Abstract
This review paper explores the potential of bioleaching as a sustainable alternative for recovering metals from solid matrices. With over 12 billion tons of solid waste annually worldwide, bioleaching provides a promising opportunity to extract metals from solid waste, avoiding harmful chemical processes. It explains bacterial and fungal bioleaching techniques that extract copper, gold, zinc, and other metals from solid matrices. Fungal bioleaching effectively extracts a wide range of valuable metals, including nickel, vanadium, aluminium, molybdenum, cobalt, iron, manganese, silver, platinum, and palladium. The review highlights different solid matrices with metal contents that have the potential to be recovered by bioleaching, presenting promising bioprocess alternatives to current industrially available technologies for metal recovery. The optimal conditions for bioleaching, including pH, temperature, agitation–aeration, and pulp density are also discussed. The review shows that bioleaching has the potential to play a crucial role in the transition to a more sustainable and circular economy by providing an efficient, cost-effective, and environmentally friendly method for metal recovery from solid matrices.
1. Introduction
The global market size of metals and their manufactured products was valued at $11.2 trillion in 2020, and it is projected to reach $18.5 trillion by 2030 [1]. This significant growth is driven by the rising demand from diverse end-use industries, such as healthcare, aviation, energy and power, electricity, and electronics [2]. Additionally, metals play a crucial role in the construction of buildings, structures, transportation networks (including bridges and railways), as well as industries like mechanical engineering, transportation, cosmetics, catalysis, and environmental cleanup [3].
An assured supply of metals is critical to economic abundance of countries and national defense. Currently, the United States and other developed nations must import metals, causing tensions between countries partly because supply chains are diminished due to the impact of the COVID-19 pandemic [4].
The market demands metals as sustainable raw materials with high recycled content to reduce the carbon footprint of the final product [5]. Countries such as the United States, Japan, Australia, and Canada support initiatives to secure material supply through diversification, development, and recycling. The agricultural, industrial, and domestic sectors are economically interested in recovering valuable minerals, processing them, and converting them into marketable products [3].
Metal recovery through recycling refers to reprocessing waste into new metal products to reduce greenhouse gas emission levels, conserve natural resources, and manage energy consumption. In Europe, metal recycling in household solid waste is integral to sustainable waste management [6]. Countries such as Canada, the United States, and the United Kingdom, among others, promote the recycling industry with waste collection systems, separation processes, and sorting [7]. Waste management is one of the critical steps to achieving sustainable development and a circular economy [8]. Governments are tightening restrictions on waste generation and opening new landfills, putting pressure on companies to produce more environmentally friendly and sustainable goods [9].
Several metal product manufacturing companies use recyclable and recycled materials to manufacture new metal products [7]. Studies by Moreau et al. show that metals used in renewable energy technologies retain their properties and can, in principle, be recycled. This fact offers more significant potential for a circular economy [10].
It is believed that there will be the availability of material to be recycled, as the recycling market was 217.0 billion dollars in 2020 with a projection of 368.7 billion dollars by 2030 [7], added to the global generation of waste such as electronic waste with values of 42 million tons in 2014 [11], 53.6 million tons in 2019 and an estimated 74.7 million tons by 2030 [12].
Studies show that the amount of metallic fraction in unsorted mixed household waste is 5.4 kg/capita/year, which represents approximately 1.6% by weight of the total solid household waste produced. The metallic fraction of unsorted mixed household waste contains about 0.78 kg/capita/year of aluminum packaging, 2.08 kg/capita/year of metallic packaging, and 2.55 kg/capita/year of other metals. The estimated metal fraction collected from solid household waste is 1.1 kg/capita/year [6].
Recoverable material profit in e-waste was estimated at USD 57 billion in 2019 [12]. For the same year, 70.9% of steel cans were recycled along with other steel packaging types, including strapping and drums, with a 3.6% increase in steel scrap used by country and region [13].
Mixed waste is incinerated, and some metals are recovered before or after the municipal solid waste incineration process. However, the recoverability is limited since a large part of the aluminum is melted and oxidized in these, and the quality of the steel scrap is reduced [6]. In addition, when pyrometallurgical processes are used for metal recovery, organic materials, carbon, and other materials such as graphite anodes are lost after burning in the smelting furnace and cannot be recycled [14].
According to studies by Inman et al., leaching is the most common method for recovering metals from e-waste at different concentrations [4]. Leaching uses inorganic acids, including hydrochloric acid, nitric acid, sulfuric acid, sulfuric acid, phosphoric acid, and organic acids, including oxalic acid, formic acid, citric acid, tartaric acid, maleic acid, ascorbic acid, malic acid. Inorganic acids have advantages such as low cost and high leaching efficiency, although they cause equipment corrosion and secondary contamination. Leaching with organic acids can achieve the same efficiency in a milder environment, although their price is higher than that of inorganic acids, which restricts their large-scale industrial application [15].
Hydrometallurgy provides advantages for the recycling of materials, such as higher efficiency, excellent metal selectivity, lower energy consumption, and reduced emissions of hazardous gases; it faces challenges such as the production of wastewater [16] and costs due to its multiple stages in the process [14], which provides a strong motivation to study more sustainable chemistries [16].
In contrast, biohydrometallurgy brings added value to these processes, as they can recover quantities of metals using aqueous solutions and biological metabolites produced by certain microorganisms [17]; bioleaching, for example, is considered one of the green technologies for metal recovery, offering low cost in terms of installation and operation, low energy consumption, no toxic waste generation and low capital investment [1,18,19,20].
In the bioleaching process, metals are solubilized from insoluble solid substrates [1], either directly by the metabolism of microorganisms or indirectly by the products of their metabolism, such as the production of organic acids, chelating agents, amino acids, and complexing agents obtained from heterotrophic bacteria or fungi [21].
Bioleaching has received attention in a variety of industrial areas, especially in mineral and solid industrial waste materials (e.g., galvanic sludge, sewage sludge, fly ash, electronic waste, spent petrochemical catalysts, medical waste, spent batteries, residual slag), where the concentration of metals is low, where the presence of certain elements would result in smelter damage, or where environmental considerations favor biological treatment options. This process also allows the recovery of metals from low-grade sulfide ores and concentrates that cannot be economically processed by conventional techniques, as well as the production of concentrated solutions of metal salts, which could be recycled [18].
This work is a review of the bioleaching process as a sustainable alternative for the recovery of metals from solid matrices such as agricultural, municipal, and industrial waste such as animal feces, peel, and vegetable waste, crop residues, municipal solid waste, mining waste, especially of low grade, ash from combustion processes, electronic waste, used batteries, metallurgical slag, among others. The bio-extraction of metals from these matrices contributes to cycle closures and, in turn, to sustainable processes.
2. Recovery of Metals of Interest from Solid Matrices
The concept of solid matrices refers to a diverse range of waste materials generated from various sources, including agricultural, municipal, and industrial activities. These solid matrices often contain a significant amount of valuable metals, but their recovery can be challenging due to their complex composition and low metal concentration.
Biotechnology is a promising alternative to current industrially available technologies. One of the emerging technologies is the bioprocessing of waste materials to recover metals [11].
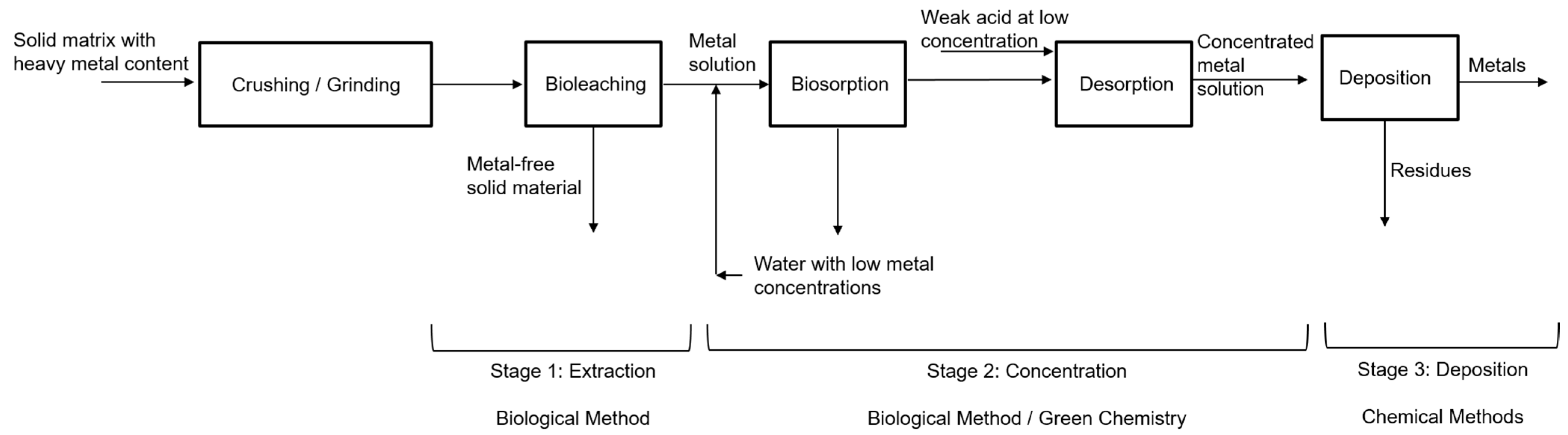
The recovery processes of metals of interest by biological methods involve three stages: the first is the extraction of the solute from the solvent using bioleaching, the second is the concentration process using biosorption/desorption, and the third is the deposition of the metal as a species in concentrated solution as a precipitated solid phase. Figure 1 shows a block diagram summarizing the process.
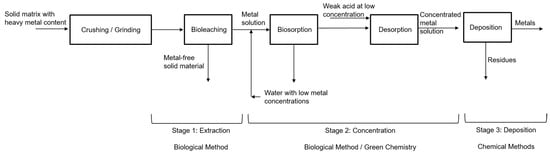
Figure 1.
Recovery processes of metals of interest. Source: Authors.
Bioleaching is the first stage where the metals of interest, such as Au, Ag, As, Co, Cu, Mn, Mo, Ni, U, V and W, and Zn, among others, are extracted from primary ores and solid matrices in general. The growing academic and commercial interest in the bioprocess is attributed to its better environmental profile, ease, and practicality of operation, better profitability, the potential for future development, and excellent selectivity with metals. Metals are extracted from solid matrices even in low concentrations [11]. In this bioprocess, solid matrices with metal content (SMMC) such as mining waste (tailings—low-grade ore), consumer waste such as electronic scrap, used batteries, municipal solid waste, and agricultural waste are used as raw materials, moving towards a circular economy, which is considered a green alternative with lower energy costs and environmental impacts compared to traditional metallurgical processes [12].
The second phase is the concentration of metals in the solution, where the biosorption/desorption process is employed. Biosorption is carried out in continuous systems in packed bed columns with microorganisms immobilized in porous matrices, as shown in the study by Ramírez et al. [22]. The use of residual yeast or fungi from the [23] industry is recommended, especially from the brewing industry, due to their volume of generation, easy acquisition, and low cost.
After the adsorption process, desorption is carried out; in this process, the aim is to separate and recover the metals retained in the packed bed column so that a concentrated solution of these metals is generated. For the desorption process, various extracting solutions or eluting agents are used, such as chelating agents (EDTA), acidic solutions (HCl, H2SO4, HNO3), inorganic and organic salts (NaNO3, Ca(NO3)2, sodium citrate), among others [24,25]. This process protonates the active sites of the microorganisms immobilized in the column, activating them again [25,26], which makes the development of the following adsorption process more efficient and allows the reuse of the packed column [24,27].
In the third phase, the metal as a concentrated solution species is deposited as a stable solid phase using conventional chemical methods such as precipitation and electrodeposition or reduction processes [12], which employ reducing agents that lower the redox potential such as SnCl2, FeSO4, SO2. Recently, organic reducing agents such as thiourea, glucose, sucrose, lactose [28], and even cellulose, hemicellulose, and lignin present in different biomasses are being explored, which are “greener” due to their low toxicity and produce less harmful gas emissions compared to inorganic reductants [29,30].
3. Solid Matrices as a Metal Source
Metal supply depends on specific geological, physical, and industrial conditions, so one supply metric does not fit all metals [10]. With waste production, a growing pool of metals is created, a pool of renewable resources that can replace limited metals from enrichment deposits in the geosphere [5].
