Abstract
Doubly Uniparental Inheritance (DUI) is considered one of the major exceptions to the common rules of eukaryotic cell biology and germline formation. DUI is known in bivalves, which belong to the phylum Mollusca; conversely, no DUI evidence was found in some gastropod species. Investigating the presence of DUI in Nemertea is of particular interest given the fact that these spiralian animals are suggested to be a sister group of Neotrochozoa (i.e., Mollusca and Annelida). DUI species are normally detected as having two sex-associated mtDNAs, which can be highly divergent. In this work, the presence of sex-associated mitochondrial genomes was investigated in the nemertean species Notospermus geniculatus and no evidence was found for DUI. Even if these are preliminary results, negative evidence is still interesting because of the high importance of the DUI phenomenon in many research fields, where it plays a pivotal role in understanding eukaryotic evolution. For this reason, further research on DUI species detection should be highly encouraged, as well as the publication of negative results beside positive ones, as is the case for the present study, improving the knowledge on the biology and ecology of a broad spectrum of marine species.
1. Introduction
Marine biodiversity is extensively declining due to human pressures and impacts [1]; however, understanding the extension and consequences of these multiple impacts is difficult since we still have limited scientific knowledge regarding some marine taxa [1]. Therefore, adding to the knowledge on the biology and ecology of a broad spectrum of marine species is of primary importance to investigate their extraordinary biodiversity and to understand their complex evolutionary patterns before it is too late.
The phylum Nemertea comprises roughly 1300 species also known as ribbon worms, nemertini, or rhyncocoeles [2,3,4,5,6]. Nemerteans usually inhabit salt waters and are bilaterian, triblastic, and coelomatic animals with an unsegmented worm-like body that is often extensible and dorso-ventrally flattened. They can span from a few millimetres in length to several meters [2]. Lineus longissimus (Gunnerus, 1770) is a well-known representative of the phylum as it is considered to be the longest animal in the world [3], reaching 60 m in length [4]. The phylum Nemertea is further divided in two superclasses: Pronemertea, which includes “unarmed” species that have a proboscis without an apical stylet and are not morphologically specialised in three regions; Neonemertea, in which most species have a proboscis with an apical stylet and a mouth separated or fused to the proboscis pore [5]. Two classes have been described inside Neonemertea: Hoplonemertea and Pilidiophora. Notospermus geniculatus (Delle Chiaje, 1822 Order Heteronemertea, Family Lineidae) belongs to the latter with several synonymised names [6].
The phylogenetic position of the relatively small phylum Nemertea makes it very interesting for comparative study. Nemerteans are spiralian animals and are regarded as strictly related with a large group comprising Brachiopoda, Phoronida, Annelida, and Mollusca [7,8,9,10,11]. Specifically, Nemertea was suggested to be the sister group of the remaining Eutrochozoa termed Neotrochozoa: Mollusca and Annelida [12]. The bewildering biodiversity of these phyla spans over countless morphological traits, adaptations, niche occupancies, natural oddities, and uncommon phenomena. For example, these two phyla are among the few animal phyla that adapted to terrestrial life; gastropods of the species Elysia chlorotica (Gould, 1870) maintain symbiosis with plastids stolen from the alga Vaucheria litorea Hofman ex. C. Agardh [13]; the fundamental metameric architecture has been lost at least twice among annelids [10,14,15,16,17].
One of the major exceptions to the common rules of eukaryotic cell biology, early development, and germline formation is mitochondrial DNA heteroplasmy, a phenomenon in which both maternal and paternal mtDNAs are transmitted to the offspring. This happens in several taxa, from arthropods [18,19] to vertebrates [20,21], and sometimes it is related to pathological manifestation [22]. However, the most outstanding example is from the phylum Mollusca and, specifically, from bivalves: it is called Doubly Uniparental Inheritance (DUI) [23,24,25,26]. In species with DUI, two mitochondrial lineages are maintained: the female (F) lineage being carried by eggs and the male (M) lineage being carried by sperm. After fertilisation, all embryos are heteroplasmic since both lineages are found in the zygote. In female embryos, dispersion and degradation of M mitochondria occurs, whereas in male embryos they are preserved together in the primordial male germline [24,25,26,27,28,29,30,31]. Therefore, adult females are generally homoplasmic for F mitochondria, while the F lineage dominates in the adult male soma but not for the male germline, which is homoplasmic for M mitochondria [32,33,34,35,36,37,38]. Nonetheless, many patterns of F and M lineage distribution are known from different DUI species [30,39]).
