Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices
Abstract
:1. Introduction
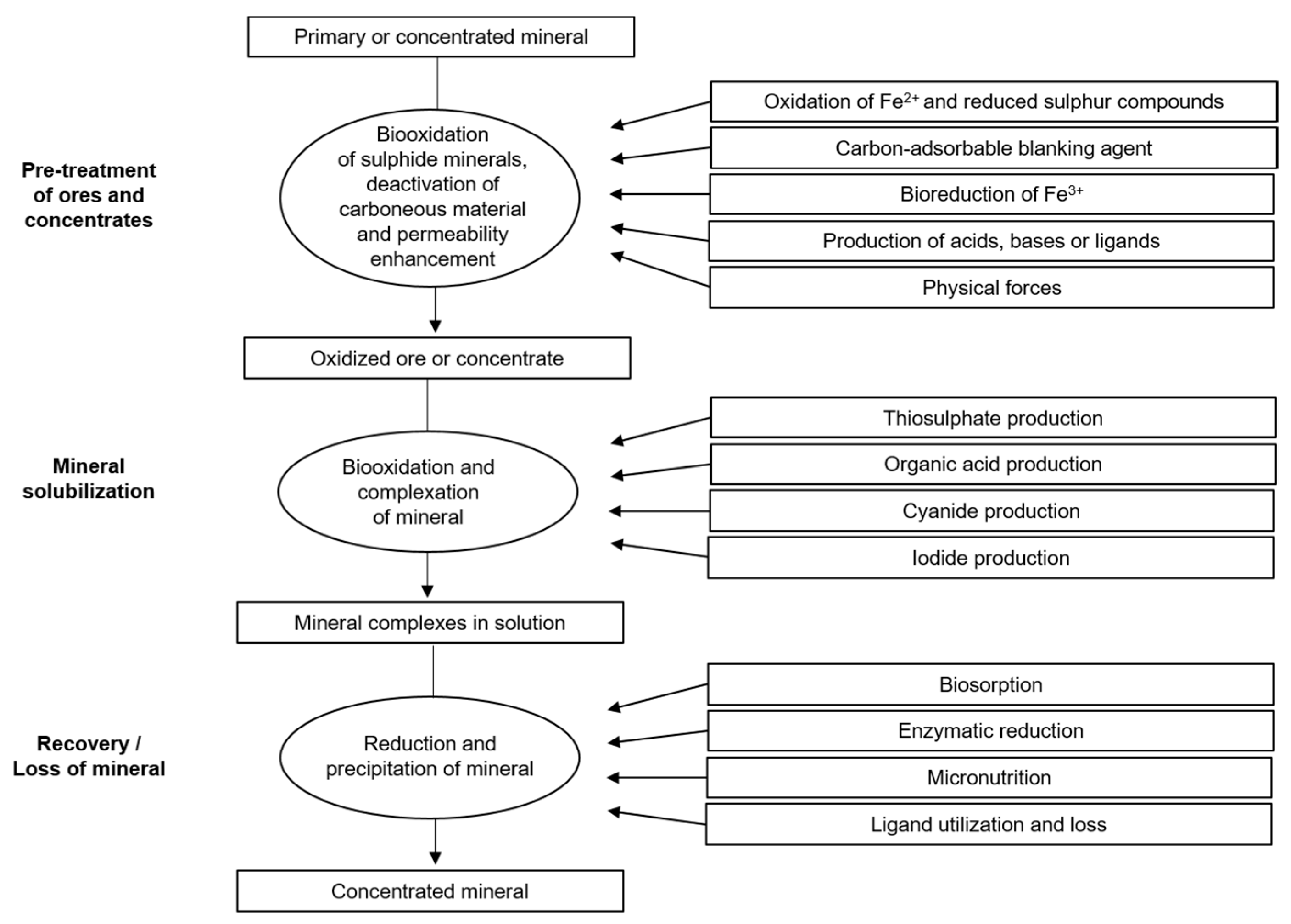
2. Recovery of Metals of Interest from Solid Matrices
3. Solid Matrices as a Metal Source
3.1. Agricultural Residues
3.2. Municipal Solid Waste
3.3. Industrial Wastes
4. Bioleaching
- A: solid solute that goes into the solution;
- B: inert solid (insoluble in S);
- S: extracting solvent.
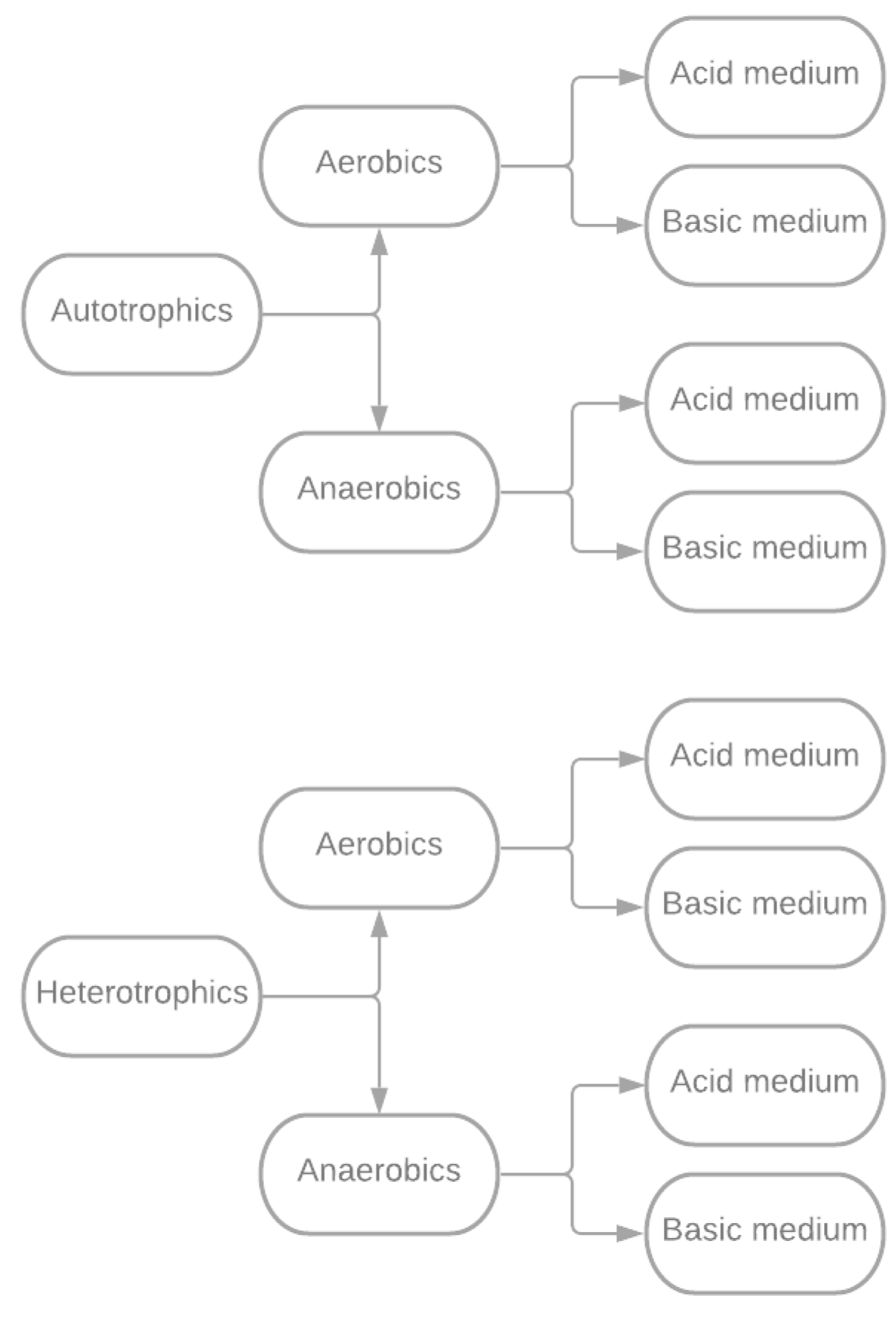
4.1. Bacterial Bioleaching
- Autotrophic: These microorganisms obtain nutrients and energy for their life cycles from the inorganic matter surrounding them.
- Heterotrophic: They require the availability of organic matter to complete their life cycles.