Waste is defined as an object or substance that is discarded after its initial use. The broadest category of waste is solid waste, which can be divided into different types, agricultural waste, municipal solid waste, and industrial waste [31].
More than 12 billion tons of solid waste are produced annually worldwide [31], including approximately 1.55 billion tons of agricultural waste [32] and about 2 billion tons of municipal solid waste [31]. The generation of industrial waste is almost 18 times greater than that of municipal solid waste [33].
The wastes mentioned above contain metals in various concentrations [3,6,33,34,35,36,37,38,39,40], which have the potential as matrices to be subjected to the process of extraction of metals in the form of dissolution using the bioleaching process.
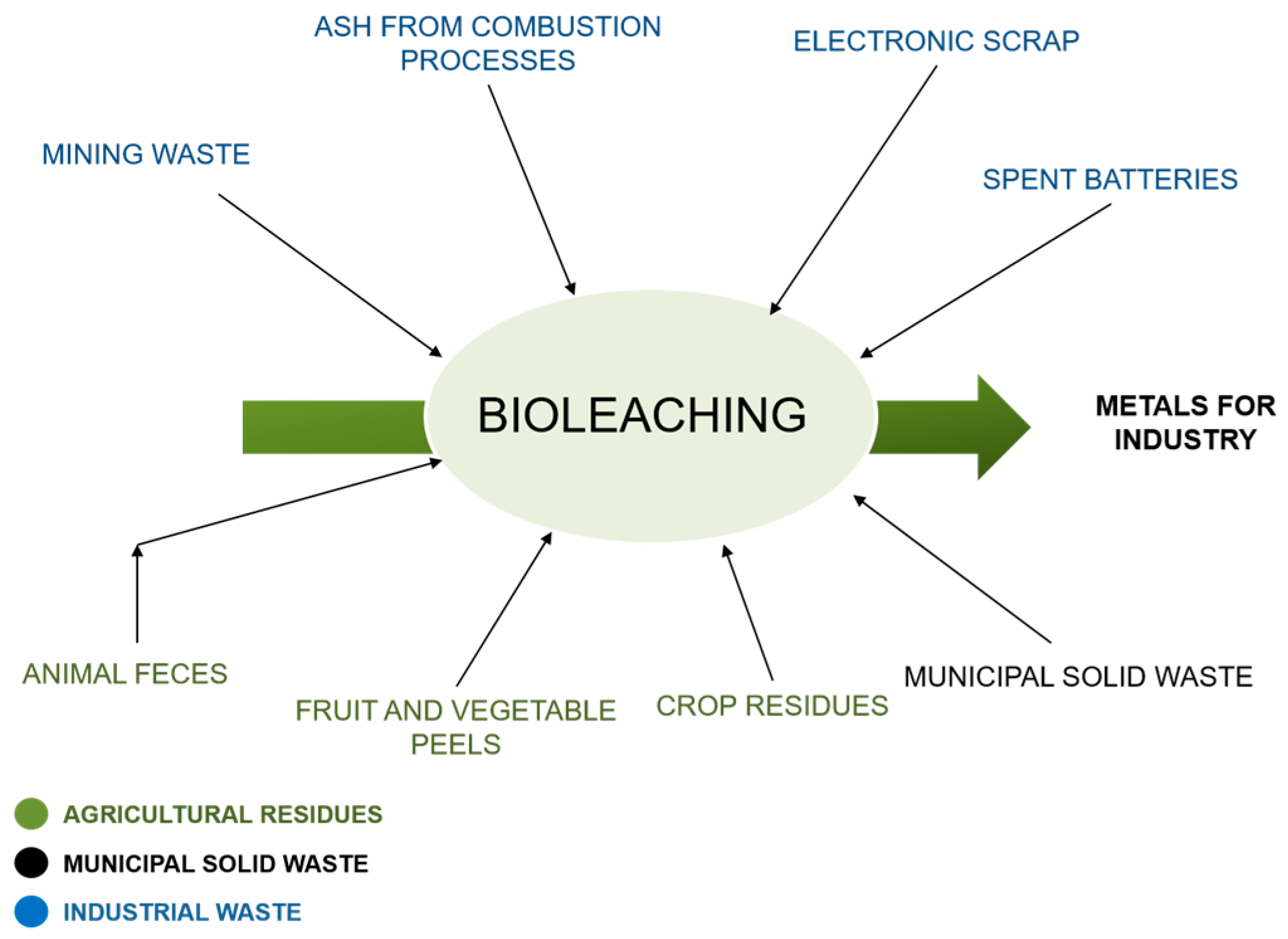
Figure 2 shows the different solid matrices with metal contents (SMMC) with the potential to be recovered by bioleaching.
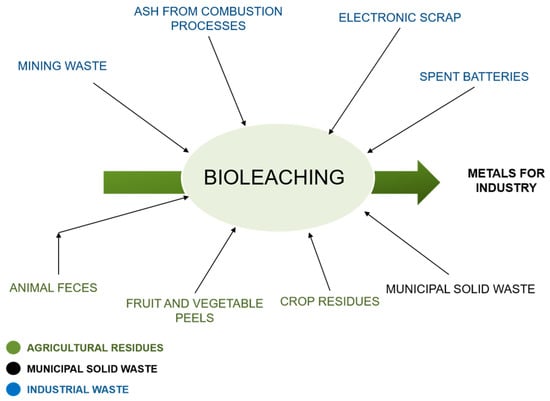
Figure 2.
SMMC with the potential to be recovered by bioleaching. Source: Authors.
3.1. Agricultural Residues
Crop residues are relevant raw materials for metal extraction. Residues such as peanut shells, cow horn, corn cob ash, sugar cane bagasse, and rice husk are rich in silicon, calcium, magnesium [3,41], and other trace elements such as Fe, Al, P, Mg, Zn, and Mn [42].
Meanwhile, based on the FAO’s State of Food and Agriculture (2019) report, approximately 14 percent of global food (estimated to be worth USD 400 billion annually) still becomes lost post-harvest and before it reaches the market. Furthermore, as per the UNEP’s Food Waste Index Report, an additional 17 percent of our food is wasted in retail and by consumers, primarily in households. According to FAO’s calculations, the combined amount of lost and wasted food could provide sustenance for 1.26 billion undernourished individuals each year [43].
At a regional level, Sub-Saharan Africa has the highest losses at 21.4 percent. Least Developed Countries (LDCs) and Small Island Developing States (SIDS) also experience significant losses, with 18.9 percent and 17.3 percent, respectively. Structural deficiencies in these regions result in substantial food losses between the farm and retail levels. East and Southeast Asia also report significant food losses at 15.1 percent, mainly due to significant losses in the fruit and vegetable value chains. The lowest losses occur in Latin America and the Caribbean (12.3 percent) and Europe and North America (9.9 percent). All regions, except for Central and Southern Asia, record an increase in estimated losses in 2020 compared to 2016, with the highest increases observed in SIDS (an additional 1 percent), Oceania (an additional 1.2 percent), and North Africa and Western Asia (an additional 1.7 percent) [43].
It is estimated that the global percentage of food lost after harvest in farming, transportation, storage, wholesale, and processing was 13% in 2016 and 13.3% in 2020. These percentages correspond to a food loss index of 98.7 in 2016 and 101.2 in 2020 [44].
Even some typical wastes, such as ashes from sugarcane bagasse, rice husk, corn cob, bamboo leaf, corn stalk, and palm kernel husk, are regularly employed as metal matrices to reinforce materials [41].
On the other hand, the chemical composition of coconut shell contains mainly calcium oxide, followed by silica, along with traces of Al, K, Fe, and P, in the form of oxides or chlorides [3]. Corn leaves and cacao plants contain Cd [39], cattle manure constitutes an essential source of metallic elements such as Zn and Cu [35], and pig manure contains Cu, Zn, and Mn [36].
Studies by Ramírez-Carmona et al. resulted in a map of agricultural biomass residues in Antioquia, Colombia, showing the metal concentrations of 92 species and the Tm/ha produced per crop. For example, metals are found in papaya residues, 1993 mg/kg of P and 6800 mg/kg Mg; branch onion residues, 1060 mg/kg of P and 10,807 mg/kg of Fe; paprika residues, 680 mg/kg of P; and citron residues, 104,000 mg/kg of Ca, among others [42].
3.2. Municipal Solid Waste
Over 2 billion tons of solid urban waste is generated annually, with approximately 33% being improperly managed. This mismanagement poses significant health and environmental risks, including water, soil, and air contamination. Improper disposal methods such as open burning of hazardous waste not only harm waste workers and neighboring communities but also increase the vulnerability of children to adverse health outcomes [45,46].
Thirty-seven percent of municipal solid waste is disposed of in landfills, 33% in open spaces; 19% is recycled, and 11% is incinerated. These wastes contain metals due to industrial and anthropic activities [47], involving metals such as Zn, Pb, Cu, Cr, Hg [37,48,49,50], Cd [37,48,49], Ni [49], Co and As [37]. In addition, household wastes such as eggshells are rich in Ca [33]. In the study by Kuusiola et al., the compositions of the metal fractions of 54% tinned steel, 15% stainless steel, 24% aluminum, and 8% other metals are shown [6]; likewise, in landfills, the concentrations of leached metals are 108.5 g/Mg of Pb, 90 g/Mg of Cu, 560 g/Mg of Zn, 101.5 g/Mg of Cr and 2.24 g/Mg of Cd [48].
In municipal solid waste incineration processes, metals such as Zn, Cu, Cr, Pb, Ni, Cd, and Hg are found [49,50]. Zhang et al. reported that after 15 days of bioleaching, 80.7–82.1% of Cd, 72.5–74.1% of Zn, 42.8–43.9% of Cu, and 24.1–25.2% of Cr and 12.4–13.0% of Pb were removed [49].
Similarly, studies in Poland showed that metal concentrations in the soil near the municipal solid waste landfill were similar to geochemical reference levels in forest and agricultural soils [48].
3.3. Industrial Wastes
Industrial waste is produced by industrial activity, including any material rendered useless during manufacturing, such as from factories, industries, mills, and mining operations. Industrial waste includes soil and gravel, masonry and concrete, and electronic waste, among others [8].
Every year, American industrial facilities generate and dispose of around 7.6 billion tons of industrial solid waste. Additionally, approximately 54 million tons of e-waste, including electronic devices like TVs, computers, and phones, are produced annually, with a projected increase to 75 million tons by 2030. However, in 2019, only 17% of e-waste was documented as being adequately collected and recycled. Improper management of e-waste and its components poses significant risks, leading to adverse health and developmental effects, particularly in young children [46,51].
Metallurgical slag is among the most abundant by-products generated by such industries [3,9]. The production of this waste is used to recover iron [52]. On the other hand, titanium is concentrated in the blast furnace titanium slag as vanadium titanomagnetite, which contains SiO2, Al2O3, CaO, and MgO [53], and the solid residues from silica sand purification contain metals such as Ba and Cd [3].
Raw materials for vanadium products include the vanadium–titanium magnetite, vanadium slag, coal, petroleum coke, fly ash, and spent catalysts [54].
The WEEE, commonly called e-waste, contains up to 60 different elements in various concentrations, comprising base metals, critical metals, and platinum group metals mixed in a complex matrix of metallic and non-metallic materials [11,55]. For example, Au, Ag and Pd, Cu, and Ni are recovered from used cell phone printed circuit boards, with 540–880 mg/kg Ag, 168 mg/kg Au and 110 mg/kg Pd [33,34].
Industrial wastes such as fly ash, gypsum, and red mud are alumina, silica, and zeolite sources. Likewise, sewage sludge ashes contain metals such as Cu, Cr, Cd, Ni, Pb, Zn, and Co [39,40,56].
Finally, depleted mines, although they increase the percentage of waste generated [9], contain a diversity of metals in low concentrations [57] that can be extracted by biological rather than chemical methods, as mentioned above.
4. Bioleaching
Leaching is a chemical method for extracting metals of interest [58]. Leaching, peroration, or solid/liquid extraction all involve an operation that consists of extracting with the help of a potentially soluble component a solute that is in solid form accompanied by other undesirable solids [59].
In general, in a leaching process, there are three components:
- A: solid solute that goes into the solution;
- B: inert solid (insoluble in S);
- S: extracting solvent.
In bioleaching, the function of the solvent is performed by microorganisms, by the action of either bacteria or fungi, as they participate in the biogeochemical cycle of minerals in direct ways by the metabolism of the microorganisms or indirectly by the products of their metabolism [21,60].