Obviously, DUI involves a complex crosstalk between the nuclear and the mitochondrial genome (mtDNA); in these species, mitochondrial inheritance is associated with sex determination and reproductive strategy [27,28]. However, the DUI distribution among bivalves does not appear to have a precise phylogenetic meaning [40]. Recently, even a hermaphroditic species was shown to have sex-associated mitochondrial lineages [41]; multiple origins of DUI are currently the most supported scenario [29,42,43,44]. Indeed, the discovery of small non-coding RNAs transcribed from the mitochondrial genome that regulate nuclear gene expression may explain the common evolutionary switch between DUI and strict maternal inheritance across bivalves [45]. Therefore, a better characterisation of the distribution of this complex phenomenon is crucial to achieve a proper understanding of its evolution, which, in turn, would entail stimulating insights into the evolution of mitochondrial inheritance, sex determination, genomic conflicts, and eukaryogenesis.
Moreover, it is important to determine whether DUI is present only among bivalves or not. DUI species are normally detected through the evidence of two sex-associated mtDNAs, which can be highly divergent [40,46]. Using the same approach, no evidence has been found for DUI in the handful of gastropod species that have been explored [47,48]. Unfortunately, negative results are seldom published; typically, they are provided along with positive results in other species [39,43]. However, even negative results are highly valuable for achieving effective characterisation of DUI distribution. In the present study, we investigated the presence of sex-associated mitochondrial genomes in a nemertean species, Notospermus geniculatus. In fact, the invention as well as the absence of DUI in nemerteans, which form the sister group of the two neotrochozoan phyla Annelida and Mollusca, does constitute a key piece of information towards the understanding of DUI evolution in marine invertebrates.
2. Materials and Methods
2.1. Sample Collection, Preparation, and Conservation
Six Notospermus geniculatus specimens were kindly provided by Mayuko Hamada and Tatsuya Sakamoto of the Okayama University. All animals were sampled in June 2018 near Ushimado, Japan and kept in a 1:1 mixture of sea water and dihydrated MgCl2 7% for 15′ until dissection in dihydrated MgCl2 7% in an ice bath. Male and female gonads were excised and then stored at −80 °C.
2.2. DNA Extraction and Purification
DNA was extracted using the commercial QIAGEN Blood & Cell Culture DNA Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. Samples were incubated for 5 h at 50 °C to improve RNAse and protease activities. Total genomic DNA was precipitated with isopropanol and then stored at −20 °C in 200 μL ethanol 70°.
2.3. Amplification and Sequencing
PCR amplifications were carried out from DNA extracted from female and male gonads in an Applied Biosystems Thermal Cycler 2720 (ThermoFisher Scientific, Waltham, MA, USA) using the GoTaq® Flexi DNA Polymerase kit (Promega, Madison, WI, USA), with a final volume of 30 μL, following manufacturer’s instructions. For each sample, the reaction mixture was as follows: 6 μL 5× Green GoTaq® Flexi Buffer, MgCl2 (3 mM), nucleotides (800 μM each), primers (500 nM each), 0.75 U Go-Taq DNA Polymerase, and 2–5 μL template DNA. Three loci were selected for PCR amplification, Cytochrome Oxidase I (cox1) and two ribosomal RNA genes, 16 S rRNA (rrnL) and 12 S rRNA (rrnS), using the primers reported in Table 1.

Table 1.
Primers used for PCR amplifications. Target loci, primer names with relative forward (F) or reverse (R) employment, 5′-3′sequence, primer length (bp), and related references are reported.
Different cycling conditions were used for each of the three amplified loci. The primer pairs COX1F674/COX1R1420 and RRNLF189/RRNLR917 were used in a program based on a first denaturation step for 2′ at 94 °C followed by 35 cycles of denaturation for 1′ at 94 °C, annealing for 1′ at 48 °C, and extension for 1′ at 72 °C; a final extension step for 5′ at 72 °C was performed. All the other primer pairs were used with a touchdown approach. The touchdown PCR program was designed as follows: 95°C for 2′, then 21 cycles at 95 °C for 1′, annealing at a decreasing temperature from 52 °C to 42 °C for 1′, and 72 °C for 1′; then, we used another denaturation step at 95 °C for 1′, followed by 20 cycles at 95 °C for 1′, 44 °C for 1′, and 72 °C for 1′, with a final extension step for 5′ at 72 °C.
All amplification products were checked on 1% agarose gel and run in 1x TAE. Amplified products were then purified with the commercial kit Wizard® SV Gel and PCR (Promega, Madison, WI, USA), and Sanger sequencing reactions were outsourced at the Macrogen Inc. facility (World Meridian Center, Seoul, Republic of Korea).
2.4. Data Analysis
Electropherograms obtained through Sanger sequencing procedures were manually handled with the software MEGA11 (Molecular Evolutionary Genetics Analysis Version 11 [51]) to underline differences among sequences, especially highlighting differences found between males and females. Three alignments—one for each gene—were produced, aligning the sequences from all the analysed samples. Similarity analysis was performed on an alignment made by concatenating the three gene-specific alignments [52] and obtaining an alignment of six sequences, three from males and three from females. The relationships among these sequences were inferred with the software MEGA11 using the Neighbour-Joining method [53], as in Soroka and Burzynski [52], estimating standard error with a bootstrap procedure (1000 replicates).
