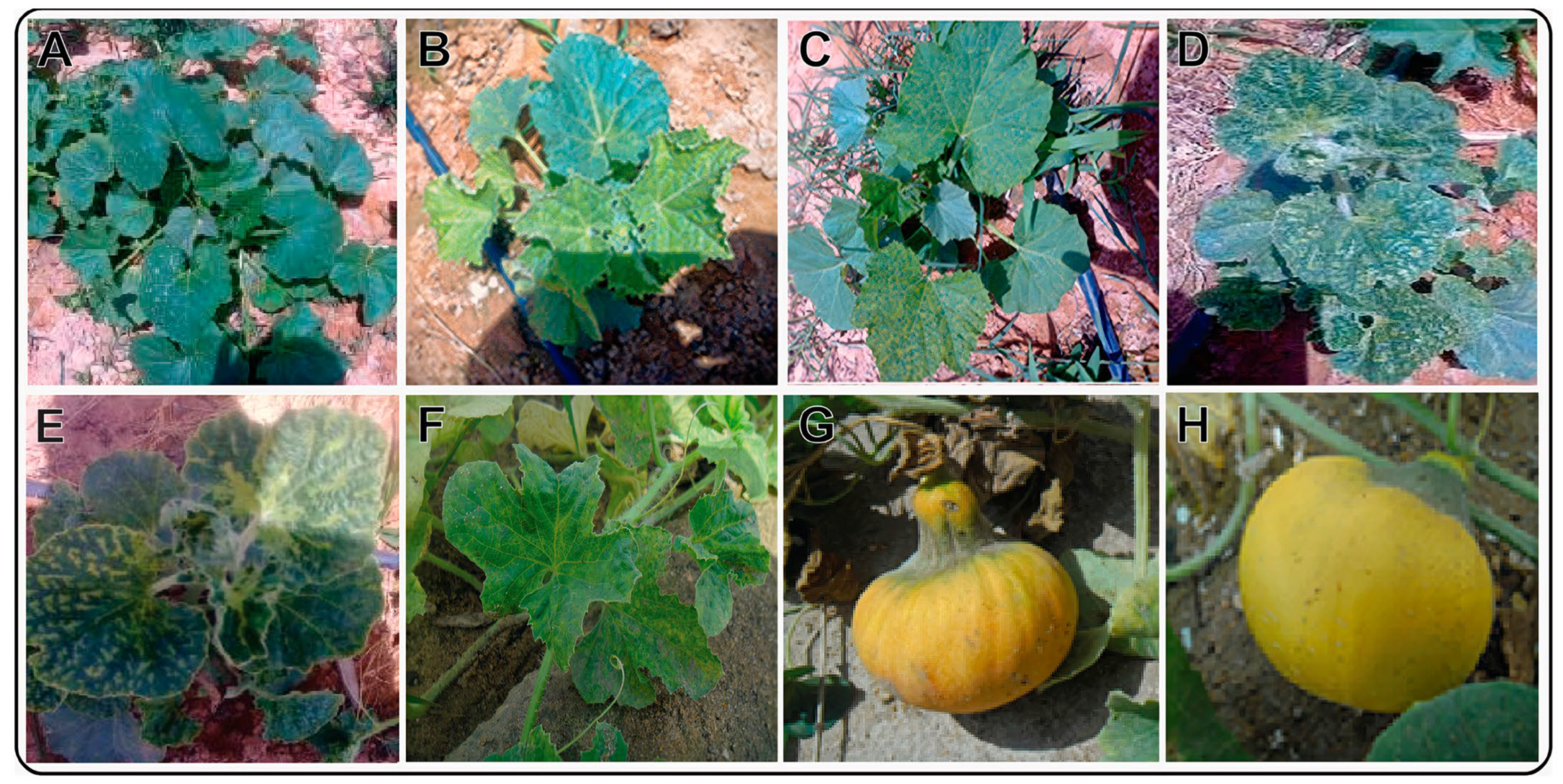
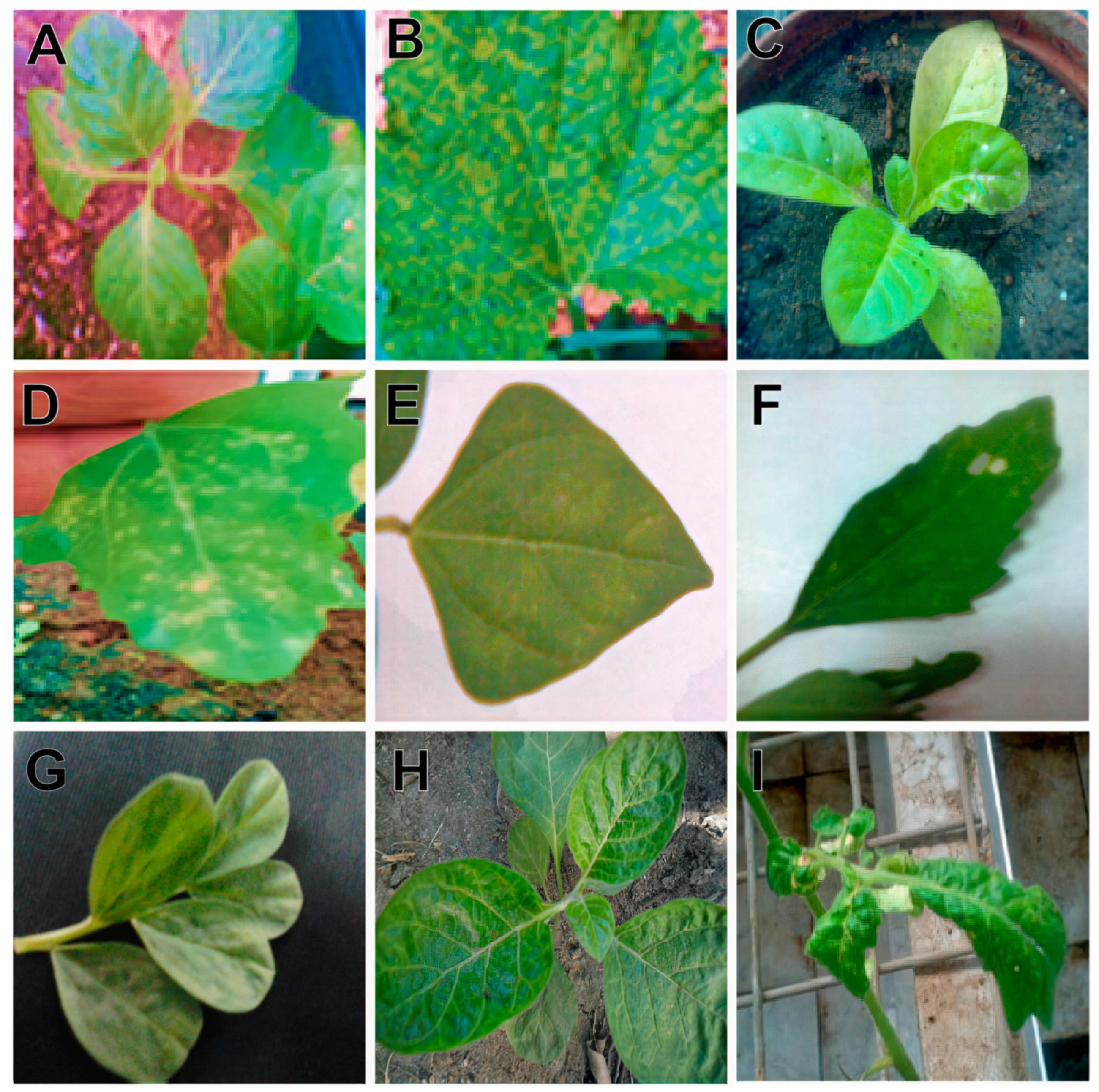
| Host plant | Phenotype * | Incubation Period (Days Post-Inoculation) | Optical Density (405 nm) | |
|---|---|---|---|---|
| Family | Species (Common Name) | |||
| Chenopodiaceae | Chenopodium amaranticolor (goosefoot) | CLL|NLL | 4 | +0.423 |
| C. quinoa (quinoa) | CLL | 5 | +0.393 | |
| Solanaceae | Nicotiana glutinosa (Peruvian tobacco) | SM | 15 | +0.591 |
| N. benthemiana (tobacco) | CR|MTM | 17 | +0.734 | |
| N. clevlandii (Cleveland’s tobacco) | SCR|M | 15 | +0.531 | |
| Solanum lycopersum (tomato) | LC | 05 | +0.417 | |
| Datura metel (thorn apple) | Chl | 16 | +0.484 | |
| Petunia hybrida (petunia) | LM|CB | 12 | +0.611 | |
| N. glauca (tree tobacco) | SLC | 15 | +0.585 | |
| Solanum melongena (brinjal) | LM | 15 | +0.743 | |
| Piper nigrum (pepper) | LM | 17 | +0.708 | |
| Leguminosae | Phaseolus vulgaris (common bean) | SM|LP | 15 | +0.593 |
| Vigna unguiculata (cowpeas) | NLL | 05 | +0.611 | |
| Vicia faba (broad bean) | VC|CH | 21 | +0.972 | |
| Cucurbitaceae | Cucumus sativus (cucumber) | SCR | 14 | +0.491 |
| Amarantheaceae | Gomphrena globosa (globe amaranth) | Chl | 9 | +0.235 |