Therefore, bioleaching is defined as the solubilization of metals from insoluble solid substrates [21]. In the case of employing bacteria, they use the mineral as a substrate, capturing electrons for their metabolic processes and releasing heat and metals without needing any external energy to carry out the process [61]. In the case of fungi, they produce organic acids, which leach metals from solid matrices. Microorganisms can also excrete ligands that stabilize the metal by forming metal-rich complexes [62].
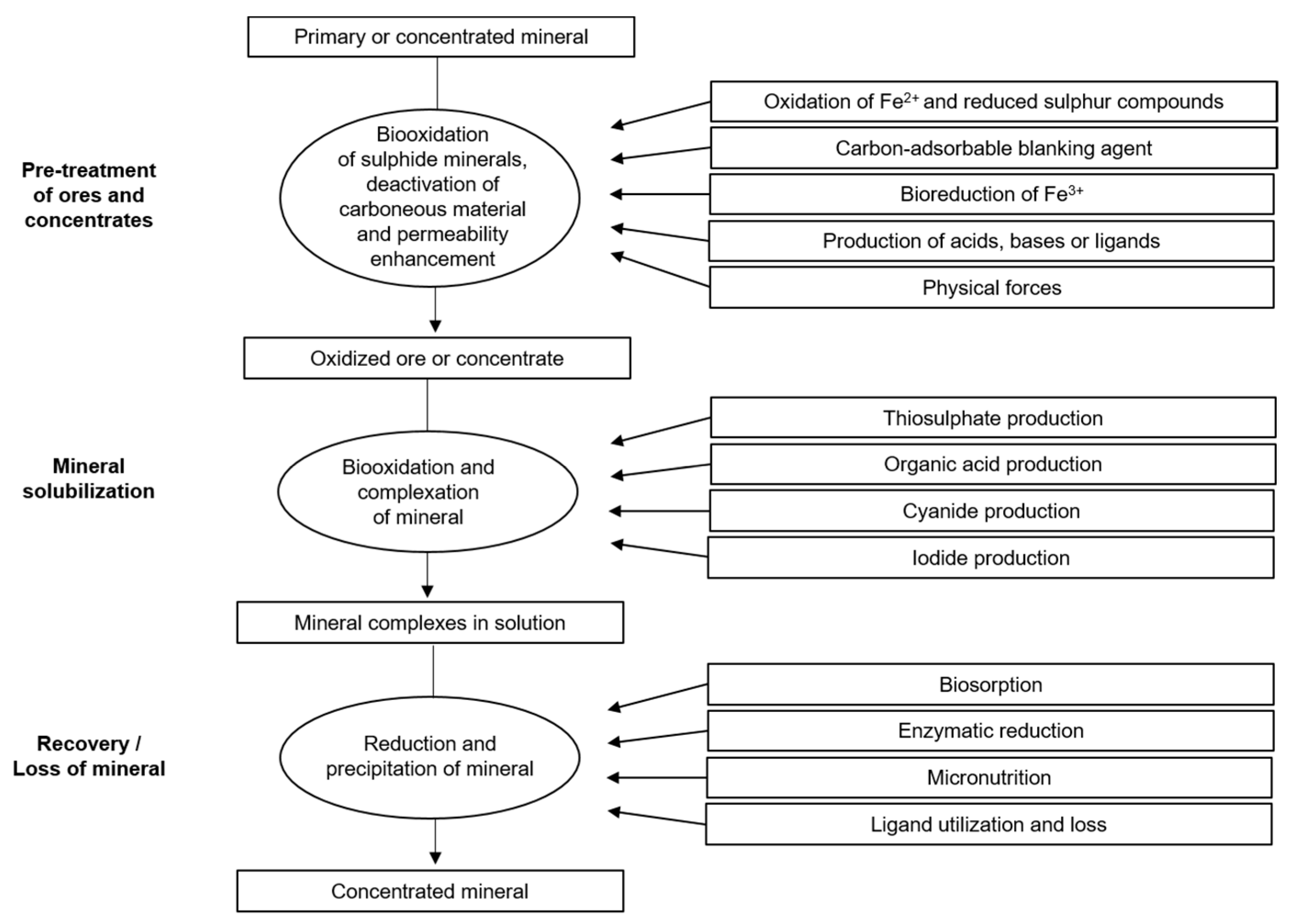
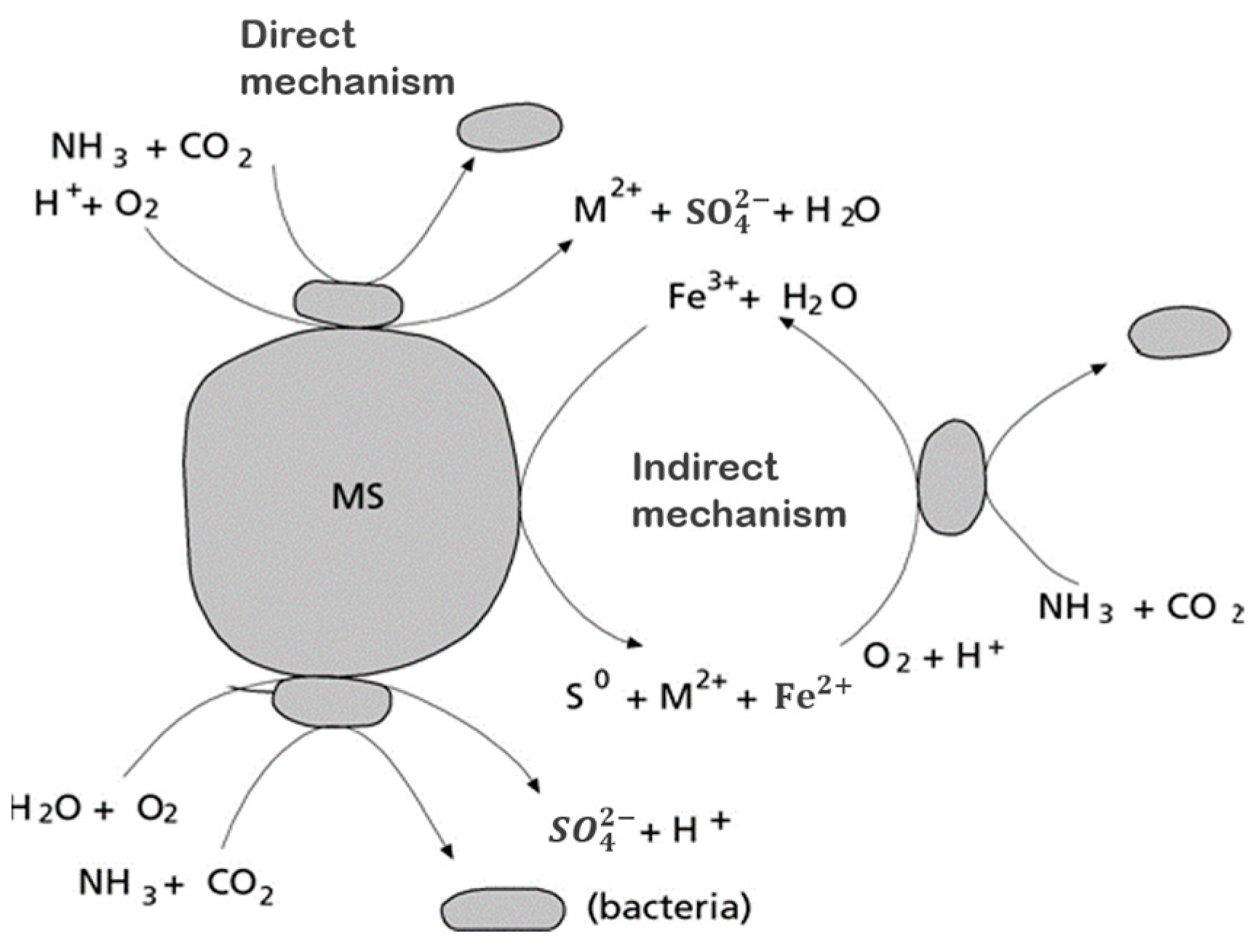
Metal solubilization can be facilitated by biologically produced amino acids, cyanide, and thiosulfate [62]. In addition, microorganisms can participate in the redox cycling of iodine [63], which is a potential alternative leachant for obtaining the metal and decreasing metal solubility by consuming ligands that are bound to the metal or by biosorption, enzymatic reduction, and precipitation, and by employing the metal as a micronutrient, as shown in Figure 3.
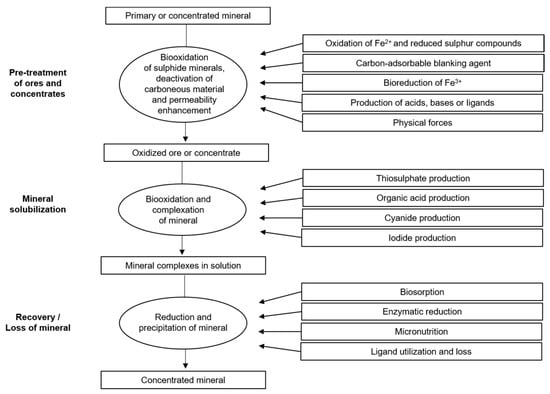
Figure 3.
Possible roles of microorganisms in mineral processing and recovery. Source: Adapted with permission from Ref. [62]. 2007, Springer Nature.
As mentioned above, SMMC metal bioleaching processes can be carried out using bacteria or fungi; the decision-making for the selection depends on the objectives to be achieved in the bioprocess. Table 1 shows the general features for selecting the bacterial or fungal bioleaching application route.

Table 1.
General features for the application of bacterial or fungal bioleaching. Source: Authors.
Bacterial bioleaching is applied when the recovery of a metal of interest is required, and it is not necessary to preserve the properties of the solid matrix. On the other hand, fungal bioleaching is applied when it is necessary to preserve the properties of the solid matrix, especially the crystalline properties, in case it is a mineral SMMC. Furthermore, sterilization and sanitization processes are required in fungal but not bacterial bioleaching due to their resistance to contamination, and because the process is usually performed in one step, which limits the exposure to external contaminants. In contrast, fungal bioleaching is performed in two steps, either by a direct or indirect method.
The operational times differ between the two methods, with bacterial bioleaching being prolonged due to the bacteria acting directly in the process. In contrast, fungal bioleaching has variable times depending on the SMMC/bacteria system and the method used. The indirect method of fungal bioleaching has a longer production time for the leaching solvents (fermented broth) but has a shorter bioleaching process time. The information provided in this table can assist researchers and industry professionals in selecting the appropriate bioleaching method according to their specific metal recovery requirements and constraints.
4.1. Bacterial Bioleaching
Bacterial leaching, also known as bioleaching, biohydro-metallurgy, or bio-oxidation of sulfides, can be defined as a natural dissolution process resulting from the action of a group of bacteria, mainly of the genus Thiobacillus, with the ability to oxidize sulfide SMMC, allowing the release of the metals contained in them [18,64].
Bacterial bioleaching is based on the ability of microorganisms to transform solid compounds into soluble and extractable elements [65] or expose metals contained in ores and concentrates by direct oxidation or indirect chemical oxidation caused by corrosive metabolic by-products generated by electrochemistry, or a combination of both [18].
The attack and solubilization of an SMMC through microorganisms are performed by different mechanisms, which depend on the matrix’s sulfur matrix [66,67].
Similarly, the bioleaching process occurs by the catalysis that microorganisms exert during the dissolution of certain SMMC; for example, microorganisms such as bacteria convert metal compounds into their water-soluble forms and are biocatalysts of these leaching processes [68,69]. The microorganism uses SMMC as fuel, electron transfer for its survival purposes, and releasing metals, without the need for external application of energy. In this type of process, high activation energies are not necessary; proof of this is that the reactions take place at low pressure and some at low temperature, depending on the type of microorganism, whereas other ways need extreme conditions for their development and performance [68].
According to Rodríguez et al. (2001), bacterial bioleaching or leaching is understood as the attack and solubilization of an SMMC through the direct or indirect action of different microorganisms [70].