3. Results
The primer pair COX1_F674/COX1_R1420 allowed the amplification of a 702 bp fragment of the cox1 gene for both male and female gonad samples. The primer pair C1-J1709/C1-N2776 allowed the amplification of a 1025 bp fragment only in female gonad samples. The two fragments were overlapped, resulting in a 1123 bp long sequence for female samples. Sequences were registered in GenBank with the following accession numbers: OQ880564 (F1–F2), OQ880565 (F3), and OQ880563 (M1–M2–M3). Four variable sites were detected, as reported in Table 2.
The primer pair RRNL_F189/RRNL_R917 allowed the amplification of a 534 bp fragment of the rrnL gene in all the samples, whereas the primer pair SbrH(32)/Sar(34) allowed the amplification of a 423 bp fragment only in female samples. The overlapping of the obtained fragments allowed the analysis of a 956 bp fragment in females and of a 530 bp fragment in males, which showed four variable sites overall, as reported in Table 2. Sequences were registered in GenBank with the following accession numbers: OQ891216 (F1–F2), OQ891217 (F3), OQ891218 (M1–M2), and OQ891219 (M3).
The primer pair SR-J14197/SR-N14745 allowed the amplification of a 557 bp fragment of the gene rrnS in females and of a 428 bp fragment in males. Only two mutations were found, as reported in Table 2. Mutations detected in the obtained sequences are as follows: at position 326, M2 shows a G, whereas in all other individuals there is an A; at position 536, M1 shows an A, whereas in all other males there is a G (females were not amplified in that fragment). Sequences were registered in GenBank with the following accession numbers: OQ909092 (F1–F2–F3), OQ880553 (M1), OQ880554 (M2), and OQ880555(M3).

Table 2.
Variable sites in Notospermus geniculatus cox1, rrnL, and rrnS analysed fragments. Nucleotide numbering follows GenBank entries.
Table 2.
Variable sites in Notospermus geniculatus cox1, rrnL, and rrnS analysed fragments. Nucleotide numbering follows GenBank entries.
| Gene | cox1 | rrnL | rrnS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specimens’ Positions | 327 | 862 | 946 | 1050 | 384 | 459 | 725 | 951 | 326 | 536 |
| F1 | A | C | K | C | T | G | C | A | A | - |
| F2 | A | C | K | C | T | G | C | A | A | - |
| F3 | G | C | T | T | G | G | T | C | A | - |
| M1 | - | T | T | T | T | G | - | - | A | A |
| M2 | - | T | T | T | T | G | - | - | G | G |
| M3 | - | T | T | T | T | T | - | - | A | G |
Figure 1 shows the results of the Neighbour-Joining analysis. Indeed, the tree shows two main branches that do not exhibit a sex-related segregation of analysed samples.
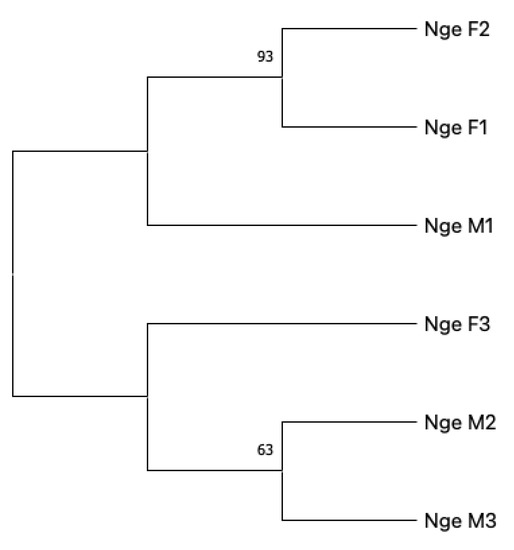
Figure 1.
Neighbour-Joining tree of the six samples analysed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches [54]. The evolutionary distances were computed using the Jukes–Cantor method [55]. Included codon positions were 1st + 2nd + 3rd + Non-coding.
