Application of Hydrogen-Based Denitrification: Simultaneous Removal of Nitrate and Reactive Black 5 Dye from Textile Wastewater Containing Organic Matter
Abstract
:1. Introduction
| Parameters | Industrial Wastewater Discharge Standards | Influent | Effluent | ||||
|---|---|---|---|---|---|---|---|
| Reference | [13] | [24] | [24] | [13] | [1] | [13] | [2] |
| pH | 5.5–9 | 6–9 | 5.5–9 | 7.0 | 6–10 | 7.77 | 7.7–8.0 |
| BOD (mg/L) | <60 | 60 | 30 | 800–1200 | 100–4000 | 1050 | 272–310 |
| COD (mg/L) | ≤400 | 200 | 250 | 6000–8000 | 150–10,000 | 3056 | 678–932 |
| TN (mg/L) | ≤100 | - | - | 100–700 | - | 154 | - |
| TSS (mg/L) | ≤150 | 150 | 100 | 1500–3000 | 100–5000 | 975 | 120–180 |
| TDS (mg/L) | ≤3000 | - | 2100 | 8725 | 1800–6000 | 4000–9000 | 1632–1902 |
| Cr3+ (mg/L) | ≤0.5 | - | 100 | - | - | 13 | - |
| Color (ADMI) | - | 800–1200 | - | - | - | - | |
| Color (mg/L) | - | - | - | 40–500 | - | - | - |
| Color (Pt-Co) | - | 80 | - | - | 50–2500 | - | 240–290 |
2. Materials and Methods
2.1. Experimental Setup
2.2. Synthetic Textile Wastewater Preparation
2.3. Investigation of Nitrogen Removal and Decolorization Efficiencies
2.4. Water Sample Analyses
2.5. Bacterial Community Analysis
2.6. Calculations and Statistical Analyses
3. Results and Discussion
3.1. HD Performance on Nitrogen Removal
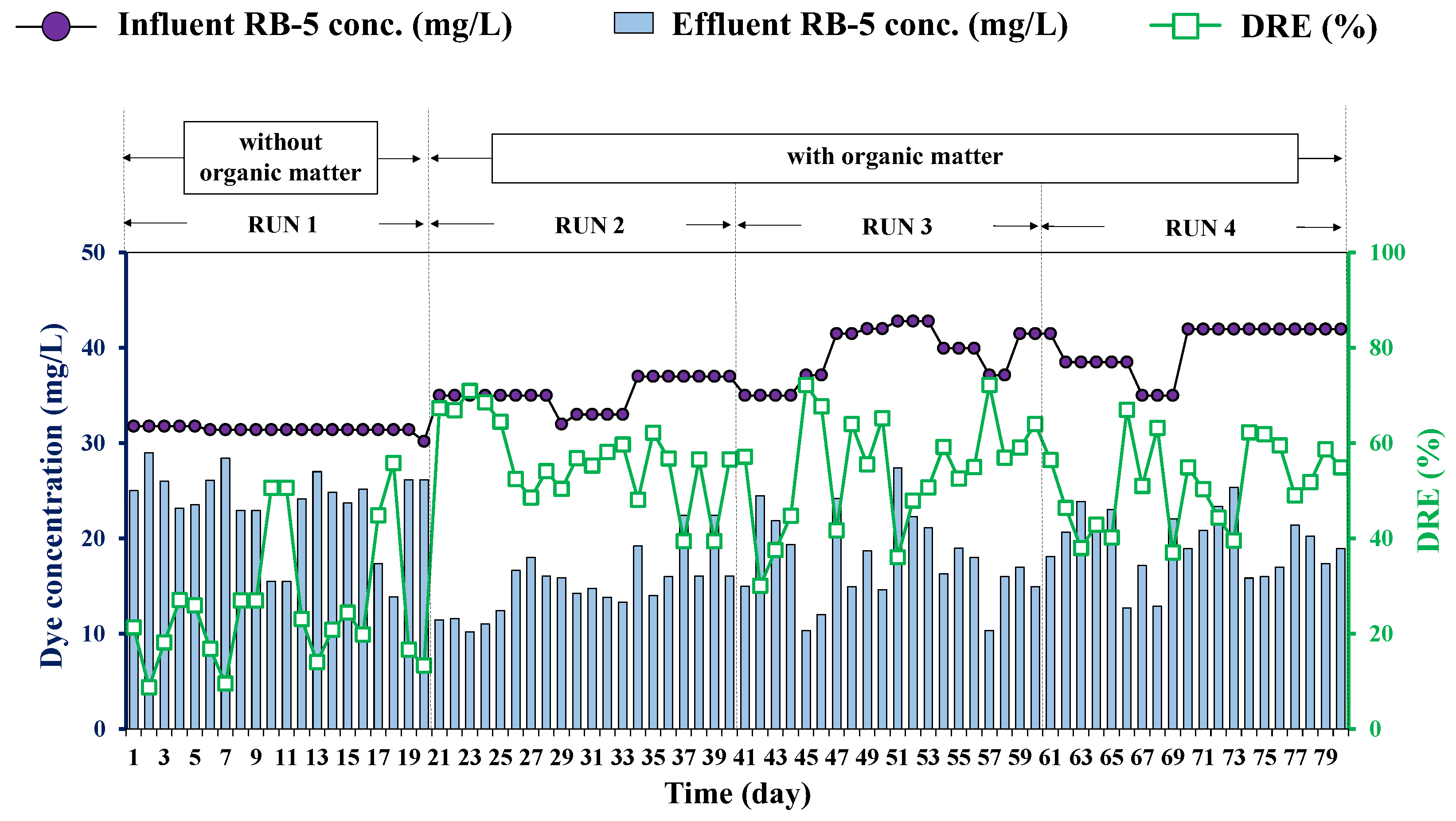
3.2. HD Performance on Decolorization of RB-5 Dye
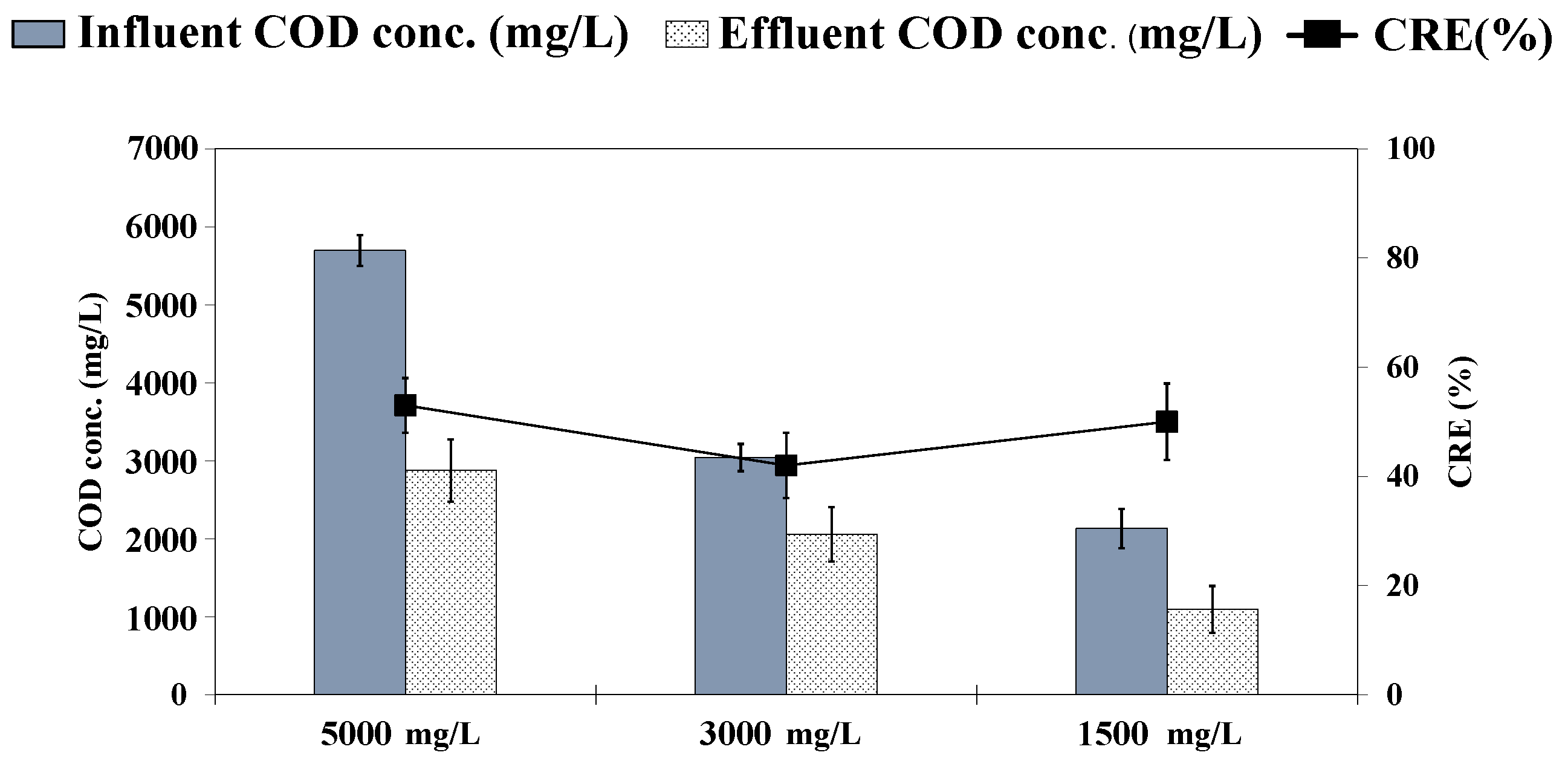
3.3. HD Performance on COD Removal
3.4. Bacterial Community under Different Operating Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meerbergen, K. Decolorization of Textile Waste Water, with an Emphasis on Microbial Treatment Processes. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2018. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- World Bank. Environment, Health and Safety Guideline for Textile Manufacturing Finance Corporation; World Bank Group: Washington, DC, USA, 2007. [Google Scholar]
- Shah, M.P. Azo dye removal technologies. Austin J. Biotechnol. Bioeng. 2018, 5, 1090–1096. [Google Scholar]
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Song, X.; Sand, W. Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: A mini-review. World J. Microbiol. Biotechnol. 2015, 34, 165–169. [Google Scholar] [CrossRef]