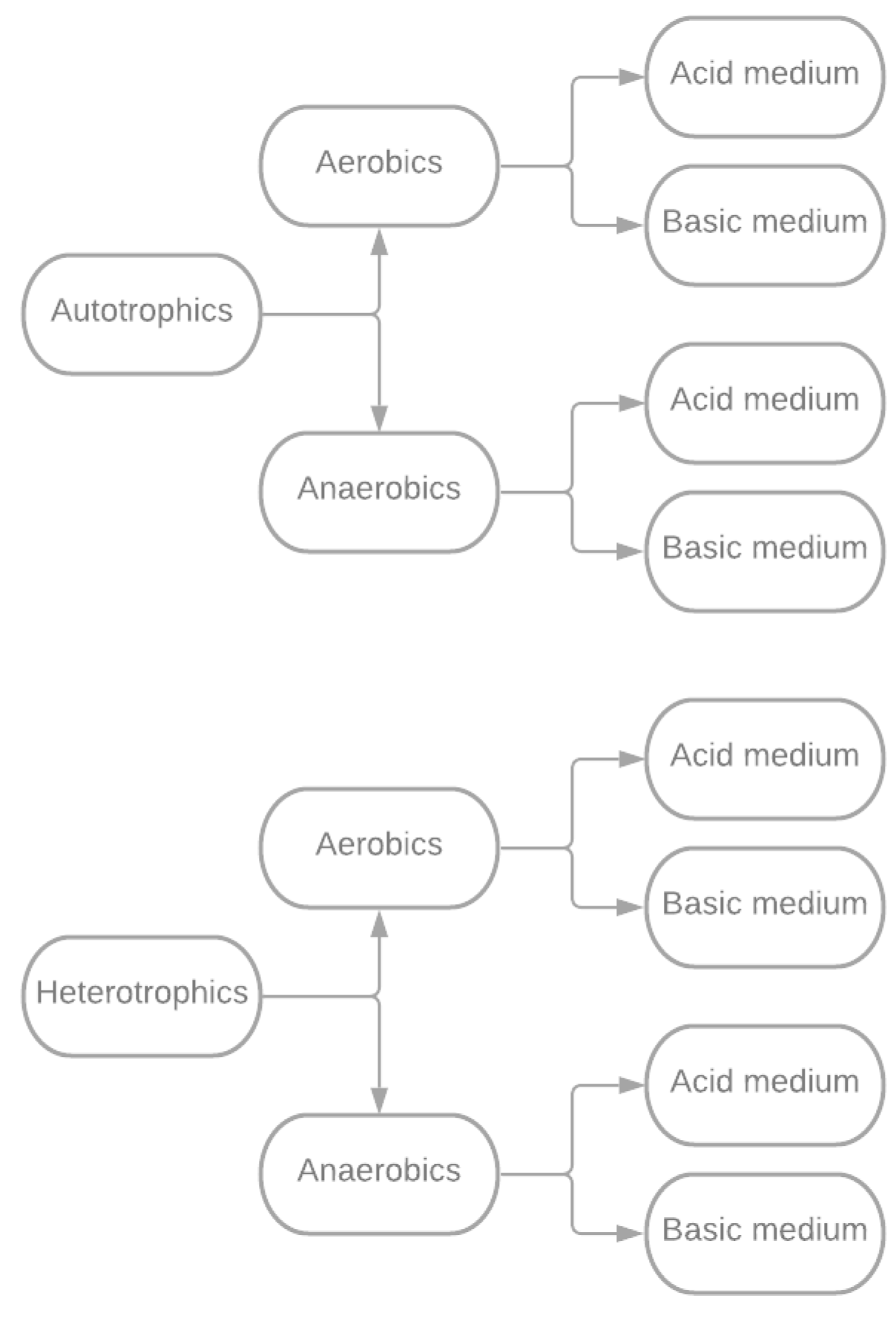
Microorganisms adapted to bioleaching processes are of two categories:
- Autotrophic: These microorganisms obtain nutrients and energy for their life cycles from the inorganic matter surrounding them.
- Heterotrophic: They require the availability of organic matter to complete their life cycles.
In both groups or categories of bacteria, some species operate predominantly in the presence of oxygen. These are the aerobic bacteria that carry out, in the first place, the oxidation reactions and have the property of oxidizing metal sulfides to soluble sulfates. Similarly, anaerobic bacteria can function and carry out their life cycles without oxygen; these batteries first conduct reduction reactions [71]. The respective classification is shown in Figure 4.
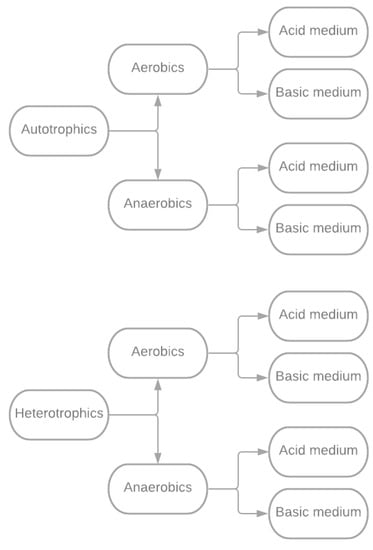
Figure 4.
The bacterial classification used in the bioleaching process.
According to Figure 4, regardless of the bacterial classification, the main characteristic of the microorganisms used in metal recovery is their capacity to evolve in environments with very aggressive or extreme temperatures, pH, and living conditions [68,70].
Some of the bacteria involved are acidophilic microorganisms capable of surviving at low pH levels, high temperatures, and high concentrations of metallic elements, and whose energy source is the oxidation of Fe2+ to Fe3+ and reduced sulfur compounds [70].
The most significant area within the biotechnological process of metals is focused on the microbial oxidation of sulfide SMMC. Low pH, high metal concentrations, and sometimes high temperatures characterize the aqueous environments associated with SMMC discharges. However, some microorganisms can live, develop and reproduce under these conditions and in these environments. In these environments, microorganisms use reduced sulfur species and certain metals in solution as a primary energy source, resulting in the solubilization of valuable metals [68,72].
According to Rodriguez et al. (2001), in recovery by bioleaching, the main action of microorganisms is direct oxidation through an enzymatic attack or indirect through regeneration of the leaching agent Fe3+ of SMMC [70].
In some cases, the bacteria contribute to the weathering of the gangue by liberating the valuable mineral and facilitating its subsequent attack since the sulfuric acid generated by the bacteria produces a rupture of the Si-O and Al-O bonds in the alumino-silicates of the mineral gangue [70].
The microorganisms used in the leaching of metals from ores and concentrates are presented in Table 2. These microorganisms are capable of oxidizing sulfides and sulfur or ferrous iron species. An oxidant (ferric sulfate) is formed when the latter is oxidized.

Table 2.
Bacteria used in bioleaching and their optimum conditions.
The bioleaching of A. thiooxidans and A. ferrooxidans contributes via both “contact” and “non-contact” mechanisms. The “contact” mechanism considers that most cells adhere to the surface of the bioleaching substrates. The “non-contact” mechanism is related to redox reactions, such as iron (III) reduction and sulfur oxidation [72,80].
In this sense, there are many and varied definitions of bioleaching. However, what should be made clear is that this leaching can be determined because the raw material studied can be under the direct or indirect action of microorganisms [81].
According to Table 2, a direct mechanism is understood as that which is mediated by bacterial action and where the chemical reactions are catalyzed enzymatically; this option also supposes the physical contact of the microorganisms with the solid matrix [81].
On the other hand, an indirect mechanism involves chemical reactions, enzymatic or non-enzymatic, with no physical contact between the microorganisms and the solid matrix. However, the microorganisms are relevant in forming chemical reagents that can participate in the process [81,82].
The first attempt to explain the mechanism of bioleaching was made by Silverman and Ehrlich in 1964 when they proposed two possible mechanisms [83], as shown in Figure 5.
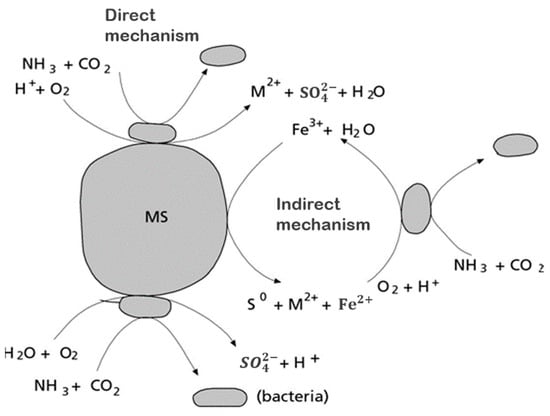
Figure 5.
Direct and indirect mechanisms of bioleaching. Adapted from [83].
According to the first possible mechanism, the bacteria attack the metal sulfide directly by adhering to the mineral surface and then enzymatically oxidize it by transporting electrons from the reduced part of the mineral, usually a sulfide, to dissolved oxygen. The general reaction is presented in Equations (1) and (2) [60,65,69].
In the case of pyrite, the above reaction would look like this:
The first mechanism proposed (Equation (1)) states that the oxidation of the mineral is carried out by direct enzymatic action, in which the role of the microorganisms is to catalyze the metal sulfide dissolution reaction. The iron-oxidizing bacteria can leach metal sulfides directly without the participation of biologically produced ferric sulfate. The M represents a bivalent metal. The second mechanism, called indirect (Equation (2)), is based on microorganisms oxidizing the minerals by generating ferric ion. In the case of pyrite, the direct transformation of sulfide to sulfate occurs through enzymatic oxidation, where the ferrous sulfate formed is oxidized to ferric sulfate in a later stage by the bacteria [74].
This theory of direct mechanism has been supported by several experimental studies that confirm the adherence of bioleaching bacteria to the surface of minerals. The work presented by Berry and Murr evidence that Acidithiobacillius spp. secrete some substances that can help the attack, although their nature is unknown [84].
Similarly, pyrite crystals exposed to the action of A. ferrooxidans were observed under the scanning electron microscope, detecting traces of corrosion in the crystals and suggesting that the bacteria caused these through a metabolite that could act in three different ways: oxidation of the ferrous ion to ferric, solubilization of the sulfur on the surface of the mineral or by direct attack. In addition, it was found that A. ferrooxidans could discern the most favorable regions of the mineral surface to obtain its energy source and select the site of attack according to the best availability of nutrients. However, the authors, far from using these results in favor of the direct mechanism, have used them to explain the indirect mechanism known as contact leaching [85].
Studies carried out by different authors also detected by electron microscopy the formation of holes on the surface of dissolved sphalerite in the presence of bacteria, and it was concluded that they occurred due to the direct attack of the bacteria on the surface of the mineral [86]. Tributsch studied by SEM the surface of lead sulfide after bacterial attack, showing depressions where the bacteria had been located. This author concluded that the bacteria produced a chemical carrier that promoted the attack at the point of adhesion of the microorganisms [87].
Studies by some authors mention that bacteria breathe using minerals, which provides a new vision of the problem, although, in this case, not related to sulfide minerals [88,89].
In contrast to the direct mechanism, the indirect mechanism considers the action of ferric ions on the sulfide ore dissolving it. Through this chemical leaching reaction, ferrous ions and elemental sulfur are produced. Finally, these chemical species are biologically oxidized to ferric iron and sulfate ions. This mechanism, in principle, does not require the adherence of cells to the sulfide mineral [81,89].
Some of the bacteria mentioned in Table 3 show extraction efficiencies in laboratory experiments with SMMC, such as minerals, sediments contaminated with metals after wastewater treatment, and printed circuit boards, among other materials that can be returned to the production chain once they fulfill their useful life and become waste.

Table 3.
Bacterial bioleaching: operating conditions and metal extraction efficiencies of different SMMCs.
4.2. Fungal Bioleaching
Leaching by fungal action is called fungal bioleaching. It occurs mainly by the action of organic acids produced in the fermentative process [2] when metals are mobilized from solid materials through ligand-induced metal solubilization [121].
The fungi used in producing organic acids, such as citric, oxalic, gluconic, and others, are shown in Table 4. They can be quickly grown using unsophisticated fermentation techniques and inexpensive growth media [122].

Table 4.
Organic acids produced by selected fungus in fermentative processes.
In the context of fungal bioleaching processes, it is important to note that while fungi can produce mycotoxins that pose risks in food matrices, these concerns are not typically relevant in industrial processes such as bioleaching. Fungal mycotoxins have been known to cause issues ranging from crop yield reductions and negative impacts on farm animal health to alterations in food quality, organoleptic characteristics, and nutritional value. The associated costs include prevention and decontamination measures [123].
Human exposure to mycotoxins primarily occurs through food consumption, although isolated cases of respiratory-related ailments have been reported. Since food represents the main source of risk for humans, it justifies the inclusion of mycotoxins as one of the molecule groups considered in the Food Safety program. However, in the context of industrial processes like bioleaching, the focus is primarily on harnessing the beneficial capabilities of fungi without significant concern for mycotoxin-related issues, as these processes involve different considerations and control measures [123,124,125].