4. Discussion
Three mitochondrial regions (namely, cox1, rrnS, and rrnL) were selected to test the presence of intraspecific, sex-associated female and male haplotypes in Notospermus geniculatus. Male and females mitogenomes, retrieved from homoplasmic regions of the nemertean bodies, were successfully amplified, demonstrating the efficacy of the primers used to detect the mtDNA. To limit the risk that, in the cases of high divergence, universal primers may not be universal enough to pick up the second genome at all, six out of the twelve used primers were specifically designed for this research and are not universal primers, as detailed in Table 1. The presence of sex-linked heteroplasmy in N. geniculatus could be excluded, at least according to the data gathered. In fact, just a single mutation in the cox1 gene (C/T in position 862) differentiates female and male gonad samples (Table 2). Furthermore, the risk of the contemporary presence of two different mitogenomes within the same amplification was ruled out upon electropherogram analyses; in fact, all electropherograms showed clear and not superimposed base calls. Moreover, contaminations from other biological entities can also be reasonably ruled out as DNA was extracted from fresh frozen tissue, which gives high yields of good-quality DNA, and this was carried out with particular attention so as to prevent not just exogenous but also, and most importantly, cross-sample contaminations. Therefore, also considering the relatively low number of species within Nemertea, it is tempting to rule out the occurrence of DUI in nemerteans. DUI has been searched outside the class Bivalvia before—for example, in gastropods—but with negative results [47,48]. The survey of gastropods is important since these animals are phylogenetically close to bivalves; similarly, the survey of nemerteans is important since these animals are phylogenetically close to molluscs. The limited number of negative publications on non-DUI species stems from the fact that negative results are usually not considered a self-standing publication and are typically provided together with positive DUI findings in other species [39,43]. However, caution is necessary before drawing definitive conclusions. Using sex-linked heteroplasmy is a very effective way to detect DUI species; however, this method is also well known to be prone to false-negative results [39,56]. The DUI system involves the establishment of two separate mitochondrial lineages that are transmitted to offspring through eggs and sperm, respectively; however, this may not imply an obvious sex-linked heteroplasmy.
For instance, it may happen that the two mitochondrial genomes are very similar and do not accumulate discriminating mutations. This may happen, for example, if the onset of DUI in the species is very recent. In fact, the hypothesis of multiple origins of DUI is supported by manifold lines of evidence [29,42,43,44], including the fact that DUI systems in different species exhibit different features [29,43]. For instance, some mitochondrial genes seem to be involved in DUI-associated signalling but with variable, somewhat opposite roles: the cox2 gene is duplicated in the clam Ruditapes philippinarum (Adams and Reeve, 1850) female mitochondrial genome but also in the male genome of the mussel Arcuatula senhousia (W. H. Benson, 1842) [57]; a male-specific 3′ extension is found in DUI species in the family Unionidae [58,59], while a male-specific, 100-amino-acid-long insertion was found in the clam Meretrix lamarckii (Deshayes, 1853) [46]. In a scenario where DUI had multiple origins, it would not be impossible to cope with a species that only recently switched to DUI, with the two mtDNAs, therefore, still being relatively similar.
Another phenomenon is known that has the potential to reset the divergence between the two genomes: masculinisation of the female mtDNA. This phenomenon is well known in DUI species of the genus Mytilus (see [29,30]): the F mtDNA can enter the male germline and then replace the expected M mtDNA, which leads to no divergence between the female mitochondrial genome and the newly masculinised one in the beginning; the two mtDNAs will then diverge again, being separate from then on. Notably, the masculinisation phenomenon was never directly observed outside Mytilidae [31].
Conversely, it may happen that they are too divergent: PCR primers would fail to amplify either, and traces of the other one may prove sufficient and be amplified. Indeed, some DUI species exhibit very high divergence between F- and M-mtDNA [48]. Moreover, in bivalves, the gonad is basically a sac filled with fluid and gametes, and harvesting the gametic fluid is close to an actual extraction of either female or male gametes. Conversely, in nemerteans, the gonads are constituted by a denser tissue that resides laterally to the feeding tube, producing either oogones or spermatogones. Therefore, gonad dissection in nemerteans does not isolate the gametes, inevitably leaving much more leakage of somatic cells. In the case of high divergence between F and M mitochondrial lineages, the F-mtDNA would be present in a significant amount (and not as a leakage of somatic tissues among maturating gametes) and would effectively compete with the M-mtDNA; it is worth remembering that PCR primers are often designed on somatic tissues—as such, in a DUI species they are normally optimised for the F mitochondrial lineage, which is expected to dominate in the soma. However, it is worth noting that multiple primer pairs have been used for the present study, both universal and specific.
To our knowledge, the only species for which DUI was excluded following the actual observation of mitochondrial movements after fertilisation is the Pacific oyster, Crassostrea gigas (Thunberg, 1793) or Magallana gigas sensu (Salvi and Mariottini, 2017) [60,61]. Nonetheless, and notwithstanding the drawbacks of the detection method based on sex-linked heteroplasmy, the species N. geniculatus analysed herein should be conservatively considered as a species with the common strict maternal inheritance of mitochondria, as depicted by the NJ tree produced. The NJ tree shows two main branches: one clade contains two females (F1, F2) and one male (M1); the other contains two males (M2, M3) and one female (F3). This partition would not be expected in the presence of DUI.