4.2. Fungal Bioleaching
5. Future Prospects
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| g/Mg | Concentration unit equivalent to gram per megagram, or Ton |
| SMMC | Solid Matrices with Metal Contents |
| SEM | Scanning Electron Microscopy |
| WEEE | Waste Electrical and Electronic Equipment |
| FCC | Fluid Catalytic Cracking |
References
- Pourhossein, F.; Mousavi, S.M. A Novel Rapid and Selective Microbially Thiosulfate Bioleaching of Precious Metals from Discarded Telecommunication Printed Circuited Boards (TPCBs). Resour. Conserv. Recycl. 2022, 187, 106599. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Gnanamoorthy, G.; Gupta, N.; Algahtani, A.; Islam, S.; Choudhary, N.; Modi, S.; Jeon, B. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials 2021, 14, 6333. [Google Scholar] [CrossRef]
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628. [Google Scholar] [CrossRef]
- Steinbach, V.; Wellmer, F.-W. Consumption and Use of Non-Renewable Mineral and Energy Raw Materials from an Economic Geology Point of View. Sustainability 2010, 2, 1408–1430. [Google Scholar] [CrossRef] [Green Version]
- Kuusiola, T.; Wierink, M.; Heiskanen, K. Comparison of Collection Schemes of Municipal Solid Waste Metallic Fraction: The Impacts on Global Warming Potential for the Case of the Helsinki Metropolitan Area, Finland. Sustainability 2012, 4, 2586–2610. [Google Scholar] [CrossRef] [Green Version]
- Business Wire the Worldwide Metal Recycling Industry Is Expected to Reach $368.7 Billion by 2030. Available online: https://www.businesswire.com/news/home/20220503005770/en/The-Worldwide-Metal-Recycling-Industry-is-Expected-to-Reach-368.7-Billion-by-2030---ResearchAndMarkets.com#:~:text=The%20global%20metal%20recycling%20market,5.2%25%20from%202021%20to%202030 (accessed on 28 February 2023).
- Psomopoulos, C.S.; Kungolos, A.; Di Nardo, A. Advances in Industrial Waste Reduction. Appl. Sci. 2023, 13, 1403. [Google Scholar] [CrossRef]
- De Colle, M.; Puthucode, R.; Karasev, A.; Jönsson, P.G. A Study of Treatment of Industrial Acidic Wastewaters with Stainless Steel Slags Using Pilot Trials. Materials 2021, 14, 4806. [Google Scholar] [CrossRef]
- Moreau, V.; Dos Reis, P.; Vuille, F. Enough Metals? Resource Constraints to Supply a Fully Renewable Energy System. Resources 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Biorecovery of Metals from Electronic Waste. In Sustainable Heavy Metal Remediation; Case Studies; Spring: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 241–278. [Google Scholar]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- The Business Research Company Metal Products Global Market Opportunities & Strategies. Available online: https://www.thebusinessresearchcompany.com/report/metal-products-global-market (accessed on 1 March 2023).
- Guo, H.; Min, Z.; Hao, Y.; Wang, X.; Fan, J.; Shi, P.; Min, Y.; Xu, Q. Sustainable Recycling of LiCoO2 Cathode Scrap on the Basis of Successive Peroxymonosulfate Activation and Recovery of Valuable Metals. Sci. Total Environ. 2021, 759, 143478. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Zhang, X.; Liang, Z.; Zhao, Q. Recycling Lithium and Cobalt from LIBs Using Microwave-Assisted Deep Eutectic Solvent Leaching Technology at Low-Temperature. Mater. Chem. Phys. 2022, 289, 126466. [Google Scholar] [CrossRef]
- Ma, C.; Svärd, M.; Forsberg, K. Recycling Cathode Material LiCo1/3Ni1/3Mn1/3O2 by Leaching with a Deep Eutectic Solvent and Metal Recovery with Antisolvent Crystallization. Resour. Conserv. Recycl. 2022, 186, 106579. [Google Scholar] [CrossRef]
- Blázquez Izquierdo, M.L.; Muñoz Sánchez, J.A.; Castro, L. Special Issue “Leaching/Bioleaching and Recovery of Metals”. Available online: https://www.mdpi.com/journal/metals/special_issues/leaching_bio_recovery (accessed on 19 January 2023).
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A Review of Sulfide Minerals Microbially Assisted Leaching in Stirred Tank Reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Deng, X.; Chai, L.; Yang, Z.; Tang, C.; Wang, Y.; Shi, Y. Bioleaching Mechanism of Heavy Metals in the Mixture of Contaminated Soil and Slag by Using Indigenous Penicillium Chrysogenum Strain F1. J. Hazard. Mater. 2013, 248–249, 107–114. [Google Scholar] [CrossRef]
- Khodadadmahmoudi, G.; Abdollahi, H.; Mohammadzadeh, A.; Saneie, R.; Mirmohammadi, M.; Rezaei, A.; Jozanikohan, G.; Naderi, H. Green Extraction of Nickel and Valuable Metals from Pyrrhotite Samples with Different Crystallographic Structures through Acidophilic Bioleaching. J. Environ. Manag. 2022, 317, 115394. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Ramírez Carmona, M.E.; Pereira da Silva, M.A.; Ferreira Leite, S.G.; Vasco Echeverri, O.H.; Ocampo-López, C. Packed Bed Redistribution System for Cr(III) and Cr(VI) Biosorption by Saccharomyces Cerevisiae. J. Taiwan Inst. Chem. Eng. 2012, 43, 428–432. [Google Scholar] [CrossRef]
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of Residual Fungal Biomass: A Review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar] [CrossRef]
- Gómez-Aguilar, D.L.; Esteban-Muñoz, J.A.; Rodríguez-Miranda, J.P.; Baracaldo-Guzmán, D.; Salcedo-Parra, O.J. Desorption of Coffee Pulp Used as an Adsorbent Material for Cr(III and VI) Ions in Synthetic Wastewater: A Preliminary Study. Molecules 2022, 27, 2170. [Google Scholar] [CrossRef]
- Lodeiro, P.; Herrero, R.; Sastre de Vicente, M.E. Batch Desorption Studies and Multiple Sorption–Regeneration Cycles in a Fixed-Bed Column for Cd(II) Elimination by Protonated Sargassum Muticum. J. Hazard. Mater. 2006, 137, 1649–1655. [Google Scholar] [CrossRef] [Green Version]
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Ocampo-López, C.; Rendón-Castrillon, L.; Vélez-Salazar, Y.; Muñoz-Blandón, O.; Salazar-Martínez, S. System for Separating Fluids and Method for the Application Thereof. 2015, pp. 1–17. Available online: https://www.freepatentsonline.com/WO2015011672.pdf (accessed on 28 February 2023).
- Benavente, O.; Hernández, M.C.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.K.; Purcell, W. Reducing Agents in the Leaching of Manganese Ores: A Comprehensive Review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Vélez-Salazar, Y. Desarrollo de un Sistema de Recuperación de Oro Proveniente de la Lixiviación con Caldos Fermentados de Origen Fúngico a Través de un Proceso Carbon-in-Leach. Ph.D. Thesis, Universidad Pontificia Bolivariana, Medellín, CO, USA, 2020. [Google Scholar]
- Kuthiala, T.; Thakur, K.; Sharma, D.; Singh, G.; Khatri, M.; Arya, S.K. The Eco-Friendly Approach of Cocktail Enzyme in Agricultural Waste Treatment: A Comprehensive Review. Int. J. Biol. Macromol. 2022, 209, 1956–1974. [Google Scholar] [CrossRef]
- Ramirez-Carmona, M.; Muñoz-Blandón, O. Agroindustrial Waste Cellulose Using Fermented Broth of White Rot Fungi. Rev. Mex. Ing. Quim. 2016, 15, 23–31. [Google Scholar]
- Medici, F. Recovery of Waste Materials: Technological Research and Industrial Scale-Up. Materials 2022, 15, 685. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Medici, F.; Pietrelli, L.; Piga, L. Effect of Acid Leaching Pre-Treatment on Gold Extraction from Printed Circuit Boards of Spent Mobile Phones. Materials 2021, 14, 362. [Google Scholar] [CrossRef]
- Sydow, M.; Chrzanowski, Ł.; Leclerc, A.; Laurent, A.; Owsianiak, M. Terrestrial Ecotoxic Impacts Stemming from Emissions of Cd, Cu, Ni, Pb and Zn from Manure: A Spatially Differentiated Assessment in Europe. Sustainability 2018, 10, 4094. [Google Scholar] [CrossRef] [Green Version]
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G. Effect of Pig and Cattle Slurry Application on Heavy Metal Composition of Maize Grown on Different Soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef] [Green Version]
- Mavakala, B.K.; Sivalingam, P.; Laffite, A.; Mulaji, C.K.; Giuliani, G.; Mpiana, P.T.; Poté, J. Evaluation of Heavy Metal Content and Potential Ecological Risks in Soil Samples from Wild Solid Waste Dumpsites in Developing Country under Tropical Conditions. Environ. Chall. 2022, 7, 100461. [Google Scholar] [CrossRef]
- Susianti, B.; Warmadewanthi, I.D.A.A.; Tangahu, B.V. Characterization and Experimental Evaluation of Cow Dung Biochar + Dolomite for Heavy Metal Immobilization in Solid Waste from Silica Sand Purification. Bioresour. Technol. Rep. 2022, 18, 101102. [Google Scholar] [CrossRef]
- Vácha, R. Heavy Metal Pollution and Its Effects on Agriculture. Agronomy 2021, 11, 1719. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K. The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and Its Speciation in Soil. Agronomy 2020, 10, 1550. [Google Scholar] [CrossRef]
- Joseph, O.O.; Babaremu, K.O. Agricultural Waste as a Reinforcement Particulate for Aluminum Metal Matrix Composite (AMMCs): A Review. Fibers 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Centro de Estudios y de Investigación en Biotecnología (CIBIOT); Centro de Investigación e Innovación Energía (CIIEN); Universidad Pontificia Bolivariana. Biomasa de Residuos Agrícolas en el Departamento de Antioquia, 2nd ed.; Universidad Pontificia Bolivariana, Ed.; Universidad Pontificia Bolivariana: Medellín, CO, USA, 2015; ISBN 9789586962292. [Google Scholar]
- FAO. Tackling Food Loss and Waste: A Triple Win Opportunity. Available online: https://www.fao.org/newsroom/detail/FAO-UNEP-agriculture-environment-food-loss-waste-day-2022/en (accessed on 29 May 2023).