Fungal cells have a specific structure or organization in the material, where various components are distinguished, such as cell wall, plasma membrane, cytoplasm, mitochondria, endoplasmic reticulum and the Golgi apparatus. Along with its structure appears the function of the cell, which is basically to remain viable, develop and multiply. This function of the cell is mediated by the various components that constitute it [122].
Studies by different authors reveal that the filamentous fungus Penicillium simplicissimum (same as Penicillium janthinellum) is one of the most versatile microorganisms for the biotechnological production of organic acids due to its adaptability to different culture media and its selectivity at a metabolic level to generate multiple organic acids.
Other filamentous fungi such as Aspergillus spp., Fusarium graminearum, Aspergillus fumigatus, and Trichoderma harzianum also produce organic acids [122,126,127,128], mainly by submerged fermentation using different carbon sources (substrates) such as cellulosic waste hydrolysates, cane molasses, glycerol, and even primary alcohols.
There are also solid-state fermentation processes that simultaneously obtain citric and gluconic acid using agro-industrial residues as substrates [129]. However, submerged fermentation systems are preferred to produce the different acids since they allow better control of the process and, therefore, greater productivity of these acids [129,130].
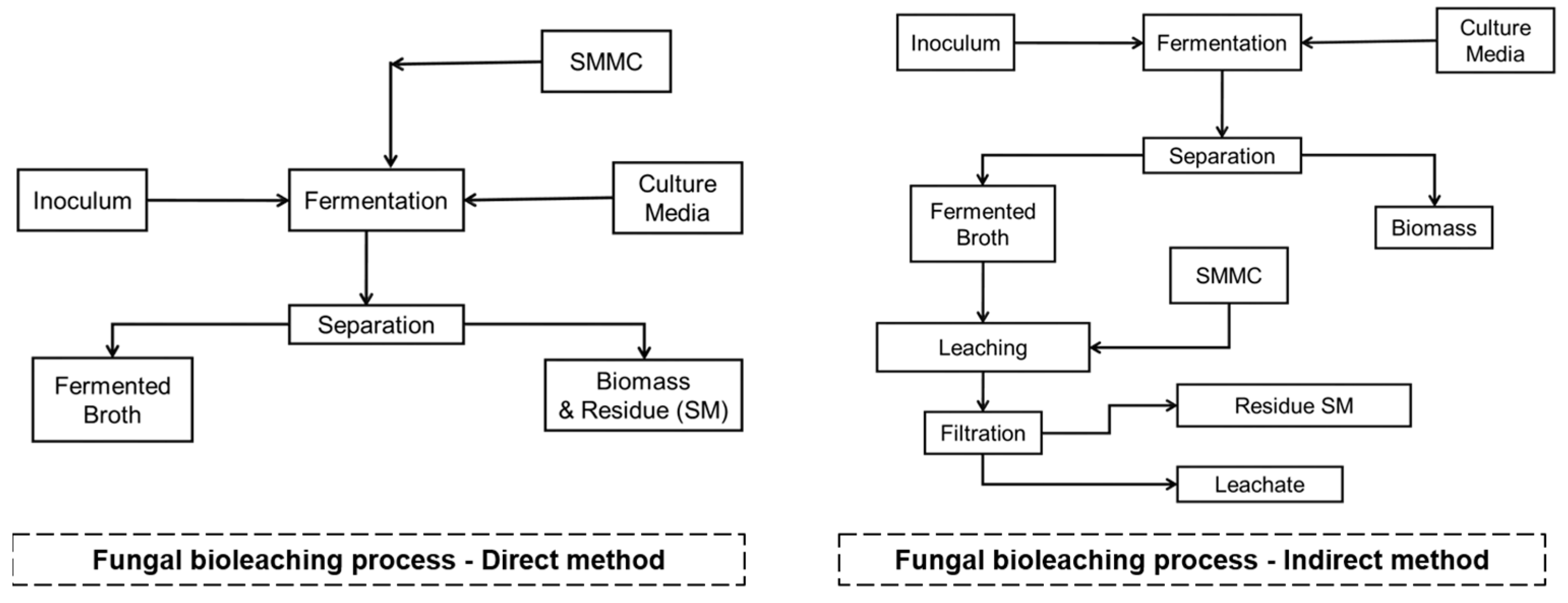
The fungal bioleaching process is carried out by direct or indirect methods, as shown in Figure 6.
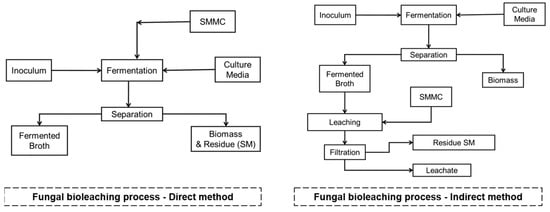
Figure 6.
Fungal bioleaching process. Source: the authors.
Direct method: this method is carried out in one stage where the fermentation occurs: SMMC/culture medium/inoculum (fungus) simultaneously. In the process, the fungus produces the leaching substances, mainly organic acids, and simultaneously extracts the metal contained in the SMMC. The process can take 3 to 10 days depending on the objective, and the pH value of the fermentation usually starts between 6 and 7. As the bioprocess progresses, it reaches pH values between 3.5 and 4, which leads to a decrease in the efficiency of the process because the fungus is inhibited by the presence of SMMC residues [122].
Indirect method: this approach is carried out in two stages [122].
Stage 1: Production of the solvent extractor or fermented broth. Fermentation: culture medium/inoculum (fungus) for 8–10 days. Subsequently, a separation process is carried out, where the biomass is discarded, and the focus is on the fermented broth. This stage generally presents pH values below 3.0.
Stage 2: The fermented broth is in contact with the SMMC, a process that can take 4 to 8 h depending on the SMMC. Subsequently, a separation process is carried out, where the metal-free solid residues are discarded and the focus is on the fermented broth containing the extracted metals (leachates).
Acids are excellent metal leachants from SMMC. Heterotrophic metabolism can also achieve leaching due to the excretion of siderophores and organic acids. These provide protons and metal complexing anions, as citrate and oxalate anions can form stable complexes with many metals [122], including chelates which are relevant in metal dissolution by acidolysis and complex formation mechanisms. In conclusion, the main mechanisms through which organic acids interact with metals (bioleaching reactions) are acidolysis (acid-base reaction), redoxolysis, bioaccumulation, chelates, and complex formation mechanisms [21].
The efficiency of the process depends on the acids produced and their acidity depending on their functional group (e.g., carboxylic or sulfonic). Considering that these acids are not equal in the number of carboxylic groups, hydroxyl groups, and carbon–carbon double bonds in their molecules, they are classified according to their typical characteristics of the carbon chain, saturation, substitution, and functional groups. Table S1 shows the main groups of organic acids, their formula, dissociation reactions, pKa, and corresponding structures [2,131,132].
Based on the Lewis acid-base theory, the ability to release protons is shown by the strength of the acid. The stronger the acidity, the stronger the ability to release protons. For example, oxalic acid (pKa 1.23) shows higher acidic capacity than formic acid (pKa 3.75), implying that the former has higher leaching power provided that the conjugate base of the oxalate anion does not interact strongly with the metal of interest, causing the precipitation of metal complexes [15].
Studies show that oxalic acid (pKa 1.23) leached more iron ions than lactic acid (pKa 3.86). The pKa values of each carboxylic acid indicate that oxalic acid is stronger than lactic acid, which leaches metals more efficiently. In addition, the complexation reaction and the formation of iron oxalates may play a relevant role in increasing the leaching of iron ions, which is confirmed in the leaching of iron contained in kaolin using oxalic acid, which is more effective than when organic acids such as citric, malonic, or acetic acid are used [133]. Oxalates are known for chelating metals, and this can be exploited for the dissolution and separation of metals. The increase in the solubility of simple oxalate compounds in the presence of excess oxalate provides the basis for formation of the oxalate complex [134].
Metallic oxalates display a diverse range of solubilities which are influenced by the particular metal cation and solution conditions. Insoluble oxalates include calcium oxalate (CaC2O4), silver oxalate (Ag2C2O4), and lead oxalate (PbC2O4). Conversely, sodium oxalate (Na2C2O4) and potassium oxalate (K2C2O4) exhibit high solubility, while barium oxalate (BaC2O4) is generally soluble. Transition metal oxalates like iron oxalate (FeC2O4) and nickel oxalate (NiC2O4) can display variable solubility depending on conditions. Factors such as pH, temperature, presence of other ions, and ligand complexation influence the solubility behavior of metallic oxalates [134].
In bioleaching processes, the binding behavior of oxalic acid is influenced by adsorption of oxalic acid onto metal surface which leads to the formation of an upright mono-oxalate species, with the carboxylate group binding to the surface. This upright orientation enables interactions between the carboxylic acid group and the surrounding environment, resulting in the creation of a chemically functionalized surface. The presence or absence of intramolecular hydrogen bonding determines the various surface species of mono-oxalate. The tilting of the molecules is influenced by surface coverage and temperature, leading to the observation of both unidentate and bridging species [135].
L-aspartic acid can release a total of three protons: two protons from its carboxyl groups and one proton from its amino group. On the other hand, L-tartaric acid does not possess an amino group, and therefore, two acidity constants are reported specifically for its carboxyl groups. In the case of L-glutamic acid, two protons are released from its carboxyl groups, while an additional proton is released from its amino group. Glutamate and aspartate chelate metal ions weakly via the amino nitrogen and carbonyl oxygen bind. A stronger chelation occurs upon amide-nitrogen-bound hydrogen by some metal ions such as Cu2+. This reaction occurs in neutral pH conditions (pH ≈ 7) with Cu2+ [136].
Several reports have documented the leaching of metals with fungi. Fungi of the genera Aspergillus and Penicillium are among the most effective and relevant for biological leaching due to filamentous fungi’s robust adaptability and ability to tolerate metal contamination stress [21].
Laboratory studies have been carried out using fungi, where only the leaching action is specified according to the type and species of microorganism. For example, A. niger, P. simplicissimum, and P. purpurogenum are related to the production of low-molecular-weight metabolites, mainly organic acids such as gluconic acid, pyruvic acid, citric acid, oxalic acid, malic acid, and succinic acid, different from the mechanism reported for R. rubra, A. thiooxidans and A. ferrooxidans [122].
Some of the most common organisms for fungal bioleaching are the genera Acidithiobacillus, Aspergillus, and Penicillium. For example, Penicillium simplicissimum is used to extract elemental Zn from ZnO. Fungi are also particularly suitable for phosphorus bioleaching, where organic acids such as citric and oxalic acid solubilize P from Fe and Al phosphates. In this process, carboxylic anions compete with binding sites that chelate Al3+ and Fe3+ [121].
Many microorganisms are reported to solubilize metals in soils, including Aspergillus niger, Penicillium purpurogenum, Rhodotorula rubra, Acidithiobacillus thiooxidans, and Acidithiobacillus ferrooxidans. The mechanism of metal solubilization during the process is related to a chemical process where the binding of microorganisms to the mineral can enhance dissolution [19,69].
Despite successful records of fungal tolerance to metals at the laboratory scale, no documentation of their use at the industrial scale is available [21,137].
In Table 5, fungal bioleaching experiments are presented: operating conditions and metal extraction efficiencies of different SMMC.

Table 5.
Fungal bioleaching: operating conditions and metal extraction efficiencies of different SMMCs.
According to Table 5, the most important and significant area within the biotechnological process of metals is focused on the microbial oxidation of sulfide minerals. The aqueous environments associated with mine discharges and mining sites are typically characterized by low pH, high concentrations of heavy metals, and, in some cases, elevated temperatures. Despite these conditions and environments, certain microorganisms not only survive but also thrive and reproduce. In these environments, microorganisms utilize reduced sulfur species and certain metals in solution as their primary energy source, resulting in the solubilization of valuable metals [68,72].
Fungal species such as A. niger, P. simplicissimum, and P. purpurogenum are among the most used species in bioleaching and offer advantages over bacterial bioleaching, including the ability to thrive at high pH values and a faster leaching rate [19]. In these studies, pH is a control variable that indirectly indicates the presence or absence of organic acid production during the fermentation processes. pH influences the growth and excretion of organic acids in filamentous fungi. When grown in an unbuffered medium, filamentous fungi often rapidly acidify their environment to very low, and sometimes detrimental, pH values [155,156].