This would provide a further clue towards the restriction of DUI within bivalves, and it would be very stimulating to understand whether DUI is present among bivalves only for some structural/genomic reason or for a mere historical contingency. Recently, smithRNAs were described as promising players in the mitonuclear crosstalk [45]. If smithRNAs are to be found among bivalves only for some genomic features of their mtDNAs, and if they are in some way necessary for DUI, this would explain the distribution of DUI in a single molluscan class. However, smithRNAs have been suggested throughout the animal diversity [42] and are not a bivalve mitochondrial oddity. Therefore, it is tempting to conclude that the smithRNA machinery has been (at least partly) co-opted (or newly evolved) in bivalves to regulate DUI, but this may have taken place in other phyla as well, and possibly along with other molecular mechanisms.
5. Conclusions
To conclude, we suggest retaining the DUI phenomenon as restricted to many bivalves at present. However, the detection of DUI is hampered by many factors and is particularly prone to false-negatives. Since the DUI phenomenon may prove highly interesting in many research fields (mitochondrial inheritance, sex determination, genomic conflicts, retrograde signalling, mitonuclear crosstalk, and genome evolution), the investigation on DUI distribution plays a pivotal role to understand eukaryotic evolution: further research on DUI species detection should be highly encouraged, as well as the publication of negative results beside positive ones, as is the case for the present study.
Moreover, all data will improve our understanding of complex evolutionary patters of marine species; therefore, the investigation of understudied marine taxa should also be encouraged.
It is important to underline that the results here presented should be considered as preliminary, since the proxy of sex-linked heteroplasmy has intrinsic drawbacks, and only the observation of organellar movements can rule out the presence of DUI in each species. Nonetheless, as reported by related literature, most DUI species were detected on the basis of sex-linked heteroplasmy and no ultra-structural observations have been carried out. For these reasons, the authors feel it is important to publish negative results so that scholars throughout the world can avoid repeating similar experiments on the same species and, if interested, know that microscopy may be needed to unravel the issue among nemertean worms.
Author Contributions
Conceptualization, F.P. and M.P.; methodology, F.P.; software, L.L.; validation, F.P., L.L. and M.P.; formal analysis, D.S.; investigation, D.S. and L.L.; resources, M.P.; data curation, D.S., L.B., L.L. and F.P.; writing—original draft preparation, D.S., L.B., L.L., F.P. and S.C.; writing—review and editing, L.B., L.L., F.P. and S.C.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Canziani Bequest, grant number “CANZIANI PASSAMONTI” funded to M.P. by University of Bologna.
Institutional Review Board Statement
Ethical review and approval were waived for this study since no ethical issues are raised by species of the phylum Nemertea, no specific permits were required, and all sampling was carried out in compliance with the relevant general guidelines. Sampling localities were not privately owned or protected in any way.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Mayuko Hamada and Tatsuya Sakamoto of the Okayama University for their fundamental support in the field sampling of specimens of Notospermus geniculatus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tosetto, L.; McNab, J.M.; Hutchings, P.A.; Rodríguez, J.; Williamson, J.E. Fantastic Flatworms and Where to Find Them: Insights into Intertidal Polyclad Flatworm Distribution in Southeastern Australian Boulder Beaches. Diversity 2023, 15, 393. [Google Scholar] [CrossRef]
- Brusca, R.C.; Moore, W.; Shuster, F.M. Invertebrates, 3rd ed.; Sinauer Associates: Oxford, UK, 2016; pp. 435–452. [Google Scholar]
- Carwardine, M. The Guinness Book of Animal Records, 1st ed.; Guinness Publishing: Milan, Italy, 1995. [Google Scholar]
- Moen, F.E.; Svensen, E. Marine Fish & Invertebrates of Northern Europe; Kom: Kristiansund, Norway, 2004. [Google Scholar]
- Chernyshev, A.V. CLSM analysis of phallodin-stained muscle system of the nemertean proboscis and rhynchocoel. Zoolog. Sci. 2015, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Norenburg, J.; Gibson, R.; Herrera Bachiller, A.; Strand, M. World Nemertea Data-base. Notospermus geniculatus (Delle Chiaje, 1828). Accessed through: World Register of Marine Species. 2019. Available online: http://www.marinespecies.org/aphia.php?p=tax-details&id=122586 (accessed on 12 May 2023).