- Cirik, K.; Kitis, M.; Cinar, O. Effect of nitrate on anaerobic azo dye reduction. Bioprocess. Biosyst. Eng. 2013, 36, 69–79. [Google Scholar] [CrossRef]
- Konsowa, A.H. Decolorization of wastewater containing direct dye by ozonation in a batch bubble column reactor. Desalination 2003, 158, 233–240. [Google Scholar] [CrossRef]
- Tizaoui, C.; Bouselmi, L.; Mansouri, L.; Ghrabi, A. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems. J. Hazard. Mater. 2007, 140, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Coagulation-flocculation-decantation of dye house effluents: Concentrated effluents. J. Hazard. Mater. 2004, 116, 57–64. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Anodic oxidation of dyes at Nobel Ti/B-Diamond electrodes. Chem. Eng. Sci. 2003, 58, 995–1001. [Google Scholar] [CrossRef]
- Singhopon, T.; Shinoda, K.; Rujakom, S.; Kazama, F. Hydrogenotrophic denitrification for treating nitrate contaminated without/with reactive black 5 dye. J. Water Reuse Desalination 2020, 10, 200–213. [Google Scholar] [CrossRef]
- Singhopon, T.; Shinoda, K.; Rujakom, S.; Kazama, F. Factors Affecting the Simultaneous Removal of Nitrate and Reactive Black 5 Dye via Hydrogen-Based Denitrification. Water 2021, 13, 922. [Google Scholar] [CrossRef]
- Lu, C.; Gu, P.; He, P.; Zhng, G.; Song, C. Characteristics of Hydrogenotrophic denitrification in a combined system of gas-permeable membrane and a biofilm reactor. J. Hazard. Mater. 2009, 168, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Eamrat, R.; Tsutsumi, Y.; Kamei, T.; Khanichaidecha, W.; Tanaka, Y.; Kazama, F. Optimization of hydrogenotrophic denitrification behavior using continuous and intermittent hydrogen gas supply. J. Water Environ. Technol. 2017, 15, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Khanitchaidecha, W.; Kazama, F. Hydrogenotrophic denitrification in an attached growth reactor under various operating conditions. Water Supply 2012, 12, 72–81. [Google Scholar] [CrossRef]
- Chen, D.; Yang, K.; Wang, H.; Lv, B. Nitrate removal from groundwater by hydrogen-fed autotrophic denitrification in a bio-ceramsite reactor. Water Sci. Technol. 2014, 69, 2417–2422. [Google Scholar] [CrossRef]