- FAO. Sustainable Development Goals. Available online: https://www.fao.org/sustainable-development-goals/indicators/1231/en/ (accessed on 29 May 2023).
- United Nations Mensaje del Secretario General Para. 2023. Available online: https://www.un.org/es/observances/zero-waste-day/messages (accessed on 30 May 2023).
- World Health Organization. Compendium of WHO and Other UN Guidance on Health and Environment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022.
- Sharma, P.; Dutta, D.; Udayan, A.; Nadda, A.K.; Lam, S.S.; Kumar, S. Role of Microbes in Bioaccumulation of Heavy Metals in Municipal Solid Waste: Impacts on Plant and Human Being. Environ. Pollut. 2022, 305, 119248. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Koda, E.; Marecka, M.; Baczewska, A.; Brągoszewska, P.; Sieczka, A.; Osiński, P. Impact of the Municipal Solid Waste Łubna Landfill on Environmental Pollution by Heavy Metals. Water 2016, 8, 470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wei, X.; Hao, Q.; Si, R. Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals. Metals 2020, 10, 815. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, X.; Zhao, Y.; Yan, J. Disposal Technology and New Progress for Dioxins and Heavy Metals in Fly Ash from Municipal Solid Waste Incineration: A Critical Review. Environ. Pollut. 2022, 311, 119878. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. EPA’s Guide for Industrial Waste Management; U.S. Environmental Protection Agency: Philadelphia, PA, USA, 2023.
- Lan, M.; He, Z.; Hu, X. Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation. Metals 2022, 12, 742. [Google Scholar] [CrossRef]
- Zheng, F.; Guo, Y.; Chen, F.; Wang, S.; Zhang, J.; Yang, L.; Qiu, G. Fluoride Leaching of Titanium from Ti-Bearing Electric Furnace Slag in [NH4+]-[F−] Solution. Metals 2021, 11, 1176. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Wang, L. Extraction of the Rare Element Vanadium from Vanadium-Containing Materials by Chlorination Method: A Critical Review. Metals 2021, 11, 1301. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological Strategies for the Recovery of Valuable and Critical Raw Materials from Waste Electrical and Electronic Equipment (WEEE)–A Review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Latosińska, J.; Czapik, P. The Ecological Risk Assessment and the Chemical Speciation of Heavy Metals in Ash after the Incineration of Municipal Sewage Sludge. Sustainability 2020, 12, 6517. [Google Scholar] [CrossRef]
- Čech, V.; Gregorová, B.; Krokusová, J.; Košová, V.; Hronček, P.; Molokáč, M.; Hlaváčová, J. Environmentally Degraded Mining Areas of Eastern Slovakia As a Potential Object of Geotourism. Sustainability 2020, 12, 6029. [Google Scholar] [CrossRef]
- Sousa, R.; Futuro, A.; Fiúza, A.; Vila, M.C.; Dinis, M.L. Bromine Leaching as an Alternative Method for Gold Dissolution. Miner. Eng. 2018, 118, 16–23. [Google Scholar] [CrossRef]
- Brown, G.; Katz, D.; Foust, A. Unit Operations, 1st ed.; John Wiley & Sons: New York, NY, USA, 1960. [Google Scholar]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The Role of Microorganisms in Gold Processing and Recovery—A Review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Latorre, M.; Cortés, M.P.; Travisany, D.; Di Genova, A.; Budinich, M.; Reyes-Jara, A.; Hödar, C.; González, M.; Parada, P.; Bobadilla-Fazzini, R.A.; et al. The Bioleaching Potential of a Bacterial Consortium. Bioresour. Technol. 2016, 218, 659–666. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The Geomicrobiology of Gold. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef]
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes. Environ. 2008, 23, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Lambert, F.; Gaydardzhiev, S.; Léonard, G.; Lewis, G.; Bareel, P.-F.; Bastin, D. Copper Leaching from Waste Electric Cables by Biohydrometallurgy. Miner. Eng. 2015, 76, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Potysz; Kierczak Prospective (Bio)Leaching of Historical Copper Slags as an Alternative to Their Disposal. Minerals 2019, 9, 542. [CrossRef] [Green Version]
- Guerrero, L.; Esbrí, J. Aplicabilidad de la Biolixiviación Como un Método Sustitutivo de la Amalgamación con Mercurio para la Recuperación del Oro en la Minería Artesanal del Sur de Perú. Bachelor’s Thesis, Escola Politècnica Superior d’Enginyeria de Manresa, Lima, Peru, 2014. [Google Scholar]
- Khaing, S.Y.; Sugai, Y.; Sasaki, K. Gold Dissolution from Ore with Iodide-Oxidising Bacteria. Sci. Rep. 2019, 9, 4178. [Google Scholar] [CrossRef] [Green Version]
- Ballester, A. Minería Química, 1st ed.; Instituto Tecnológico Geominero de España, Ed.; Instituto Tecnológico Geominero de España: Madrid, Spain, 1991. [Google Scholar]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial Leaching of Critical Metal Values from Polish Copper Metallurgical Slags Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Ballester, A.; Blázquez, M.L.; González, F.; Muñoz, J.A. Mecanismo de Biolixiviación de Sulfuros Metálicos. Rev. Metal. 2001, 37, 665–672. [Google Scholar] [CrossRef]
- Chuquipoma, M.; Sergio, F. Biolixiviación; Osinergmin: Lima, Peru, 2016; Available online: https://www.osinergmin.gob.pe/seccion/centro_documental/mineria/Documentos/Publicaciones/Biolixiviacion.pdf (accessed on 10 May 2023).