The production of organic acids and other metabolites in the fermentation process is also influenced by the agitation rate. Agitation at low to intermediate levels, ranging from 100 to 300 rpm, increases with agitation speed, whereas agitation speeds between 500 and 800 rpm further enhance it. Citrate synthase activity decreases with increasing agitation speed, while aconitase and isocitrate dehydrogenase activities increase with agitation speed, favoring the transformation of citrate into oxoglutarate [129].
Amino acids such as glycine, histidine, and alanine have been used to test their effect on gold solutions, revealing that the initial dissolution of gold in histidine solution is faster than in glycine and alanine solutions. However, upon extended leaching, it was found that glycine dissolves gold more rapidly and to a greater extent than histidine and alanine [157].
Pulp density plays a crucial role in determining the feasibility of applying bioleaching on a commercial scale. Increasing the pulp density from 1% to 2% (w/v) leads to a significant reduction of 50% in both the volume of leaching media required and the size of the bioreactor. This reduction in size and resource consumption directly translates into a substantial decrease in bioleaching costs. In the bio-hydrometallurgical treatment of low-grade ores, it is common to utilize pulp densities of 10% or higher to maximize efficiency and productivity [158].
5. Future Prospects
The recovery of metals from solid matrices using bioleaching is a promising alternative to traditional metallurgical processes due to its better environmental profile, effectiveness, and high selectivity with metals.
Solid matrices with metal content, such as mining waste, electronic scrap, used batteries, municipal solid waste, and agricultural waste, are potential raw materials moving toward a circular economy. This approach is considered a green alternative with lower energy costs and environmental impacts than traditional metallurgical processes and industrially available technologies for metal recovery.
Additionally, the biomasses produced by these biotechnological processes can be used as soil improvers and can even be used in the biosorption/desorption processes for the concentration of metals in the recovery of metals [23], where these processes are relevant due to the formation of non-soluble compounds between metals and metabolites generated by certain microorganisms.
The use of bioleaching avoids the depletion of non-renewable resources and reduces raw material acquisition costs.
6. Conclusions
Bacterial bioleaching is applied when the aim is to recover a metal of interest without preserving the properties of the solid matrix. In contrast, fungal bioleaching is preferred when it is necessary to preserve the properties of the solid matrix, especially its crystalline properties.
Fungal bioleaching involves a two-step process (direct or indirect), whereas bacterial bioleaching is a single-step process.
Optimal pH for fungal bioleaching varies depending on the microorganism and metal source, ranging from pH 2.5 to pH 10–12.
Temperature and agitation–aeration conditions significantly affect the efficiency of bioleaching, with temperatures ranging from 25 °C to 95 °C and agitation–aeration ranging from 0 to 400 rpm and 100–400 mL/min, respectively.
The pulp density used in bioleaching, ranging from 1% to 10% (w/v), influences the extraction efficiency of metals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151310222/s1, Table S1: Leading groups of organic acids, formula, dissociation reactions, and corresponding pKa [2,131,132].
Author Contributions
Conceptualization, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; methodology, C.O.-L.; software, C.O.-L. and L.G.-A.; validation, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; formal analysis, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; investigation, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; resources, C.O.-L. and L.G.-A.; data curation, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; writing—original draft preparation, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; writing—review and editing, C.O.-L., L.G.-A., M.R.-C. and L.R.-C.; visualization, C.O.-L. and L.R.-C.; supervision, C.O.-L.; project administration, C.O.-L.; funding acquisition, C.O.-L., L.G.-A. and M.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Bioexplora S.A.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| g/Mg | Concentration unit equivalent to gram per megagram, or Ton |
| SMMC | Solid Matrices with Metal Contents |
| SEM | Scanning Electron Microscopy |
| WEEE | Waste Electrical and Electronic Equipment |
| FCC | Fluid Catalytic Cracking |
References
- Pourhossein, F.; Mousavi, S.M. A Novel Rapid and Selective Microbially Thiosulfate Bioleaching of Precious Metals from Discarded Telecommunication Printed Circuited Boards (TPCBs). Resour. Conserv. Recycl. 2022, 187, 106599. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Gnanamoorthy, G.; Gupta, N.; Algahtani, A.; Islam, S.; Choudhary, N.; Modi, S.; Jeon, B. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials 2021, 14, 6333. [Google Scholar] [CrossRef]
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628. [Google Scholar] [CrossRef]
- Steinbach, V.; Wellmer, F.-W. Consumption and Use of Non-Renewable Mineral and Energy Raw Materials from an Economic Geology Point of View. Sustainability 2010, 2, 1408–1430. [Google Scholar] [CrossRef]
- Kuusiola, T.; Wierink, M.; Heiskanen, K. Comparison of Collection Schemes of Municipal Solid Waste Metallic Fraction: The Impacts on Global Warming Potential for the Case of the Helsinki Metropolitan Area, Finland. Sustainability 2012, 4, 2586–2610. [Google Scholar] [CrossRef]
- Business Wire the Worldwide Metal Recycling Industry Is Expected to Reach $368.7 Billion by 2030. Available online: https://www.businesswire.com/news/home/20220503005770/en/The-Worldwide-Metal-Recycling-Industry-is-Expected-to-Reach-368.7-Billion-by-2030---ResearchAndMarkets.com#:~:text=The%20global%20metal%20recycling%20market,5.2%25%20from%202021%20to%202030 (accessed on 28 February 2023).
- Psomopoulos, C.S.; Kungolos, A.; Di Nardo, A. Advances in Industrial Waste Reduction. Appl. Sci. 2023, 13, 1403. [Google Scholar] [CrossRef]
- De Colle, M.; Puthucode, R.; Karasev, A.; Jönsson, P.G. A Study of Treatment of Industrial Acidic Wastewaters with Stainless Steel Slags Using Pilot Trials. Materials 2021, 14, 4806. [Google Scholar] [CrossRef]
- Moreau, V.; Dos Reis, P.; Vuille, F. Enough Metals? Resource Constraints to Supply a Fully Renewable Energy System. Resources 2019, 8, 29. [Google Scholar] [CrossRef]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Biorecovery of Metals from Electronic Waste. In Sustainable Heavy Metal Remediation; Case Studies; Spring: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 241–278. [Google Scholar]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- The Business Research Company Metal Products Global Market Opportunities & Strategies. Available online: https://www.thebusinessresearchcompany.com/report/metal-products-global-market (accessed on 1 March 2023).
- Guo, H.; Min, Z.; Hao, Y.; Wang, X.; Fan, J.; Shi, P.; Min, Y.; Xu, Q. Sustainable Recycling of LiCoO2 Cathode Scrap on the Basis of Successive Peroxymonosulfate Activation and Recovery of Valuable Metals. Sci. Total Environ. 2021, 759, 143478. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Zhang, X.; Liang, Z.; Zhao, Q. Recycling Lithium and Cobalt from LIBs Using Microwave-Assisted Deep Eutectic Solvent Leaching Technology at Low-Temperature. Mater. Chem. Phys. 2022, 289, 126466. [Google Scholar] [CrossRef]
- Ma, C.; Svärd, M.; Forsberg, K. Recycling Cathode Material LiCo1/3Ni1/3Mn1/3O2 by Leaching with a Deep Eutectic Solvent and Metal Recovery with Antisolvent Crystallization. Resour. Conserv. Recycl. 2022, 186, 106579. [Google Scholar] [CrossRef]
- Blázquez Izquierdo, M.L.; Muñoz Sánchez, J.A.; Castro, L. Special Issue “Leaching/Bioleaching and Recovery of Metals”. Available online: https://www.mdpi.com/journal/metals/special_issues/leaching_bio_recovery (accessed on 19 January 2023).
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A Review of Sulfide Minerals Microbially Assisted Leaching in Stirred Tank Reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Deng, X.; Chai, L.; Yang, Z.; Tang, C.; Wang, Y.; Shi, Y. Bioleaching Mechanism of Heavy Metals in the Mixture of Contaminated Soil and Slag by Using Indigenous Penicillium Chrysogenum Strain F1. J. Hazard. Mater. 2013, 248–249, 107–114. [Google Scholar] [CrossRef]
- Khodadadmahmoudi, G.; Abdollahi, H.; Mohammadzadeh, A.; Saneie, R.; Mirmohammadi, M.; Rezaei, A.; Jozanikohan, G.; Naderi, H. Green Extraction of Nickel and Valuable Metals from Pyrrhotite Samples with Different Crystallographic Structures through Acidophilic Bioleaching. J. Environ. Manag. 2022, 317, 115394. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Ramírez Carmona, M.E.; Pereira da Silva, M.A.; Ferreira Leite, S.G.; Vasco Echeverri, O.H.; Ocampo-López, C. Packed Bed Redistribution System for Cr(III) and Cr(VI) Biosorption by Saccharomyces Cerevisiae. J. Taiwan Inst. Chem. Eng. 2012, 43, 428–432. [Google Scholar] [CrossRef]
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of Residual Fungal Biomass: A Review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar] [CrossRef]
- Gómez-Aguilar, D.L.; Esteban-Muñoz, J.A.; Rodríguez-Miranda, J.P.; Baracaldo-Guzmán, D.; Salcedo-Parra, O.J. Desorption of Coffee Pulp Used as an Adsorbent Material for Cr(III and VI) Ions in Synthetic Wastewater: A Preliminary Study. Molecules 2022, 27, 2170. [Google Scholar] [CrossRef]
- Lodeiro, P.; Herrero, R.; Sastre de Vicente, M.E. Batch Desorption Studies and Multiple Sorption–Regeneration Cycles in a Fixed-Bed Column for Cd(II) Elimination by Protonated Sargassum Muticum. J. Hazard. Mater. 2006, 137, 1649–1655. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Ocampo-López, C.; Rendón-Castrillon, L.; Vélez-Salazar, Y.; Muñoz-Blandón, O.; Salazar-Martínez, S. System for Separating Fluids and Method for the Application Thereof. 2015, pp. 1–17. Available online: https://www.freepatentsonline.com/WO2015011672.pdf (accessed on 28 February 2023).
- Benavente, O.; Hernández, M.C.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W. Reducing Agents in the Leaching of Manganese Ores: A Comprehensive Review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Vélez-Salazar, Y. Desarrollo de un Sistema de Recuperación de Oro Proveniente de la Lixiviación con Caldos Fermentados de Origen Fúngico a Través de un Proceso Carbon-in-Leach. Ph.D. Thesis, Universidad Pontificia Bolivariana, Medellín, CO, USA, 2020. [Google Scholar]
- Kuthiala, T.; Thakur, K.; Sharma, D.; Singh, G.; Khatri, M.; Arya, S.K. The Eco-Friendly Approach of Cocktail Enzyme in Agricultural Waste Treatment: A Comprehensive Review. Int. J. Biol. Macromol. 2022, 209, 1956–1974. [Google Scholar] [CrossRef]
- Ramirez-Carmona, M.; Muñoz-Blandón, O. Agroindustrial Waste Cellulose Using Fermented Broth of White Rot Fungi. Rev. Mex. Ing. Quim. 2016, 15, 23–31. [Google Scholar]
- Medici, F. Recovery of Waste Materials: Technological Research and Industrial Scale-Up. Materials 2022, 15, 685. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Medici, F.; Pietrelli, L.; Piga, L. Effect of Acid Leaching Pre-Treatment on Gold Extraction from Printed Circuit Boards of Spent Mobile Phones. Materials 2021, 14, 362. [Google Scholar] [CrossRef]
- Sydow, M.; Chrzanowski, Ł.; Leclerc, A.; Laurent, A.; Owsianiak, M. Terrestrial Ecotoxic Impacts Stemming from Emissions of Cd, Cu, Ni, Pb and Zn from Manure: A Spatially Differentiated Assessment in Europe. Sustainability 2018, 10, 4094. [Google Scholar] [CrossRef]
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G. Effect of Pig and Cattle Slurry Application on Heavy Metal Composition of Maize Grown on Different Soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef]
- Mavakala, B.K.; Sivalingam, P.; Laffite, A.; Mulaji, C.K.; Giuliani, G.; Mpiana, P.T.; Poté, J. Evaluation of Heavy Metal Content and Potential Ecological Risks in Soil Samples from Wild Solid Waste Dumpsites in Developing Country under Tropical Conditions. Environ. Chall. 2022, 7, 100461. [Google Scholar] [CrossRef]
- Susianti, B.; Warmadewanthi, I.D.A.A.; Tangahu, B.V. Characterization and Experimental Evaluation of Cow Dung Biochar + Dolomite for Heavy Metal Immobilization in Solid Waste from Silica Sand Purification. Bioresour. Technol. Rep. 2022, 18, 101102. [Google Scholar] [CrossRef]
- Vácha, R. Heavy Metal Pollution and Its Effects on Agriculture. Agronomy 2021, 11, 1719. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K. The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and Its Speciation in Soil. Agronomy 2020, 10, 1550. [Google Scholar] [CrossRef]
- Joseph, O.O.; Babaremu, K.O. Agricultural Waste as a Reinforcement Particulate for Aluminum Metal Matrix Composite (AMMCs): A Review. Fibers 2019, 7, 33. [Google Scholar] [CrossRef]
- Centro de Estudios y de Investigación en Biotecnología (CIBIOT); Centro de Investigación e Innovación Energía (CIIEN); Universidad Pontificia Bolivariana. Biomasa de Residuos Agrícolas en el Departamento de Antioquia, 2nd ed.; Universidad Pontificia Bolivariana, Ed.; Universidad Pontificia Bolivariana: Medellín, CO, USA, 2015; ISBN 9789586962292. [Google Scholar]
- FAO. Tackling Food Loss and Waste: A Triple Win Opportunity. Available online: https://www.fao.org/newsroom/detail/FAO-UNEP-agriculture-environment-food-loss-waste-day-2022/en (accessed on 29 May 2023).