- Lee, K.C.; Rittmann, B.E. Applying a novel autohydrogenotrophic hollow-fiber membrane biofilm reactor for denitrification of drinking water. Water Res. 2022, 36, 2040–2052. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Elliott, D.J.; Zazouli, M.A. Biological nitrate removal processes from drinking water supply a review. J. Environ. Health Sci. Eng. 2013, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xing, W.; Zuo, J.; Tang, L.; Wang, Y.; Lin, J. Hygrogenotrophic denitrification for tertiary nitrogen removal from municipal wastewater using membrane diffusion packed-bed bioreactor. Bioresour. Technol. 2013, 144, 452–459. [Google Scholar] [CrossRef]
- Visvanathan, C.; Hung, N.Q.; Jegatheesan, V. Hydrogenotrophic denitrification of synthetic aquaculture wastewater using membrane bioreactor. Process. Biochem. 2008, 43, 673–682. [Google Scholar] [CrossRef]
- Omata, J. Biological Wastewater Treatment under Hydrogen Gas Diffusion. Master’s Thesis, University of Yamanashi, Yamanashi, Japan, 2019. [Google Scholar]
- Mostafa, M. Waste water treatment in textile industries-the concept and current removal technologies. J. Biodivers. Environ. Sci. 2015, 7, 501–525. [Google Scholar]
- Panswad, T.; Luangdilok, W. Decolorization of reactive dyes with different molecular structures under different environmental conditions. Water Res. 2002, 34, 4177–4184. [Google Scholar] [CrossRef]
- Sairiam, S.; Thuptimdang, P.; Painmanakul, P. Decolorization of reactive black 5 from synthetic dye wastewater by fenton process. Environ. Asia 2019, 12, 1–8. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22th ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Supaka, N.; Juntongjin, K.; Damronglerd, S.; Delia, M.L.; Strehaiano, P. Microbial decolorization of reactive azo dyes in a sequential anaerobic-aerobic system. Chem. Eng. J. 2004, 99, 169–176. [Google Scholar] [CrossRef]
- Ni, S.Q.; Ni, J.Y.; Hu, D.L.; Sung, S. Effect of organic matter on the performance of granular anammox process. Bioresour. Technol. 2012, 110, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Rujakom, S.; Singhopon, T.; Eamrat, R.; Kamei, T.; Kazama, F. Effect of carrier filling ration on hydrogenotrophic denitrification performance. Jpn. J. Water Treat. Biol. 2020, 56, 67–78. [Google Scholar] [CrossRef]