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and Its Potential Application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Boon, M.; Snijder, M.; Hansford, G.S.; Heijnen, J.J. The Oxidation Kinetics of Zinc Sulphide with Thiobacillus ferrooxidans. Hydrometallurgy 1998, 48, 171–186. [Google Scholar] [CrossRef]
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of Leptospirillum Ferrooxidans for Leaching. Appl. Environ. Microbiol. 1992, 58, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.K.; Ha, M.-G.; Shin, S.; Seo, M.; Jang, J.; Jo, S.; Kim, D.; Lee, S.; Jung, Y.; Kang, P.; et al. Electrochemical Effect on Bioleaching of Arsenic and Manganese from Tungsten Mine Wastes Using Acidithiobacillus spp. J. Environ. Manag. 2018, 223, 852–859. [Google Scholar] [CrossRef]
- Sreekrishnan, T.R.; Tyagi, R.D. A Comparative Study of the Cost of Leaching out Heavy Metals from Sewage Sludges. Process Biochem. 1996, 31, 31–41. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gupta, A. Current Trends in Applicability of Thermophiles and Thermozymes in Bioremediation of Environmental Pollutants. In Microbial Extremozymes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–176. [Google Scholar]
- Borje Lindstrom, E.; Wold, S.; Kettaneh-Wold, N. Optimization of Pyrite Bioleaching Using Sulfolobus acidocaldarius. Appl. Microbiol. Biotechnol. 1993, 38, 702–707. [Google Scholar] [CrossRef]
- Brierley, C.L.; Brierley, J.A. Anaerobic Reduction of Molybdenum by Sulfolobus Species. Zent. Bakteriol. Mikrobiol. Hyg. I. Abt. Orig. C Allg. Angew. Okol. Mikrobiol. 1982, 3, 289–294. [Google Scholar] [CrossRef]
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of Iron, Copper, Lead, and Zinc from the Sludge Mining Sediment at Different Particle Sizes, PH, and Pulp Density Using Acidithiobacillus ferrooxidans. Minerals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Acevedo, F.; Gentina, J. Fundamentos y Perspectivas de las Tecnologías Biomineras, 1st ed.; Acevedo, F., Gentina, J., Eds.; Ediciones Universitarias de Valparaíso: Valparaiso, Chile, 2005. [Google Scholar]
- Rossi, G. Biohydrometallurgy, 1st ed.; McGraw-Hill: Hamburg, Germany, 1990. [Google Scholar]
- Rodríguez, Y. Contribución al Estudio Del Mecanismo de Biolixiviación de Distintos Sulfuros Metálicos Con Bacterias Mesófilas y Termófilas. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 2000. [Google Scholar]
- Berry, V.; Murr, L. Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena, 1st ed.; Murr, L., Torma, A., Brierley, J., Eds.; Elsevier: New York, NY, USA, 1978; ISBN 9780125111508. [Google Scholar]
- Bennett, J.C.; Tributsch, H. Bacterial Leaching Patterns on Pyrite Crystal Surfaces. J. Bacteriol. 1978, 134, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Hansford, G.S.; Drossou, M. A Propagating Pore Model for the Batch Bioleach Kinetics of Refractory Gold-Bearing Pyrite. In Proceedings of the Biohydrometallurgy International Symposium, Warwick, UK, 12–16 July 1987; Norris, P.R., Kelly, D.P., Eds.; pp. 345–358. [Google Scholar]
- Tributsch, H. The Oxidative Desintegration of Sulfide Crystals by Thiobacillus ferrooxidans. Naturwissenschaften 1976, 63, 88. [Google Scholar] [CrossRef]
- Newman, D.K. How Bacteria Respire Minerals. Science 2001, 292, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
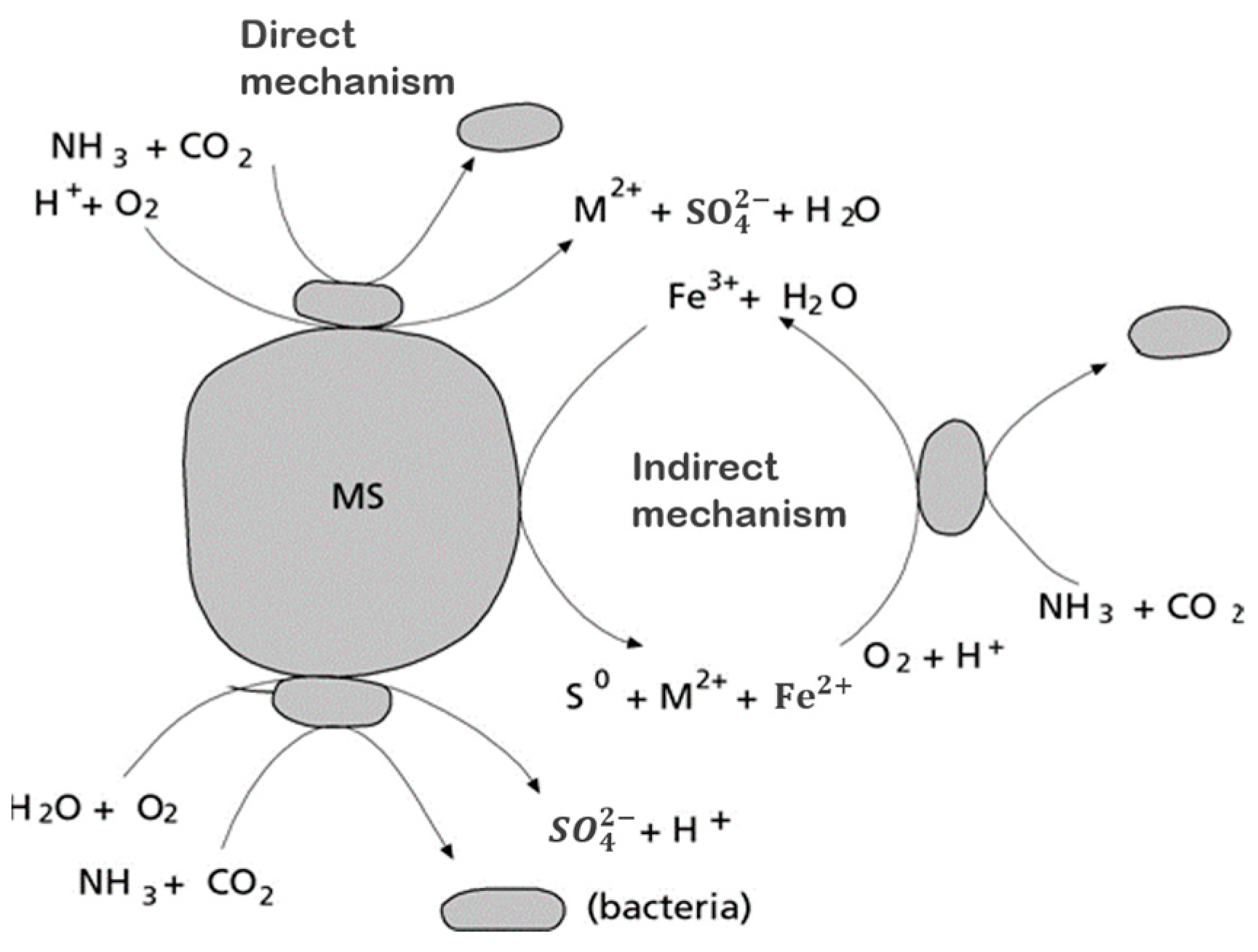
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)Chemistry of Bacterial Leaching—Direct vs. Indirect Bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Masloboev, V.; Seleznev, S.; Svetlov, A.; Makarov, D. Hydrometallurgical Processing of Low-Grade Sulfide Ore and Mine Waste in the Arctic Regions: Perspectives and Challenges. Minerals 2018, 8, 436. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-J.; Koh, Y.-K.; Kwon, J.-S. Bioleaching of Pyrrhotite with Bacterial Adaptation and Biological Oxidation for Iron Recovery. Metals 2021, 11, 295. [Google Scholar] [CrossRef]
- Kovaříková, H.; Janáková, I.; Čablík, V.; Vrlíková, V. Bacterial Leaching of Polymetallic Ores from Zlatý Chlum Locality. J. Pol. Miner. Eng. Soc. 2019, 1, 145–158. [Google Scholar] [CrossRef]
- Zhappar, N.K.; Shaikhutdinov, V.M.; Kanafin, Y.N.; Ten, O.A.; Balpanov, D.S.; Korolkov, I.V.; Collinson, S.R.; Erkasov, R.S.; Bakibaev, A.A. Bacterial and Chemical Leaching of Copper-Containing Ores with the Possibility of Subsequent Recovery of Trace Silver. Chem. Pap. 2019, 73, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Huang, T.; Zhang, X.; Zhang, S. Bioelectrochemical-Assisted Bioleaching of Chalcopyrite: Effect of Pulp Density, Anode Material, and Sliver Ion. Process Saf. Environ. Prot. 2022, 159, 740–748. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wu, J.-Q.; Sung, S. Effects of Sulfur Dosage on Continuous Bioleaching of Heavy Metals from Contaminated Sediment. J. Hazard. Mater. 2022, 424, 127257. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A Review on Recent Advancements in Recovery of Valuable and Toxic Metals from E-Waste Using Bioleaching Approach. Chemosphere 2022, 287, 132230. [Google Scholar] [CrossRef]
- Diaz, M.A.; De Ranson, I.U.; Dorta, B.; Banat, I.M.; Blazquez, M.L.; Gonzalez, F.; Muñoz, J.A.; Ballester, A. Metal Removal from Contaminated Soils Through Bioleaching with Oxidizing Bacteria and Rhamnolipid Biosurfactants. Soil Sediment Contam. Int. J. 2015, 24, 16–29. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching Remediation of Heavy Metal-Contaminated Soils Using Burkholderia sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152. [Google Scholar] [CrossRef]
- Tran, T.M.; Han, H.-J.; Ko, J.-I.; Lee, J.-U. Effect of Indigenous Microbial Consortium on Bioleaching of Arsenic from Contaminated Soil by Shewanella Putrefaciens. Sustainability 2020, 12, 3286. [Google Scholar] [CrossRef] [Green Version]
- Praburaman, L.; Park, J.-H.; Govarthanan, M.; Selvankumar, T.; Oh, S.-G.; Jang, J.-S.; Cho, M.; Kamala-Kannan, S.; Oh, B.-T. Impact of an Organic Formulation (Panchakavya) on the Bioleaching of Copper and Lead in Contaminated Mine Soil. Chemosphere 2015, 138, 127–132. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Deng, Y.; Zhang, S.; Hao, X.; Zhu, P.; Zhou, J.; Yin, H.; Liang, Y.; Liu, H.; et al. Bioremediation of Cadmium-Contaminated Paddy Soil Using an Autotrophic and Heterotrophic Mixture. RSC Adv. 2020, 10, 26090–26101. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, P.; Zhang, H.; Liang, Y.; Yin, H.; Liu, X.; Bai, L.; Liu, H.; Jiang, H. Mixotrophic Acidophiles Increase Cadmium Soluble Fraction and Phytoextraction Efficiency from Cadmium Contaminated Soils. Sci. Total Environ. 2019, 655, 347–355. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of Bioleaching of Heavy Metals from Sediment with Indigenous Bacteria Using Agricultural Sulfur Soil Conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef]
- Gan, M.; Jie, S.; Li, M.; Zhu, J.; Liu, X. Bioleaching of Multiple Metals from Contaminated Sediment by Moderate Thermophiles. Mar. Pollut. Bull. 2015, 97, 47–55. [Google Scholar] [CrossRef]