- Helmkampf, M.; Bruchhaus, I.; Hausdorf, B. Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proc. Biol. Sci. 2008, 275, 1927–1933. [Google Scholar] [CrossRef]
- Struck, T.H.; Fisse, F. Phylogenetic Position of Nemertea Derived from Phylogenomic Data. Mol. Biol. Evol. 2008, 25, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Nesnidal, M.P.; Helmkampf, M.; Meyer, A.; Witek, A.; Bruchhaus, I.; Ebersberger, I.; Hankeln, T.; Lieb, B.; Struck, T.H.; Hausdorf, B. New phylogenomic data support the monophyly of Lophophorata and an Ectoproct-Phoronid clade and indicate that Polyzoa and Kryptrochozoa are caused by systematic bias. BMC Evol. Biol. 2013, 13, 253. [Google Scholar] [CrossRef]
- Weigert, A.; Helm, C.; Meyer, M.; Nickel, B.; Arendt, D.; Hausdorf, B.; Santos, S.R.; Halanych, K.M.; Purschke, G.; Bleidorn, C.; et al. Illuminating the Base of the Annelid Tree Using Transcriptomics. Mol. Biol. Evol. 2014, 31, 1391–1401. [Google Scholar] [CrossRef]
- Kocot, K.M.; Struck, T.H.; Merkel, J.; Waits, D.S.; Todt, C.; Brannock, P.M.; Weese, D.A.; Cannon, J.T.; Moroz, L.L.; Lieb, B.; et al. Phylogenomics of Lophotrochozoa with Consideration of Systematic Error. Syst. Biol. 2016, 66, 256–282. [Google Scholar] [CrossRef]
- Peterson, K.J.; Eernisse, D.J. Animal phylogeny and the ancestry of bilaterians: Inferences from morphology and 18s rDNA gene sequences. Evol. Dev. 2001, 3, 170–205. [Google Scholar] [CrossRef]
- Chan, C.X.; Vaysberg, P.; Price, D.C.; Pelletreau, K.N.; Rumpho, M.E.; Bhattacharya, D. Active Host Response to Algal Symbionts in the Sea Slug Elysia chlorotica. Mol. Biol. Evol. 2018, 35, 1706–1711. [Google Scholar] [CrossRef]
- Struck, T.H.; Schult, N.; Kusen, T.; Hickman, E.; Bleidorn, C.; McHugh, D.; Halanych, K.M. Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evol. Biol. 2007, 7, 57. [Google Scholar] [CrossRef]
- Shen, X.; Ma, X.; Ren, J.; Zhao, F. A close phylogenetic relationship between Sipuncula and Annelida evidenced from the complete mitochondrial genome sequence of Phascolosoma esculenta. BMC Genom. 2009, 10, 136. [Google Scholar] [CrossRef]
- Zrzavý, J.; Říha, P.; Piálek, L.; Janouškovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-evidence analysis of morphology and six genes. BMC Evol. Biol. 2009, 9, 189. [Google Scholar] [CrossRef]
- Struck, T.H.; Paul, C.; Hill, N.; Hartmann, S.; Hösel, C.; Kube, M.; Lieb, B.; Meyer, A.; Tiedemann, R.; Purschke, G.; et al. Phylogenomic analyses unravel annelid evolution. Nature 2011, 471, 95–98. [Google Scholar] [CrossRef]
- Davies, O.K.; Dorey, J.B.; Stevens, M.I.; Gardner, M.G.; Bradford, T.M.; Schwarz, M.P. Unparalleled mitochondrial heteroplasmy and Wolbachia co-infection in the non-model bee, Amphylaeus morosus. Curr. Res. Insect Sci. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, C.; Raborn, R.T.; Lin, M.; Wei, W.; Hao, Y.; Lynch, M. Genetic Diversity, Heteroplasmy, and Recombination in Mitochondrial Genomes of Daphnia pulex, Daphnia pulicaria, and Daphnia obtusa. Mol. Biol. Evol. 2022, 39, msac059. [Google Scholar] [CrossRef]
- Radojičic, J.M.; Krizmanić, I.; Kasapidis, P.; Zouros, E. Extensive mitochondrial heteroplasmy in hybrid water frog (Pelophylax spp.) populations from Southeast Europe. Ecol. Evol. 2015, 5, 4529. [Google Scholar]
- Pizzirani, C.; Viola, P.; Gabbianelli, F.; Fagotti, A.; Simoncelli, F.; Di Rosa, I.; Salvi, P.; Amici, A.; Lucentini, L. First evidence of heteroplasmy in Grey Partridge (Perdix perdix). Avian Res. 2020, 11, 27. [Google Scholar] [CrossRef]
- Parakatselaki, M.E.; Ladoukakis, E.D. mtDNA Heteroplasmy: Origin, Detection, Significance, and Evolutionary Consequences. Life 2021, 11, 633. [Google Scholar] [CrossRef]
- Skibinski, D.O.F.; Gallagher, C.; Beynon, C.M. Mitochondrial DNA inheritance. Nature 1994, 368, 817–818. [Google Scholar] [CrossRef]
- Skibinski, D.O.F.; Gallagher, C.; Beynon, C.M. Sex-limited mitochondrial DNA transmission in the marine mussel Mytilus edulis. Genetics 1994, 138, 801–809. [Google Scholar] [CrossRef]