- Hamdani, A.; Mountadar, M.; Assobhei, O. Simultaneous nitrate and organic matter removal from a dairy effluent by biodenitrification. Rev. Des. Sci. De. L’Eau. 2018, 31, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Wu, S.; Liu, J.; Li, B. Denitrification capacity of a landfilled refuse in response to the variations of COD/NO3-N in the injected leachate. Bioresour. Technol. 2012, 103, 109–115. [Google Scholar] [CrossRef]
- Henze, M.; Kristensen, G.H.; Strube, R. Rate-Capacity Characterization of Wastewater for Nutrient Removal Processes. Water Sci. Technol. 1994, 29, 101–107. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.H.; Park, K.Y.; Meng, S.K. Hydrogentrophic denitrification in a packed bed reactor: Effect of hydrogen-to-water flow rate ratio. Bioresour. Technol. 2012, 101, 3940–3946. [Google Scholar] [CrossRef]
- Saroyan, H.; Ntagiou, D.; Rekos, K.; Deliyanni, E. Reactive black 5 degradation on manganese oxides supported on sodium hydroxide modified graphene oxide. Appl. Sci. 2019, 9, 2167. [Google Scholar] [CrossRef] [Green Version]
- Rasool, K.; Mahmoud, K.A.; Lee, D.S. Influence of co-substrate on textile wastewater treatment and microbial community changes in the anaerobic biological sulfate reduction process. J. Hazard. Mater. 2015, 299, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Y.; Zhang, J.; Sun, L.; Quan, X. Performance of a ZVI-UASB reactor for azo dye wastewater treatment. J. Chem. Technol. Biotechnol. 2011, 86, 99–204. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Kocyigit, H.; Ugurlu, A. Biological decolorization of reactive azo dye by anaerobic/aerobic-sequencing batch reactor system. Glob. Nest J. 2015, 17, 210–219. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shang, H.; Li, Q.; Zhou, W. Treatment of azo dye wastewater by the self-flocculating marine bacterium aliiglaciecola lipolytica. Environ. Technol. Innov. 2020, 19, 100810. [Google Scholar] [CrossRef]
- Costa, M.C.; Mota, F.S.; Santos, A.B.D.; Mendonça, G.L.F.; Nascimento, R.F. Effect of dye structure and redox mediators on anaerobic azo and anthraquinone dye reduction. Quim. Nova 2012, 35, 482–486. [Google Scholar] [CrossRef] [Green Version]
- Wijetunga, S.; Li, X.F.; Jian, C. Effect of organic load on decolourization of textile wastewater containing acid dyes in upflow anaerobic sludge blanket reactor. J. Hazard. Mater. 2010, 177, 792–798. [Google Scholar] [CrossRef]