- Qayyum, S.; Meng, K.; Pervez, S.; Nawaz, F.; Peng, C. Optimization of PH, Temperature and Carbon Source for Bioleaching of Heavy Metals by Aspergillus Flavus Isolated from Contaminated Soil. Main Group Met. Chem. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Porzionato, N.; Tufo, A.; Candal, R.; Curutchet, G. Metal Bioleaching from Anaerobic Sediments from Reconquista River Basin (Argentina) as a Potential Remediation Strategy. Environ. Sci. Pollut. Res. 2017, 24, 25561–25570. [Google Scholar] [CrossRef]
- Gan, M.; Song, Z.; Zhu, J.; Liu, X. Efficient Bioleaching of Heavy Metals from Contaminated Sediment in Batch Method Coupled with the Assistance of Heterotrophic Microorganisms. Environ. Earth Sci. 2016, 75, 457. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J.-U. A Comparison of Microbial Leaching and Chemical Leaching of Arsenic and Heavy Metals from Mine Tailings. Biotechnol. Bioprocess Eng. 2015, 20, 91–99. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of Metals from Lead-Zinc Mine Tailings Using Bioleaching and Followed by Sulfide Precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef]
- Ye, M.; Yan, P.; Sun, S.; Han, D.; Xiao, X.; Zheng, L.; Huang, S.; Chen, Y.; Zhuang, S. Bioleaching Combined Brine Leaching of Heavy Metals from Lead-Zinc Mine Tailings: Transformations during the Leaching Process. Chemosphere 2017, 168, 1115–1125. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J.-U. Effect of Sulfur Concentration on Microbial Removal of Arsenic and Heavy Metals from Mine Tailings Using Mixed Culture of Acidithiobacillus spp. J. Geochem. Explor. 2015, 148, 241–248. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of Arsenic from Highly Contaminated Mine Tailings Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New Approaches for Extracting and Recovering Metals from Mine Tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, M.; Abdollahzadeh, A.A.; Askari, M. Bioleaching of Copper, Nickel and Cobalt from the Low Grade Sulfidic Tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of Arsenopyrite from Janggun Mine Tailings (South Korea) Using an Adapted Mixed Mesophilic Culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of Indium from Sphalerite Ore and Flotation Tailings by Bioleaching and Subsequent Precipitation Processes. Miner. Eng. 2015, 75, 94–99. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of Cobalt from Cu/Co-Rich Sulfidic Mine Tailings from the Polymetallic Rammelsberg Mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.-E.; Kinnunen, P. Bioleaching of Cobalt from Sulfide Mining Tailings; a Mini-Pilot Study. Hydrometallurgy 2020, 196, 105418. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Yang, Q.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Comparative Study on Bioleaching of Two Different Types of Low-Grade Copper Tailings by Mixed Moderate Thermophiles. Trans. Nonferrous Met. Soc. China 2018, 28, 1847–1853. [Google Scholar] [CrossRef]
- Huerta-Rosas, B.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Gómez-Castro, F.I.; Carrillo-Pedroza, F.R.; Romo-Rodríguez, P.; Gutiérrez-Corona, J.F. Aerobic Processes for Bioleaching Manganese and Silver Using Microorganisms Indigenous to Mine Tailings. World J. Microbiol. Biotechnol. 2020, 36, 124. [Google Scholar] [CrossRef]
- de Wet, M.M.M.; Brink, H.G. Fungi in the Bioremediation of Toxic Effluents. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 407–431. [Google Scholar]
- Gadd, G.M. Microbial Influence on Metal Mobility and Application for Bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Almudena, A.; Lizaso, J. Hongos y Micotoxinas; Fundación Ibérica para la Seguridad Alimentaria: Madrid, Spain, 2001. [Google Scholar]
- Serrano-Coll, H.; Cardona-Castro, N. Mycotoxicosis and Mycotoxins: Generalities and Basic Aspects. Rev. CES Med. 2015, 29, 143–151. [Google Scholar]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Grewal, H.S.; Kalra, K.L. Fungal Production of Citric Acid. Biotechnol. Adv. 1995, 13, 209–234. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar] [PubMed]
- Khan, I.; Qayyum, S.; Maqbool, F.; Mujaddad-ur-Rehman; Hayat, A.; Farooqui, M.S. Microbial Organic Acids Production, Biosynthetic Mechanism and Applications -Mini Review. Indian J. Geomarine. Sci. 2017, 46, 2165–2174. [Google Scholar]
- Roukas, T. Citric and Gluconic Acid Production from Fig by Aspergillus Niger Using Solid-State Fermentation. J. Ind. Microbiol. Biotechnol. 2000, 25, 298–304. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in Citric Acid Fermentation by Aspergillus Niger: Biochemical Aspects, Membrane Transport and Modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef]
- Alongi, K.S.; Shields, G.C. Theoretical Calculations of Acid Dissociation Constants: A Review Article. Annu. Rep. Comput. Chem. 2010, 6, 113–138. [Google Scholar]
- Kütt, A.; Selberg, S.; Kaljurand, I.; Tshepelevitsh, S.; Heering, A.; Darnell, A.; Kaupmees, K.; Piirsalu, M.; Leito, I. PKa Values in Organic Chemistry–Making Maximum Use of the Available Data. Tetrahedron. Lett. 2018, 59, 3738–3748. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Martin, D.S.; Cole, R.J.; Haq, S. Investigating the Adsorption of Oxalic Acid onto Cu(110) to Create a Chemically Functionalised Surface. Surf. Sci. 2003, 539, 171–181. [Google Scholar] [CrossRef]
- Sajadi, S.A.A. Metal Ion-Binding Properties of L-Glutamic Acid and L-Aspartic Acid, a Comparative Investigation. Nat. Sci. 2010, 2, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-López, C.; Giraldo-Aristizabal, R. Proceso Para La Separación de Metales En Una Matriz Sólida Mediante Lixiviación, Que Emplea Una Composición Que Contiene Ácidos Carboxílicos, Monosacáridos, Disacáridos, Aminoácidos, Ácidos Grasos, Alcoholes y Compuestos Fenólicos. Patent Number NC2019/0013648, 2022. 13. [Google Scholar]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging Role of Organic Acids in Leaching of Valuable Metals from Refinery-Spent Hydroprocessing Catalysts, and Potential Techno-Economic Challenges: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of Low-Grade Waste Printed Circuit Boards by Mixed Fungal Culture and Its Community Structure Analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal Bioleaching of WPCBs Using Aspergillus Niger: Observation, Optimization and Kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Nili, S.; Arshadi, M.; Yaghmaei, S. Fungal Bioleaching of E-Waste Utilizing Molasses as the Carbon Source in a Bubble Column Bioreactor. J. Environ. Manag. 2022, 307, 114524. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect Bioleaching of Low-Grade Nickel Limonite and Saprolite Ores Using Fungal Metabolic Organic Acids Generated by Aspergillus Niger. Hydrometallurgy 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M.; Rastegar, S.O.; Azargoshasb, H. Fungal Leaching of Valuable Metals from a Power Plant Residual Ash Using Penicillium Simplicissimum: Evaluation of Thermal Pretreatment and Different Bioleaching Methods. Waste Manag. 2016, 52, 309–317. [Google Scholar] [CrossRef]