- Zouros, E.; Oberhauser Ball, A.; Saavedra, C.; Freeman, K.R. An unusual type of mitochondrial DNA inheritance in the blue mussel Mytilus. Proc. Natl. Acad. Sci. USA 1994, 91, 7463–7467. [Google Scholar] [CrossRef]
- Zouros, E.; Oberhauser Ball, A.; Saavedra, C.; Freeman, K.R. Mitochondrial DNA inheritance. Nature 1994, 368, 818. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Beaupré, H.D.; Stewart, D.T.; Hoeh, W.R.; Blier, P.U. The unusual system of doubly uniparental inheritance of mtDNA: Isn’t one enough? Trends Genet. 2007, 23, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, M.; Ghiselli, F. Doubly Uniparental Inheritance: Two mitochondrial genomes, one precious model for organelle DNA inheritance and evolution. DNA Cell Biol. 2009, 28, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zouros, E. Biparental inheritance through uniparental transmission: The doubly uniparental inheritance (DUI) of mitochondrial DNA. Evol. Biol. 2013, 40, 1–31. [Google Scholar] [CrossRef]
- Zouros, E.; Rodakis, G.C. Doubly Uniparental Inheritance of mtDNA: An Unappreciated Defiance of a General Rule. Adv. Anat. Embryol. Cell Biol. 2019, 231, 25–49. [Google Scholar]
- Wang, R.; Li, X.; Qi, J. The complete paternally inherited mitochondrial genomes of three clam species in genus Macridiscus (Bivalvia: Veneridae): A TDRL model of dimer-mitogenome rearrangement of doubly uniparental inheritance. Front. Mar. Sci. 2022, 9, 1016779. [Google Scholar] [CrossRef]
- Garrido-Ramos, M.A.; Stewart, D.T.; Sutherland, B.W.; Zouros, E. The distribution of male-transmitted and female-transmitted mitochondrial DNA types in somatic tissues of blue mussels: Implications for the operation of doubly uniparental inheritance of mitochondrial DNA. Genome 1998, 41, 818–824. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Walker, J.M.; Chapman, E.G.; Shepardson, S.P.; Trdan, R.J.; Curole, J.P.; Watters, G.T.; Stewart, D.T.; Vijayaraghavan, S.; Hoeh, W.R. Reproductive Function for a C-terminus Extended, Male-Transmitted Cytochrome c Oxidase Subunit II Protein Expressed in Both Spermatozoa and Eggs. FEBS Lett. 2007, 581, 5213–5219. [Google Scholar] [CrossRef]
- Kyriakou, E.; Zouros, E.; Rodakis, G.C. The atypical presence of the paternal mitochondrial DNA in somatic tissues of male and female individuals of the blue mussel species Mytilus galloprovincialis. BMC Res. Notes 2010, 3, 222. [Google Scholar] [CrossRef]
- Batista, F.M.; Lallias, D.; Taris, N.; Guerdes-Pinto, H.; Beaumont, A.R. Relative quantification of the M and F mitochondrial DNA types in the blue mussel Mytilus edulis by real-time PCR. J. Molluscan Stud. 2011, 77, 24–29. [Google Scholar] [CrossRef]
- Ghiselli, F.; Milani, L.; Passamonti, M. Strict sex-specific mtDNA segregation in the germ line of the DUI species Venerupis philippinarum (Bivalvia: Veneridae). Mol. Biol. Evol. 2011, 28, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Obata, M.; Sano, N.; Komaru, A. Different transcriptional ratios of male and female transmitted mitochondrial DNA and tissue-specific expression patterns in the blue mussel, Mytilus galloprovincialis. Dev. Growth Diff. 2011, 53, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Brannock, P.M.; Roberts, M.A.; Hilbish, T.J. Ubiquitous heteroplasmy in Mytilus spp. resulting from disruption in doubly uniparental inheritance regulation. Mar. Ecol. Prog. Ser. 2013, 480, 131–143. [Google Scholar] [CrossRef]
- Lucentini, L.; Plazzi, F.; Sfriso, A.A.; Pizzirani, C.; Sfriso, A.; Chiesa, S. Additional taxonomic coverage of the doubly uniparental inheritance in bivalves: Evidence of sex-linked heteroplasmy in the razor clam Solen marginatus Pulteney, 1799, but not in the lagoon cockle Cerastoderma glaucum (Bruguière, 1789). J. Zool. Syst. Evol. Res. 2020, 58, 561–570. [Google Scholar] [CrossRef]
- Gusman, A.; Lecomte, S.; Stewart, D.T.; Passamonti, M.; Breton, S. Pursuing the quest for better understanding the taxonomic distribution of the system of doubly uniparental inheritance of mtDNA. PeerJ 2016, 4, e2760. [Google Scholar] [CrossRef] [PubMed]