- FAO. Sustainable Development Goals. Available online: https://www.fao.org/sustainable-development-goals/indicators/1231/en/ (accessed on 29 May 2023).
- United Nations Mensaje del Secretario General Para. 2023. Available online: https://www.un.org/es/observances/zero-waste-day/messages (accessed on 30 May 2023).
- World Health Organization. Compendium of WHO and Other UN Guidance on Health and Environment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022.
- Sharma, P.; Dutta, D.; Udayan, A.; Nadda, A.K.; Lam, S.S.; Kumar, S. Role of Microbes in Bioaccumulation of Heavy Metals in Municipal Solid Waste: Impacts on Plant and Human Being. Environ. Pollut. 2022, 305, 119248. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Koda, E.; Marecka, M.; Baczewska, A.; Brągoszewska, P.; Sieczka, A.; Osiński, P. Impact of the Municipal Solid Waste Łubna Landfill on Environmental Pollution by Heavy Metals. Water 2016, 8, 470. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, X.; Hao, Q.; Si, R. Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals. Metals 2020, 10, 815. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, X.; Zhao, Y.; Yan, J. Disposal Technology and New Progress for Dioxins and Heavy Metals in Fly Ash from Municipal Solid Waste Incineration: A Critical Review. Environ. Pollut. 2022, 311, 119878. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. EPA’s Guide for Industrial Waste Management; U.S. Environmental Protection Agency: Philadelphia, PA, USA, 2023.
- Lan, M.; He, Z.; Hu, X. Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation. Metals 2022, 12, 742. [Google Scholar] [CrossRef]
- Zheng, F.; Guo, Y.; Chen, F.; Wang, S.; Zhang, J.; Yang, L.; Qiu, G. Fluoride Leaching of Titanium from Ti-Bearing Electric Furnace Slag in [NH4+]-[F−] Solution. Metals 2021, 11, 1176. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Wang, L. Extraction of the Rare Element Vanadium from Vanadium-Containing Materials by Chlorination Method: A Critical Review. Metals 2021, 11, 1301. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological Strategies for the Recovery of Valuable and Critical Raw Materials from Waste Electrical and Electronic Equipment (WEEE)–A Review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Latosińska, J.; Czapik, P. The Ecological Risk Assessment and the Chemical Speciation of Heavy Metals in Ash after the Incineration of Municipal Sewage Sludge. Sustainability 2020, 12, 6517. [Google Scholar] [CrossRef]
- Čech, V.; Gregorová, B.; Krokusová, J.; Košová, V.; Hronček, P.; Molokáč, M.; Hlaváčová, J. Environmentally Degraded Mining Areas of Eastern Slovakia As a Potential Object of Geotourism. Sustainability 2020, 12, 6029. [Google Scholar] [CrossRef]
- Sousa, R.; Futuro, A.; Fiúza, A.; Vila, M.C.; Dinis, M.L. Bromine Leaching as an Alternative Method for Gold Dissolution. Miner. Eng. 2018, 118, 16–23. [Google Scholar] [CrossRef]
- Brown, G.; Katz, D.; Foust, A. Unit Operations, 1st ed.; John Wiley & Sons: New York, NY, USA, 1960. [Google Scholar]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The Role of Microorganisms in Gold Processing and Recovery—A Review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Latorre, M.; Cortés, M.P.; Travisany, D.; Di Genova, A.; Budinich, M.; Reyes-Jara, A.; Hödar, C.; González, M.; Parada, P.; Bobadilla-Fazzini, R.A.; et al. The Bioleaching Potential of a Bacterial Consortium. Bioresour. Technol. 2016, 218, 659–666. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The Geomicrobiology of Gold. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef]
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes. Environ. 2008, 23, 269–276. [Google Scholar] [CrossRef]
- Lambert, F.; Gaydardzhiev, S.; Léonard, G.; Lewis, G.; Bareel, P.-F.; Bastin, D. Copper Leaching from Waste Electric Cables by Biohydrometallurgy. Miner. Eng. 2015, 76, 38–46. [Google Scholar] [CrossRef]
- Potysz; Kierczak Prospective (Bio)Leaching of Historical Copper Slags as an Alternative to Their Disposal. Minerals 2019, 9, 542. [CrossRef]
- Guerrero, L.; Esbrí, J. Aplicabilidad de la Biolixiviación Como un Método Sustitutivo de la Amalgamación con Mercurio para la Recuperación del Oro en la Minería Artesanal del Sur de Perú. Bachelor’s Thesis, Escola Politècnica Superior d’Enginyeria de Manresa, Lima, Peru, 2014. [Google Scholar]
- Khaing, S.Y.; Sugai, Y.; Sasaki, K. Gold Dissolution from Ore with Iodide-Oxidising Bacteria. Sci. Rep. 2019, 9, 4178. [Google Scholar] [CrossRef]
- Ballester, A. Minería Química, 1st ed.; Instituto Tecnológico Geominero de España, Ed.; Instituto Tecnológico Geominero de España: Madrid, Spain, 1991. [Google Scholar]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial Leaching of Critical Metal Values from Polish Copper Metallurgical Slags Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Ballester, A.; Blázquez, M.L.; González, F.; Muñoz, J.A. Mecanismo de Biolixiviación de Sulfuros Metálicos. Rev. Metal. 2001, 37, 665–672. [Google Scholar] [CrossRef]
- Chuquipoma, M.; Sergio, F. Biolixiviación; Osinergmin: Lima, Peru, 2016; Available online: https://www.osinergmin.gob.pe/seccion/centro_documental/mineria/Documentos/Publicaciones/Biolixiviacion.pdf (accessed on 10 May 2023).
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and Its Potential Application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Boon, M.; Snijder, M.; Hansford, G.S.; Heijnen, J.J. The Oxidation Kinetics of Zinc Sulphide with Thiobacillus ferrooxidans. Hydrometallurgy 1998, 48, 171–186. [Google Scholar] [CrossRef]
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of Leptospirillum Ferrooxidans for Leaching. Appl. Environ. Microbiol. 1992, 58, 85–92. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Ha, M.-G.; Shin, S.; Seo, M.; Jang, J.; Jo, S.; Kim, D.; Lee, S.; Jung, Y.; Kang, P.; et al. Electrochemical Effect on Bioleaching of Arsenic and Manganese from Tungsten Mine Wastes Using Acidithiobacillus spp. J. Environ. Manag. 2018, 223, 852–859. [Google Scholar] [CrossRef]
- Sreekrishnan, T.R.; Tyagi, R.D. A Comparative Study of the Cost of Leaching out Heavy Metals from Sewage Sludges. Process Biochem. 1996, 31, 31–41. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gupta, A. Current Trends in Applicability of Thermophiles and Thermozymes in Bioremediation of Environmental Pollutants. In Microbial Extremozymes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–176. [Google Scholar]
- Borje Lindstrom, E.; Wold, S.; Kettaneh-Wold, N. Optimization of Pyrite Bioleaching Using Sulfolobus acidocaldarius. Appl. Microbiol. Biotechnol. 1993, 38, 702–707. [Google Scholar] [CrossRef]
- Brierley, C.L.; Brierley, J.A. Anaerobic Reduction of Molybdenum by Sulfolobus Species. Zent. Bakteriol. Mikrobiol. Hyg. I. Abt. Orig. C Allg. Angew. Okol. Mikrobiol. 1982, 3, 289–294. [Google Scholar] [CrossRef]
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of Iron, Copper, Lead, and Zinc from the Sludge Mining Sediment at Different Particle Sizes, PH, and Pulp Density Using Acidithiobacillus ferrooxidans. Minerals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Acevedo, F.; Gentina, J. Fundamentos y Perspectivas de las Tecnologías Biomineras, 1st ed.; Acevedo, F., Gentina, J., Eds.; Ediciones Universitarias de Valparaíso: Valparaiso, Chile, 2005. [Google Scholar]
- Rossi, G. Biohydrometallurgy, 1st ed.; McGraw-Hill: Hamburg, Germany, 1990. [Google Scholar]
- Rodríguez, Y. Contribución al Estudio Del Mecanismo de Biolixiviación de Distintos Sulfuros Metálicos Con Bacterias Mesófilas y Termófilas. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 2000. [Google Scholar]
- Berry, V.; Murr, L. Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena, 1st ed.; Murr, L., Torma, A., Brierley, J., Eds.; Elsevier: New York, NY, USA, 1978; ISBN 9780125111508. [Google Scholar]
- Bennett, J.C.; Tributsch, H. Bacterial Leaching Patterns on Pyrite Crystal Surfaces. J. Bacteriol. 1978, 134, 310–317. [Google Scholar] [CrossRef]
- Hansford, G.S.; Drossou, M. A Propagating Pore Model for the Batch Bioleach Kinetics of Refractory Gold-Bearing Pyrite. In Proceedings of the Biohydrometallurgy International Symposium, Warwick, UK, 12–16 July 1987; Norris, P.R., Kelly, D.P., Eds.; pp. 345–358. [Google Scholar]
- Tributsch, H. The Oxidative Desintegration of Sulfide Crystals by Thiobacillus ferrooxidans. Naturwissenschaften 1976, 63, 88. [Google Scholar] [CrossRef]
- Newman, D.K. How Bacteria Respire Minerals. Science 2001, 292, 1312–1313. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)Chemistry of Bacterial Leaching—Direct vs. Indirect Bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Masloboev, V.; Seleznev, S.; Svetlov, A.; Makarov, D. Hydrometallurgical Processing of Low-Grade Sulfide Ore and Mine Waste in the Arctic Regions: Perspectives and Challenges. Minerals 2018, 8, 436. [Google Scholar] [CrossRef]
- Kim, B.-J.; Koh, Y.-K.; Kwon, J.-S. Bioleaching of Pyrrhotite with Bacterial Adaptation and Biological Oxidation for Iron Recovery. Metals 2021, 11, 295. [Google Scholar] [CrossRef]
- Kovaříková, H.; Janáková, I.; Čablík, V.; Vrlíková, V. Bacterial Leaching of Polymetallic Ores from Zlatý Chlum Locality. J. Pol. Miner. Eng. Soc. 2019, 1, 145–158. [Google Scholar] [CrossRef]
- Zhappar, N.K.; Shaikhutdinov, V.M.; Kanafin, Y.N.; Ten, O.A.; Balpanov, D.S.; Korolkov, I.V.; Collinson, S.R.; Erkasov, R.S.; Bakibaev, A.A. Bacterial and Chemical Leaching of Copper-Containing Ores with the Possibility of Subsequent Recovery of Trace Silver. Chem. Pap. 2019, 73, 1357–1367. [Google Scholar] [CrossRef]
- Jin, Z.; Huang, T.; Zhang, X.; Zhang, S. Bioelectrochemical-Assisted Bioleaching of Chalcopyrite: Effect of Pulp Density, Anode Material, and Sliver Ion. Process Saf. Environ. Prot. 2022, 159, 740–748. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wu, J.-Q.; Sung, S. Effects of Sulfur Dosage on Continuous Bioleaching of Heavy Metals from Contaminated Sediment. J. Hazard. Mater. 2022, 424, 127257. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A Review on Recent Advancements in Recovery of Valuable and Toxic Metals from E-Waste Using Bioleaching Approach. Chemosphere 2022, 287, 132230. [Google Scholar] [CrossRef]
- Diaz, M.A.; De Ranson, I.U.; Dorta, B.; Banat, I.M.; Blazquez, M.L.; Gonzalez, F.; Muñoz, J.A.; Ballester, A. Metal Removal from Contaminated Soils Through Bioleaching with Oxidizing Bacteria and Rhamnolipid Biosurfactants. Soil Sediment Contam. Int. J. 2015, 24, 16–29. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching Remediation of Heavy Metal-Contaminated Soils Using Burkholderia sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152. [Google Scholar] [CrossRef]
- Tran, T.M.; Han, H.-J.; Ko, J.-I.; Lee, J.-U. Effect of Indigenous Microbial Consortium on Bioleaching of Arsenic from Contaminated Soil by Shewanella Putrefaciens. Sustainability 2020, 12, 3286. [Google Scholar] [CrossRef]