- Biswas, S.; Bhattacharjee, K. Fungal Assisted Bioleaching Process Optimization and Kinetics: Scenario for Ni and Co Recovery from a Lateritic Chromite Overburden. Sep. Purif. Technol. 2014, 135, 100–109. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Ting, Y.-P. Metal Extraction from Municipal Solid Waste (MSW) Incinerator Fly Ash—Chemical Leaching and Fungal Bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Alavi, N.; Partovi, K.; Majlessi, M.; Rashidi, M.; Alimohammadi, M. Bioleaching of Metals from Cellphones Batteries by a Co-Fungus Medium in Presence of Carbon Materials. Bioresour. Technol. Rep. 2021, 15, 100768. [Google Scholar] [CrossRef]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Enhanced Bio-Recovery of Aluminum from Low-Grade Bauxite Using Adapted Fungal Strains. Braz. J. Microbiol. 2020, 51, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium Bioleaching: An Emerging Approach for the Recovery of Li from Spent Lithium Ion Batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of Heavy Metal (Cd and Cr)–Polluted Soil through Indigenous Metallotolerant Fungal Isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Alvarez, R. Removal of Copper, Chromium and Arsenic from Preservative-Treated Wood by Chemical Extraction-Fungal Bioleaching. Waste Manag. 2009, 29, 1885–1891. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Gao, W.; Zheng, Y. Bioremediation of Hexavalent Chromium Contaminated Soil by a Bioleaching System with Weak Magnetic Fields. Int. Biodeterior. Biodegrad. 2018, 128, 41–47. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, S.; Sun, L.; Jacques, D.A.; Tang, J.; Lian, M.; Ji, Z.; Wang, J.; Zhu, J.; Xu, Z. Bioleaching of Heavy Metals from Contaminated Sediments by the Aspergillus Niger Strain SY1. J. Soils Sediments 2015, 15, 1029–1038. [Google Scholar] [CrossRef]
- Seh-Bardan, B.J.; Othman, R.; Wahid, S.A.; Husin, A.; Sadegh-Zadeh, F. Bioleaching of Heavy Metals from Mine Tailings by Aspergillus Fumigatus. Bioremediat. J. 2012, 16, 57–65. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by Filamentous Fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Springer: Boston, MA, USA, 2004; pp. 307–340. [Google Scholar]
- Shankar, T.; Sivakumar, T. Optimization of Citric Acid Production Using Aspergillus Niger Isolated from the Leaf Litter Soil of Sathuragiri Hills. Univers. J. Microbiol. Res. 2016, 4, 79–87. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The Selective Leaching of Copper from a Gold–Copper Concentrate in Glycine Solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef]



| Feature | Bacterial Bioleaching | Fungal Bioleaching |
|---|---|---|
| Application | Applied when it is required to recover a metal of interest, and it is not necessary to preserve the properties of the solid matrix | Applied when it is necessary to preserve the properties of the solid matrix, especially the crystalline properties, in case it is a mineral SMMC. |
| Applied if the final color of the solid matrix is not of interest. | ||
| Applied if not required to separate the fungus from the solid matrix. | ||
| Sterilization and Sanitization | Not required | Required |
| Stages | Performed in one step | Performed in two steps: Direct method (See Section 4.2). |
| Performed in two steps: Indirect method (See Section 4.2). | ||
| Operational times | Prolonged due to bacteria acting directly in the process | Direct method: Simultaneous fermentation with leaching can take 3 to 10 days, depending on the target. |
| Process times depend on the SMMC/bacteria system | Indirect method: Production of the leaching solvents (fermented broth): can have a production time of 8 to 10 days, with possible constant production and storage for consumption, without this being the limiting stage. The bioleaching process takes approximately 4 to 6 h, depending on the solid matrix and the metal to be extracted. |
| Microorganism | Energy Source | pH | T (°C) | References |
|---|---|---|---|---|
| Acidithiobacillus ferrooxidans | Ferrous iron, sulfide minerals, sulfur, thiosulfate | 1.7–3.5 | 28–30 | [73] |
| Leptospirillum ferrooxidans | Ferrous iron | 3.0 | 30 | [74] |
| Acidithiobacillus thiooxidans | Elemental sulfur, thiosulfate | 1.0–3.5 | 28–30 | [75] |
| Thiobacillus thioparus | Elemental sulfur, thiosulfate | 7.0–8.5 | 28–30 | [76] |
| Sulfobacillus thermosulfidoxidans | Ferrous iron, elemental sulfur, sulfide minerals | 2.1–2.5 | 50–55 | [77] |
| Sulfolobus acidocaldarius | Elemental sulfur, yeast extract | 2.0–3.0 | 70–75 | [78] |
| Sulfolobus brierly | Elemental sulfur, ferrous iron, yeast extract | 2.0–3.0 | 60 | [79] |
| Acidiphilium acidophilum | Elemental sulfur, thiosulfate, yeast extract, salts, sugars, amino acids | 2.0–3.0 | 28–30 | [79] |
| Microorganism | SMMC | Metal | pH | T (°C) | Agitation | Pulp Density | Efficiencies | References |
|---|---|---|---|---|---|---|---|---|
| A. ferrooxidans, Desulfotomaculum geothermicum | Crushed and screened graphitic schist with a diameter of 8 mm | Iron, zinc, nickel, copper, and cobalt | 1.7–2.0 | 40–50 | - | - | In 500 days, the recoveries were Ni 92%, Zn 82%, Co 14%, and Cu 2%. | [90] |
| A. ferrooxidans | Pyrrhotite, chalcopyrite and arsenopyrite | Iron, copper | 2.8–3.2 | – | - | - | In 41 days, recoveries were 47.4 mg/L at a pH of 3.2. | [91] |
| A. ferrooxidans | Dried and crushed sludge at different particle sizes | Gold, copper, zinc, lead | 1.8–2.2 | 30 | 100 rpm | 6.0% (w/v) | In 14 days, the extractions were 4.71%, 9.01% Pb, 12.98% Cu, and 31.88% Zn. | [80] |
| A. ferrooxidans | Quartz, chlorite, chalcopyrite, albite, pyrite | Aluminum, iron, copper | 1.8 | 30 | 150 rpm | - | In 5 weeks, metal recoveries were 47.29% Al, 54.41% Fe, and 28.08% Cu. | [92] |
| A. ferrooxidans FT-22, A. ferrooxidans FT-23, A. ferrooxidans BF, and A. ferrivorans | Albite, quartz, clinochlore, muscovite, illite | Silver, copper | 10.5–11.0 | 25 | 20–30 rpm | 40% (w/v) | In 48 h, the recovery of metals was 51% Ag and 70% Cu. | [93] |
| Sulfobacillus thermosulfidooxidans, A. thiooxidans, Acidiphilum multivorum, and Leptospirillum Ferriphilum. | Chalcopyrite | Iron, copper | 2.0 | 30 | - | 1–6% (w/v) | In 11 days, the metal recovery was 28.57% Fe and 39.55% Cu. | [94] |
| S. Thermosulfidooxidans, A. thiooxidans/A. ferrooxidans, S. thermotolerans, and A. albertensis. | Clay, sand, silt | Zinc, copper, nickel, chromium | 1.5–3.1 | 30 | 200 rpm | 10% (w/v) | In 20 days of operation, metal recovery was 49% Zn, 50% Cu, 65% Ni and 27% Cr. | [95] |
| Sulfobacillus thermophidus oxidans | Printed circuit board (PCB) | Aluminum, lead, zinc, and tin | - | 45 | 120–145 rpm | 0.33% (w/v) | Recovery of 83% Zn, 89% Cu, and 81% Ni in 18 days. | [96] |
| A. thiooxidans and A. ferrooxidans | Soil contaminated with metals and metalloids | Cadmium, copper, lead, zinc, zinc, chromium, iron | 5.6 | 30 | 150 rpm | 10% (w/v) | In 42 days, metal recovery was 36% Fe and 70% Zn. | [97] |
| Burkholderia spp. Z-90 | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 3.0 | 35 | 180 rpm | 5% (w/v) | In 5 days, the maximum metal recovery achieved was 31.6% As, 37.7% Cd, 24.1% Cu, 52.2% Mn, 32.5% Pb, and 44% Zn. | [98] |
| Shewanella putrefaciens | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.2 | 30 | 100 rpm | 3% (w/v) | Arsenic recovery was 57.5% in 40 days. | [99] |
| Acidithiobacillus, Acetobacter, Acidophilum, Acidophilum, Arthrobacter spp., and Pseudomonas spp. | Panchakavya (soil mixture) | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.6 | 30 | 120–180 rpm | 0.2–1% (w/v) | Metal recovery in 5 days was 64% Pb and 49% Cu. | [100] |