| Compositae | Zinnia elegans (zinnia) | NS | - | −0.083 |
| No. of Cultivated Seeds | No. of Seeds without CMV Detection | No. of Seeds With CMV | Transmission% |
|---|---|---|---|
| 55 | 46 | 9 | 16.3% |
| Anatomical Attribute | Healthy Plants (µm) | Infected Plants (µm) | % Change |
|---|---|---|---|
| Thickness of the leaflet blade | 773.189 | 613.616 | −20.64 |
| Thickness of the upper epidermal layer | 17.381 | 18.102 | +4.15 |
| Thickness of the lower epidermal layer | 5.278 | 9.790 | +85.48 |
| Thickness of the palisade tissue | 28.276 | 29.196 | +3.25 |
| Thickness of the spongy tissue | 79.777 | 80.110 | +0.42 |
| Thickness of the midrib zone | 837.220 | 591.427 | −29.36 |
| Length of the protoxylem vessel | 312.112 | 210.021 | −32.71 |
| Length of the metaxylem vessel | 250.100 | 314.201 | +25.63 |
| Name of Attribute | Healthy Plants | CMV-Infected Plants | Relative Change (%) |
|---|---|---|---|
| Plant height (m²) | 3.57 a | 2.76 b | −22.68 |
| Leaf area (cm2) | 98.9 | 54.6 | −44.79 |
| Number of fruits | 12.3 a | 7.2 b | −41.46 |
| Fruit Wight (g) | 2.8 a | 0.95 b | −66.07 |
| Fruit size (cm³) | 297 a | 136 b | −54.20 |