- Lubośny, M.; Przyłucka, A.; Śmietanka, B.; Burzyński, A. Semimytilus algosus: First known hermaphroditic mussel with doubly uniparental inheritance of mitochondrial DNA. Sci. Rep. 2020, 10, 11256. [Google Scholar] [CrossRef]
- Milani, L.; Ghiselli, F.; Guerra, D.; Breton, S.; Passamonti, M. A comparative analysis of mitochondrial ORFans: New clues on their origin and role in species with doubly uniparental inheritance of mitochondria. Genome Biol. Evol. 2013, 5, 1408–1434. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.T.; Breton, S.; Chase, E.E.; Robicheau, B.M.; Bettinazzi, S.; Pante, E.; Youssef, N.; Garrido-Ramos, M.A. An unusual evolutionary strategy: The origins, genetic repertoire, and implications of doubly uniparental inheritance of mitochondrial DNA in bivalves. In Evolutionary Biology—A Transdisciplinary Approach; Pontarotti, P., Ed.; Springer International Publishing: New York, NY, USA, 2020; pp. 301–323. [Google Scholar]
- Milani, L.; Ghiselli, F.; Passamonti, M. Mitochondrial selfish elements and the evolution of biological novelties. Curr. Zool. 2016, 62, 687–697. [Google Scholar] [CrossRef]
- Pozzi, A.; Plazzi, F.; Milani, L.; Ghiselli, F.; Passamonti, M. SmithRNAs: Could mitochondria “bend” nuclear regulation? Mol. Biol. Evol. 2017, 34, 1960–1973. [Google Scholar] [CrossRef]
- Bettinazzi, S.; Plazzi, F.; Passamonti, M. The Complete Female- and Male-Transmitted Mitochondrial Genome of Meretrix lamarckii. PLoS ONE 2016, 11, e0153631. [Google Scholar] [CrossRef]
- Parakatselaki, M.E.; Saavedra, C.; Ladoukakis, E.D. Searching for doubly uniparental inheritance of mtDNA in the apple snail Pomacea diffusa. Mitochondrial DNA Part A 2016, 27, 4000–4002. [Google Scholar] [CrossRef] [PubMed]
- Gusman, A.; Azuelos, C.; Breton, S. No evidence of sex-linked heteroplasmy or doubly-uniparental inheritance of mtDNA in five gastropod species. J. Molluscan Stud. 2017, 83, 119–122. [Google Scholar] [CrossRef]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, 1st ed.; University of Hawaii: Honolulu, HI, USA, 1996. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Burzyński, A. Doubly uniparental inheritance and highly divergent mitochondrial genomes of the freshwater mussel Unio tumidus (Bivalvia: Unionidae). Hydrobiologia 2018, 810, 239–254. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Theologidis, I.; Fodelianakis, S.; Gaspar, M.B.; Zouros, E. Doubly Uniparental Inheritance (DUI) of mitochondrial DNA in Donax trunculus (Bivalvia: Donacidae) and the problem of its sporadic detection in bivalvia. Evolution 2008, 62, 959–970. [Google Scholar] [CrossRef]
- Breton, S.; Milani, L.; Ghiselli, F.; Guerra, D.; Stewart, D.T.; Passamonti, M. A resourceful genome: Updating the functional repertoire and evolutionary role of animal mitochondrial DNAs. Trends Genet. 2014, 30, 555–564. [Google Scholar] [CrossRef]
- Curole, J.P.; Kocher, T.D. Ancient sex-specific extension of the cytochrome c oxidase II gene in bivalves and the fidelity of doubly-uniparental inheritance. Mol. Biol. Evol. 2002, 19, 1323–1328. [Google Scholar] [CrossRef]
- Breton, S.; Stewart, D.T.; Shepardson, S.; Trdan, R.J.; Bogan, A.E.; Chapman, E.G.; Ruminas, A.J.; Piontkivska, H.; Hoeh, W.R. Novel protein genes in animal mtDNA: A new sex determination system in freshwater mussels (Bivalvia: Unionoida)? Mol. Biol. Evol. 2011, 28, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Obata, M.; Shimizu, M.; Sano, N.; Komaru, A. Maternal inheritance of mitochondrial DNA (mtDNA) in the Pacific oyster (Crassostrea gigas): A preliminary study using mtDNA sequence analysis with evidence of random distribution of MitoTracker-stained sperm mitochondria in fertilized eggs. Zool. Sci. 2008, 25, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Salvi, D.; Mariottini, P. Molecular taxonomy in 2D: A novel ITS2 rRNA sequence-structure approach guides the description of the oysters’ subfamily Saccostreinae and the genus Magallana (Bivalvia: Ostreidae). Zool. J. Linn. Soc.-Lond. 2017, 179, 263–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).