| Massilia spp., Alicyclobacillus spp., and Micromonospora spp. | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 3.5 | 30 | 180 rpm | 1% (w/v) | The metal extraction in 10 min was 32.09% Cd | [101] |
| Myxotrophic acidophiles | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.0 | 25 | 175 rpm | 4% (w/v) | In 14 days, the two-step bioleaching achieved the extraction of 34% Cd | [102] |
| Indigenous bacteria | Agricultural land | Zinc, copper, nickel | 8.0 | 28 | 180 rpm | 1% (w/v) | In 9 days, the maximum metal extraction achieved was 74.72% Cu, 35.35% Ni, and 69.92% Zn. | [103] |
| A. ferrooxidans, A. thiooxidans, and L. ferriphilum | Pyrite and sulfosalts | Aluminum, manganese, iron, copper, zinc, mercury, zinc, mercury | 4.0 | 30 | 180 rpm | 5% (w/v) | In 31 days, the maximum metal recovery was 93.3% Cu, 92.13% Mn, and 96.1% Zn. | [104] |
| Sulfobacillus thermosulfidooxides and A. caldus | Pyrite and sulfosalts | Aluminum, manganese, iron, copper, zinc, mercury, zinc, mercury | 7.5 | 45 | 180 rpm | 5% (w/v) | Fermentation was carried out for 31 days, and the metal recovery efficiency was 45% As, 89% Cd, 94% Cu, 34% Hg, 95% Mn, and 98% Zn. | [104] |
| Indigenous bacteria | Port sediments | Copper, chromium, cadmium, lead, zinc | 6.0 | 30 | 100 rpm | 1% (w/v) | During 30 days of processing, the recovery of metals was 29% Cu, 8% Ni, 5% Pb, and 39% Zn. | [105] |
| Bacteria from exogenous soil | Port sediments | Copper, chromium, cadmium, lead, zinc | 8.0 | 30 | 100 rpm | 4% (w/v) | During 30 days of processing, the recovery of metals was 100% Cu, 95% Cr, 100% Ni, 100% Pb, 100% Zn, 100% Cu, 95% Cr, 100% Ni, 100% Ni, 100% Pb, 100% Pb and 100% Zn. | [105] |
| A. ferrooxidans and A. thiooxidans | Anaerobic sediment from urban wastewater | Copper, chromium, cadmium, lead, zinc | 5.0 | 25 | 120 rpm | 15% (w/v) | Metal recovery during 57 days was 43% Cu and 80% Zn. | [106] |
| A. ferrooxidans, A. thiooxidans, and Leptospirillum ferriphilum | Sediment from sewage outfall | Copper, chromium, cadmium, lead, zinc | 4.0 | 30 | 180 rpm | 5% (w/v) | Metal recovery was 90.9% Cu and 94.74% Zn; elements such as Cd, Hg, Mn, and Pb were below 30%. | [107] |
| A. ferrooxidans | Mining tailings | Copper, iron, cadmium, antimony, zinc, nickel, chromium, nickel, chromium | 3.0 | 30 | 200 rpm | 5% (w/v) | In 20 days, the maximum efficiency achieved was 36.2% Cu, 65.95% Cr, 97.4% Ni, 2.2% Sb, and 34.8% Zn. | [108] |
| A. ferrooxidans and A. thiooxidans | Mining tailings | Arsenic, zinc, copper, lead | 2.5 | 30 | 200 rpm | 5% (w/v) | Metal recovery in 25 days was 72.2% As, 47.1% Cu, 99.5% Mn, and 78.9% Zn. | [108] |
| A. ferrooxidans | Mining tailings | Arsenic, zinc, copper, lead | 2.0 | 30 | 200 rpm | 20% (w/v) | The maximum metal recovery achieved in 50 days was 71.37% Fe, 0.82% Pb, and 97.38% Zn. | [109,110] |
| A. thiooxidans | Tailings from an abandoned and inactive mine | Arsenic, zinc, copper, lead | 1.8 | 40 | 150 rpm | 0.5% (w/v) | Arsenic recovery in 25 days was 47%. | [108,111,112] |
| A. ferrooxidans | Mine and metallurgical wastes | Lead, iron, copper, zinc | 1.5 | 40 | 160 rpm | 20% (w/v) | In 50 days of fermentation, the metal recovery achieved was 85.45% Fe, 4.12% Pb, and 97.85% Zn. | [109,110] |
| A. ferrooxidans and A. thiooxidans | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 6.0 | 30 | 200 rpm | 5%(w/v) | In 20.8 days, 42.4% As, 45% Cu, 47.7% Fe, 92% Mn, and 67.2% Zn were extracted. | [108,111] |
| A. ferrooxidans and A. thiooxidans | Mine waste | Arsenic, manganese | 2.5 | 30 | 200 rpm | 5% (w/v) | In 35 days, metal recovery was 96.7% As and 100% Mn. | [75] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus thermosulfidooxidan, A. sulfuroxidans, Ferroplasma acidiphilum, Acidiplasma sp., Sulfobacillus acidophilus, Acidithiobacillus spp., and Acidiphilum cryptum. | Mining waste materials | Silver, lead mercury, zinc, arsenic, manganese, indium, gallium, germanium, and cobalt. | 1.7 | 45 | 150 rpm | 5% (w/v) | Bioleaching was carried out for 50 days, and the efficiency of recovered metals was 90% Cu and 99% Zn. | [113] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus sp. and Ferroplasma sp. | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.8 | 45 | 150 rpm | 5% (w/v) | In 30 days, the metal recovery was 59.5% Co, 55% Cu, and 98.2% Ni. | [114] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus sp. and Ferroplasma sp. | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.2 | 45 | 150 rpm | 5% (w/v) | In 30 days, the metal recovery was 36.5% Co, 72% Cu, and 61.2% Ni. | [114] |
| A. caldus, Leptospirillum ferriphilum, Methylophaga spp. and Sphingomonas spp. | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.5 | 40 | 550 rpm Aeration: 5 L/min | 15% (w/v) | Fermentation was carried out for seven days, and the metal extraction achieved was 105,000 mg/kg. | [73] |
| A. caldus and Leptospirillum ferriphilum | Scorodite | Arsenic, copper, iron, sulfur | 1.2 | 45 | Aeration: 200 mL/min | 1% (w/v) | The maximum recovery achieved during 88 days was 97% As. | [115] |
| Acidophilic ferrous, iron-oxidizing, and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.6 | 30 | 100 rpm | 4% (w/v) | During 22 days, the extraction efficiencies reported were 100% As, 85% Cd, 40% Cu, 85.4% Ln, 100% Mn, 5% Pb, and 100% Zn. | [116] |
| Acidophilic ferrous iron-oxidizing and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.8 | 30 | 100 rpm | 4% (w/v) | During 22 days, the reported extraction efficiencies were 79.9% In and 94.6% Zn. | [116] |
| Acidophilic ferrous iron-oxidizing and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium gallium, germanium, cobalt | 1.8 | 30 | 100 rpm | 10% (w/v) | During 22 days, the reported extraction efficiencies were 72% As, 88% Cd, 87% In, and 81% Zn. | [116] |
| A. thiooxidans Ram 8, A. ferrooxidans Ram 6F, Leptospirillum ferrooxidans, and Ferroplasma acidiphilum BRGM 4 | Tailing and mining residues (pyrite, quartz, etc.) | Iron, zinc, silica, cobalt, cobalt, nickel, aluminum, manganese, arsenic | 2.0 | 30 | 150 rpm | 10% (w/v) | The recovery achieved in the fermentative process was 91% Co, 57% Cu. | [117] |
| Marinobacter sp., Acidithiobacillus spp., Leptospirillum sp., Cuniculiplasma sp., Nitrosotenius sp. and Ferroplasma sp. | Tailing and mining residues (pyrite, quartz, etc.) | Iron, zinc, silica, cobalt, cobalt, nickel, aluminum, manganese, arsenic | 1.5 | 30 | 300 rpm | 10% (w/v) | In 10 days of retention, the amount of metal recovered was 87% Co, 43% Cu, 67% Ni, and 100% Zn. | [118] |
| Leptospirillum ferriphilum YSK, Ferroplasma thermophilum L1, A. caldus S1, and A. thiooxidans A01. | Metallurgical industry waste | Copper, cobalt, nickel, zinc | 1.8 | 40 | 175 rpm | 5% (w/v) | In 16 days, the maximum copper recovery was 58.7%. | [119] |
| Indigenous bacterial and fungal strains | Mining waste | Silver, manganese | 2.0 | 30 | 200 rpm | 6% (w/v) | 67% Ag, 745 Mn. | [120] |
| Fungi | Organic Acids |
|---|---|
| Yarrowia lipolytica | Citric acid |
| Mucor spp. | Fumaric and gluconic acid |
| Rhizopus spp. | Lactic, fumaric and gluconic acid |
| Aspergillus niger | Citric, oxalic and gluconic acids |