- Praburaman, L.; Park, J.-H.; Govarthanan, M.; Selvankumar, T.; Oh, S.-G.; Jang, J.-S.; Cho, M.; Kamala-Kannan, S.; Oh, B.-T. Impact of an Organic Formulation (Panchakavya) on the Bioleaching of Copper and Lead in Contaminated Mine Soil. Chemosphere 2015, 138, 127–132. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Deng, Y.; Zhang, S.; Hao, X.; Zhu, P.; Zhou, J.; Yin, H.; Liang, Y.; Liu, H.; et al. Bioremediation of Cadmium-Contaminated Paddy Soil Using an Autotrophic and Heterotrophic Mixture. RSC Adv. 2020, 10, 26090–26101. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, P.; Zhang, H.; Liang, Y.; Yin, H.; Liu, X.; Bai, L.; Liu, H.; Jiang, H. Mixotrophic Acidophiles Increase Cadmium Soluble Fraction and Phytoextraction Efficiency from Cadmium Contaminated Soils. Sci. Total Environ. 2019, 655, 347–355. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of Bioleaching of Heavy Metals from Sediment with Indigenous Bacteria Using Agricultural Sulfur Soil Conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef]
- Gan, M.; Jie, S.; Li, M.; Zhu, J.; Liu, X. Bioleaching of Multiple Metals from Contaminated Sediment by Moderate Thermophiles. Mar. Pollut. Bull. 2015, 97, 47–55. [Google Scholar] [CrossRef]
- Qayyum, S.; Meng, K.; Pervez, S.; Nawaz, F.; Peng, C. Optimization of PH, Temperature and Carbon Source for Bioleaching of Heavy Metals by Aspergillus Flavus Isolated from Contaminated Soil. Main Group Met. Chem. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Porzionato, N.; Tufo, A.; Candal, R.; Curutchet, G. Metal Bioleaching from Anaerobic Sediments from Reconquista River Basin (Argentina) as a Potential Remediation Strategy. Environ. Sci. Pollut. Res. 2017, 24, 25561–25570. [Google Scholar] [CrossRef]
- Gan, M.; Song, Z.; Zhu, J.; Liu, X. Efficient Bioleaching of Heavy Metals from Contaminated Sediment in Batch Method Coupled with the Assistance of Heterotrophic Microorganisms. Environ. Earth Sci. 2016, 75, 457. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J.-U. A Comparison of Microbial Leaching and Chemical Leaching of Arsenic and Heavy Metals from Mine Tailings. Biotechnol. Bioprocess Eng. 2015, 20, 91–99. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of Metals from Lead-Zinc Mine Tailings Using Bioleaching and Followed by Sulfide Precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef]
- Ye, M.; Yan, P.; Sun, S.; Han, D.; Xiao, X.; Zheng, L.; Huang, S.; Chen, Y.; Zhuang, S. Bioleaching Combined Brine Leaching of Heavy Metals from Lead-Zinc Mine Tailings: Transformations during the Leaching Process. Chemosphere 2017, 168, 1115–1125. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J.-U. Effect of Sulfur Concentration on Microbial Removal of Arsenic and Heavy Metals from Mine Tailings Using Mixed Culture of Acidithiobacillus spp. J. Geochem. Explor. 2015, 148, 241–248. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of Arsenic from Highly Contaminated Mine Tailings Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New Approaches for Extracting and Recovering Metals from Mine Tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, M.; Abdollahzadeh, A.A.; Askari, M. Bioleaching of Copper, Nickel and Cobalt from the Low Grade Sulfidic Tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of Arsenopyrite from Janggun Mine Tailings (South Korea) Using an Adapted Mixed Mesophilic Culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of Indium from Sphalerite Ore and Flotation Tailings by Bioleaching and Subsequent Precipitation Processes. Miner. Eng. 2015, 75, 94–99. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of Cobalt from Cu/Co-Rich Sulfidic Mine Tailings from the Polymetallic Rammelsberg Mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.-E.; Kinnunen, P. Bioleaching of Cobalt from Sulfide Mining Tailings; a Mini-Pilot Study. Hydrometallurgy 2020, 196, 105418. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Yang, Q.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Comparative Study on Bioleaching of Two Different Types of Low-Grade Copper Tailings by Mixed Moderate Thermophiles. Trans. Nonferrous Met. Soc. China 2018, 28, 1847–1853. [Google Scholar] [CrossRef]
- Huerta-Rosas, B.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Gómez-Castro, F.I.; Carrillo-Pedroza, F.R.; Romo-Rodríguez, P.; Gutiérrez-Corona, J.F. Aerobic Processes for Bioleaching Manganese and Silver Using Microorganisms Indigenous to Mine Tailings. World J. Microbiol. Biotechnol. 2020, 36, 124. [Google Scholar] [CrossRef]
- de Wet, M.M.M.; Brink, H.G. Fungi in the Bioremediation of Toxic Effluents. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 407–431. [Google Scholar]
- Gadd, G.M. Microbial Influence on Metal Mobility and Application for Bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Almudena, A.; Lizaso, J. Hongos y Micotoxinas; Fundación Ibérica para la Seguridad Alimentaria: Madrid, Spain, 2001. [Google Scholar]
- Serrano-Coll, H.; Cardona-Castro, N. Mycotoxicosis and Mycotoxins: Generalities and Basic Aspects. Rev. CES Med. 2015, 29, 143–151. [Google Scholar]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Grewal, H.S.; Kalra, K.L. Fungal Production of Citric Acid. Biotechnol. Adv. 1995, 13, 209–234. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar] [PubMed]
- Khan, I.; Qayyum, S.; Maqbool, F.; Mujaddad-ur-Rehman; Hayat, A.; Farooqui, M.S. Microbial Organic Acids Production, Biosynthetic Mechanism and Applications -Mini Review. Indian J. Geomarine. Sci. 2017, 46, 2165–2174. [Google Scholar]
- Roukas, T. Citric and Gluconic Acid Production from Fig by Aspergillus Niger Using Solid-State Fermentation. J. Ind. Microbiol. Biotechnol. 2000, 25, 298–304. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in Citric Acid Fermentation by Aspergillus Niger: Biochemical Aspects, Membrane Transport and Modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef]
- Alongi, K.S.; Shields, G.C. Theoretical Calculations of Acid Dissociation Constants: A Review Article. Annu. Rep. Comput. Chem. 2010, 6, 113–138. [Google Scholar]
- Kütt, A.; Selberg, S.; Kaljurand, I.; Tshepelevitsh, S.; Heering, A.; Darnell, A.; Kaupmees, K.; Piirsalu, M.; Leito, I. PKa Values in Organic Chemistry–Making Maximum Use of the Available Data. Tetrahedron. Lett. 2018, 59, 3738–3748. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Martin, D.S.; Cole, R.J.; Haq, S. Investigating the Adsorption of Oxalic Acid onto Cu(110) to Create a Chemically Functionalised Surface. Surf. Sci. 2003, 539, 171–181. [Google Scholar] [CrossRef]
- Sajadi, S.A.A. Metal Ion-Binding Properties of L-Glutamic Acid and L-Aspartic Acid, a Comparative Investigation. Nat. Sci. 2010, 2, 85–90. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-López, C.; Giraldo-Aristizabal, R. Proceso Para La Separación de Metales En Una Matriz Sólida Mediante Lixiviación, Que Emplea Una Composición Que Contiene Ácidos Carboxílicos, Monosacáridos, Disacáridos, Aminoácidos, Ácidos Grasos, Alcoholes y Compuestos Fenólicos. Patent Number NC2019/0013648, 2022. 13. [Google Scholar]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging Role of Organic Acids in Leaching of Valuable Metals from Refinery-Spent Hydroprocessing Catalysts, and Potential Techno-Economic Challenges: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of Low-Grade Waste Printed Circuit Boards by Mixed Fungal Culture and Its Community Structure Analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal Bioleaching of WPCBs Using Aspergillus Niger: Observation, Optimization and Kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Nili, S.; Arshadi, M.; Yaghmaei, S. Fungal Bioleaching of E-Waste Utilizing Molasses as the Carbon Source in a Bubble Column Bioreactor. J. Environ. Manag. 2022, 307, 114524. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect Bioleaching of Low-Grade Nickel Limonite and Saprolite Ores Using Fungal Metabolic Organic Acids Generated by Aspergillus Niger. Hydrometallurgy 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M.; Rastegar, S.O.; Azargoshasb, H. Fungal Leaching of Valuable Metals from a Power Plant Residual Ash Using Penicillium Simplicissimum: Evaluation of Thermal Pretreatment and Different Bioleaching Methods. Waste Manag. 2016, 52, 309–317. [Google Scholar] [CrossRef]
- Biswas, S.; Bhattacharjee, K. Fungal Assisted Bioleaching Process Optimization and Kinetics: Scenario for Ni and Co Recovery from a Lateritic Chromite Overburden. Sep. Purif. Technol. 2014, 135, 100–109. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Ting, Y.-P. Metal Extraction from Municipal Solid Waste (MSW) Incinerator Fly Ash—Chemical Leaching and Fungal Bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Alavi, N.; Partovi, K.; Majlessi, M.; Rashidi, M.; Alimohammadi, M. Bioleaching of Metals from Cellphones Batteries by a Co-Fungus Medium in Presence of Carbon Materials. Bioresour. Technol. Rep. 2021, 15, 100768. [Google Scholar] [CrossRef]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Enhanced Bio-Recovery of Aluminum from Low-Grade Bauxite Using Adapted Fungal Strains. Braz. J. Microbiol. 2020, 51, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium Bioleaching: An Emerging Approach for the Recovery of Li from Spent Lithium Ion Batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of Heavy Metal (Cd and Cr)–Polluted Soil through Indigenous Metallotolerant Fungal Isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Alvarez, R. Removal of Copper, Chromium and Arsenic from Preservative-Treated Wood by Chemical Extraction-Fungal Bioleaching. Waste Manag. 2009, 29, 1885–1891. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Gao, W.; Zheng, Y. Bioremediation of Hexavalent Chromium Contaminated Soil by a Bioleaching System with Weak Magnetic Fields. Int. Biodeterior. Biodegrad. 2018, 128, 41–47. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, S.; Sun, L.; Jacques, D.A.; Tang, J.; Lian, M.; Ji, Z.; Wang, J.; Zhu, J.; Xu, Z. Bioleaching of Heavy Metals from Contaminated Sediments by the Aspergillus Niger Strain SY1. J. Soils Sediments 2015, 15, 1029–1038. [Google Scholar] [CrossRef]
- Seh-Bardan, B.J.; Othman, R.; Wahid, S.A.; Husin, A.; Sadegh-Zadeh, F. Bioleaching of Heavy Metals from Mine Tailings by Aspergillus Fumigatus. Bioremediat. J. 2012, 16, 57–65. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by Filamentous Fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Springer: Boston, MA, USA, 2004; pp. 307–340. [Google Scholar]
- Shankar, T.; Sivakumar, T. Optimization of Citric Acid Production Using Aspergillus Niger Isolated from the Leaf Litter Soil of Sathuragiri Hills. Univers. J. Microbiol. Res. 2016, 4, 79–87. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The Selective Leaching of Copper from a Gold–Copper Concentrate in Glycine Solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).