| Sugar content (mg·g−1) | 5.5 | 3.4 | −38.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, W.F.; Iqbal, Z.; Abdelbaset, T.E.; Saker, K.I.; El Shorbagy, A.E.; Soliman, A.M.; Sattar, M.N.; El-Ganainy, S.M. Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants. Sustainability 2023, 15, 9751. https://doi.org/10.3390/su15129751
Shehata WF, Iqbal Z, Abdelbaset TE, Saker KI, El Shorbagy AE, Soliman AM, Sattar MN, El-Ganainy SM. Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants. Sustainability. 2023; 15(12):9751. https://doi.org/10.3390/su15129751
Chicago/Turabian StyleShehata, Wael Fathy, Zafar Iqbal, Tarek Elsayed Abdelbaset, Khalied Ibrahiem Saker, Ahmed Elnabawy El Shorbagy, Ahmed Mohamed Soliman, Muhammad Naeem Sattar, and Sherif Mohamed El-Ganainy. 2023. "Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants" Sustainability 15, no. 12: 9751. https://doi.org/10.3390/su15129751
APA StyleShehata, W. F., Iqbal, Z., Abdelbaset, T. E., Saker, K. I., El Shorbagy, A. E., Soliman, A. M., Sattar, M. N., & El-Ganainy, S. M. (2023). Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants. Sustainability, 15(12), 9751. https://doi.org/10.3390/su15129751