| Aspergillus spp. | Citric, malic, tartaric, ketoglutaric, itaconic and aconitic acid |
| Penicillium spp. | Citric, malic, tartaric, ketoglutaric, ketoglutaric and gluconic acids |
| Schizophyllum commune | Malic acid |
| Paecilomyces variotii | Malic acid |
| Microorganism | SMMC | Metal | pH | T (°C) | Agitation-Aeration | Pulp Density | Result | References |
|---|---|---|---|---|---|---|---|---|
| Aspergillus niger | Spent FCC catalyst (zeolites), crushed and screened | Nickel, vanadium, aluminum, aluminum, antimony, molybdenum, cobalt, tungsten | 6.0 | 30 | - | 1% (w/v) | In 60 days, the recovery was 9% Ni, 23% Fe, 30% Al, 36% V, and 64% Sb. | [138] |
| Penicillium simplicissimum | Spent FCC catalyst (zeolites), crushed and screened | Nickel, vanadium, aluminum, aluminum, antimony, molybdenum, cobalt, tungsten | 4–7 | 30 | - | 3% (w/v) | In two-step bioleaching, 32% Al, 67% Co, 65% Mo, and 38% Ni were recovered in 30 days. | [139] |
| Purpureocillium lilacinum y Aspergillus niger (7:3) | Printed circuit boards, crushed with d < 40mm | Aluminum, lead, zinc, and tin | 5.0 | 30 | 150 rpm | 3 to 8% (w/v) | In 27 days, 15.7 ± 0.87% Al, 20.5 ± 0.78% Pb, 49.5 ± 0.38% Zn and 8.1 ± 0.34% Sn were extracted. | [140] |
| Aspergillus niger | Printed circuit boards, shredded and screened | Aluminum, lead, zinc, copper | 5.08 | 25 | 120 rpm | 3.9% (w/v) | In 21 days, the maximum recovery of metals was 98.57% Zn, 43.95% Ni, and 64.03% Cu. | [141] |
| P. simplicissimum | Printed circuit boards | Aluminum, lead, zinc, copper | 6.0 | 30 | 100–400 mL/min | 1–10% (w/v) | The maximum recovery achieved for Cu and Ni was 40% in 7 days. | [142] |
| Aspergillus niger | Saprolite | Iron, silica, nickel, manganese | 5.0 | 95 | 400 rpm | 10% (w/v) | The maximum recovery achieved in 24h was 65% Ni and 58% Fe. | [143] |
| Aspergillus niger | Limonite | Iron, aluminum, silica, manganese | 5.0 | 95 | 400 rpm | 10% (w/v) | Maximum recovery achieved in 24h was 78% Ni and 60% Fe. | [143] |
| Penicillium simplicissimum | Waste ash from power plant | Vanadium, iron, nickel | 4.5 | 30 | 130 rpm | 1% (w/v) | The maximum extraction achieved was 48.3% Fe, 19% V, and 12% Ni in 15 days. | [144] |
| Aspergillus niger NCIM 548 | Chromite | Nickel, cobalt | 2.5 | 30 | 150 rpm | 2% (w/v) | In 21 days of fermentation, the metal recovery was 70.49% Ni and 66.93% Co. | [145] |
| Aspergillus niger | Fly ash from municipal solid waste incinerators | Aluminum, lead, zinc, copper | 10–12 | 30 | 120 rpm | 1–8% (w/v) | After 30 days, the recovery of Cu, Pb, and Fe metals was between 60 to 70%, 55 to 70%, and 30 to 40%, respectively. | [146] |
| Aspergillus niger y Aspergillus tubingensis | Electronic waste (e-waste) | Copper, lead, tin, silver, gold, platinum, platinum, aluminum, manganese, and palladium | 5.0 | 30 | 140 rpm | 1%(w/v) | The achieved metal recovery was 80% Al, 50% Co, 90% Mn, 80% Li and 67% Ni in 27 days. | [147] |
| Aspergillus niger | Bauxite (d < 180 µm) | Aluminum, iron, silica | 6.5 | 30 | 130 rpm | 1% (w/v) | Metal recovery in 10 days was 82.80% Al. | [148] |
| Aspergillus niger adaptado | Lithium-ion batteries (LIBs) | Cobalt, lithium, nickel, manganese, copper, aluminum, graphite, and other materials | 5.4 | 30 | 120–170 rpm | 0.3–1% (w/v) | The obtained recovery efficiency from spent LIBs was 100%, 94%, 72%, 62%, 45%, and 38% for Li, Cu, Mn, Al, Ni, and Co, respectively, in 27 days. | [149] |
| Penicillium chrysogenum strain F1 | Soil contaminated with metals and metalloids | Cadmium, copper, lead, zinc | - | 25 | 120 rpm | 5% (w/v) | In 15 days, the recovery of metals was 50% Cd, 35% Cu, 9% Pb, and 40% Zn. | [19] |
| Phanerochaete chrysosporium | Waste of electrical and electronic equipment | Copper, lead, tin, silver, gold, platinum, platinum, aluminum, manganese, and palladium | 5.0 | 30 | 150 rpm | 1% (w/v) | In 14 days, the copper recovery achieved was 54%. | [15] |
| Aspergillus fumigatus (M3Ai) | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | In 3 days, the metal recovery in two-step bioleaching was 79% Cd and 69% Cr. | [150] |
| Aspergillus flavus | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | - | 30 | 130 rpm | 5%(w/v) | In 15 days, the maximum metal recovery was 39.77% Cd, 18.16% Pb, and 58.22% Zn. | [105] |
| Fibroporia vaillantii | Wood preservative: Chromated copper arsenate | Chromium, copper, arsenic | 3.1 | 30 | 150 rpm | - | In 28 days of fermentation, the maximum metal recovery efficiency was 87% Cu, 80% Cr, and 100% As. | [151] |
| Geotrichum sp. G1 y Bacillus sp. B2 | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 2.0–10 | - | - | 2% | Chromium extraction at 28 days was 94.8%. | [152] |
| Aspergillus niger (M1DGR) | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | The 3-day two-step bioleaching metal recovery was 98% Cd and 43% Cr. | [150] |
| Penicillium rubens (M2Aiii) | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | The 3-day metal recovery in two-step bioleaching was 79% Cd and 69% Cr. | [150] |
| Penicillium, Aspergillus, y Fusarium | Panchakavya (soil mixture) | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.6 | 30 | 120–180 rpm | 0.2–1% (w/v) | The 5-day metal recovery was 64% Pb and 49% Cu. | [100] |
| Aspergillus niger strain SY1 | Contaminated sediment | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 6.5 | 30 | 220 rpm | 10% (w/v) | Metal recovery in 7 days was 93.5% Cd, 62.3% Cu, 11.5% Pb, and 68% Zn. | [153] |
| Aspergillus niger strain SY1 | Contaminated sediment | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 220 rpm | 2.5% (w/v) | In 15 days of fermentation, the recovery efficiency achieved was 90% Cd, 20% Pb, 60% Cu, and 60% Zn. | [153] |
| Penicillium chrysogenum strain KBS3 | Mine tailings | Cobalt, zinc, copper, nickel, manganese, lead | 2.5 | 30 | 120 rpm | 10% (w/v) | In 25 days, the maximum metal recovery achieved was 60% Co, 67% Cu, 69% Mg, 55% Ni, and 65% Zn. | [120] |
| Aspergillus fumigatus | Mine tailings | Arsenic, iron, manganese, lead, zinc, zinc | 5.0 | 30 | 150 rpm | 8% (w/v) | In 40 days, the one-step bioleaching recovered 62.1% As, 58.4% Fe, 100% Mn, 56.1 Pb, and 54.43% Zn. | [154] |
| Aspergillus fumigatus | Mine tailings | Arsenic, iron, manganese, lead, zinc, zinc | 5.0 | 30 | 150 rpm | 8% (w/v) | The two-step bioleaching showed that the maximum metal recovery would be 32% As, 45.20% Fe, 58.4% Mn, 88.4% Pb, and 31.3% Zn. | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability 2023, 15, 10222. https://doi.org/10.3390/su151310222
Rendón-Castrillón L, Ramírez-Carmona M, Ocampo-López C, Gómez-Arroyave L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability. 2023; 15(13):10222. https://doi.org/10.3390/su151310222
Chicago/Turabian StyleRendón-Castrillón, Leidy, Margarita Ramírez-Carmona, Carlos Ocampo-López, and Luis Gómez-Arroyave. 2023. "Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices" Sustainability 15, no. 13: 10222. https://doi.org/10.3390/su151310222
APA StyleRendón-Castrillón, L., Ramírez-Carmona, M., Ocampo-López, C., & Gómez-Arroyave, L. (2023). Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability, 15(13), 10222. https://doi.org/10.3390/su151310222