- Al-Amrani, W.A.; Lim, P.E.; Seng, C.E.; Wan Ngah, W.S. Factors affecting bio-decolorization of azo dyes and COD removal in anoxic-aerobic REACT operated sequencing batch reactor. J. Taiwan Inst. Chem. Eng. 2014, 45, 609–616. [Google Scholar] [CrossRef]
- El Bouraie, M.E.; El Din, W.S. Biodegradation of reactive black 5 by Aeromonas hydrophla strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Naimabadi, A.; Movahedian Attar, H.; Shahsavani, A. Decolorization and biological degradation of azo dye reactive red 2 by anaerobic/aerobic sequential process. J. Environ. Health Sci. Eng. 2009, 6, 67–72. [Google Scholar]
- Erick, M.; Igor, P.; Firmino, M. Sequential anaerobic/aerobic treatment of dye-containing Wastewaters: Colour and COD Removals, and ecotoxicity tests. Appl. Biochem. Biotechnol. 2012, 166, 1057–1069. [Google Scholar] [CrossRef]
- Torrentó, C.; Urmeneta, J.; Otero, N.; Soler, A.; Viñas, M.; Cama, J. Enhanced denitrification in groundwater and sediments from a nitrate-contaminated aquifer after addition of pyrite. Chem. Geol. 2011, 287, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Rujakom, S.; Kamei, T.; Kazama, F. Thauera sp. in Hydrogen-Based Denitrification: Effects of Plentiful Bicarbonate Supplementation on Powerful Nitrite Reducer. Sustainability 2023, 15, 277. [Google Scholar] [CrossRef]
- Stolz, A.; Ka, P. Bacterium that is able to degrade azo dyes of the Orange II type. Int. J. Syst. Evol. Microbiol. 2001, 51, 1831–1837. [Google Scholar]
- Junghare, M.; Patil, Y.; Schink, B. Draft genome sequence of a nitrate-reducing, o-phthalate degrading bacterium, Azoarcus sp. strain PA01(T). Stand. Genom. Sci. 2015, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhold, H.B.; Hurek, T.; Gillis, M.; Hoste, B.; Vancanneyt, M.; Kersters, K. Azoarcus gen. nov., nitrogen-fixing Proteobacteria associated with roots of kallar grass (Leptochloa fusca (L.) Kunth), and description of two species, Azoarcus indigens sp. nov. and Azoarcus communis sp. nov. Int. J. Syst. Bacteriol. 1993, 43, 574–584. [Google Scholar] [CrossRef]
- Evans, W.C.; Fuchs, G. Anaerobic degradation of aromatic compounds. Ann. Rev. Microbiol. 1998, 42, 289–317. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.X.; Lu, X.; Liu, B.; Li, Y. Abundance and diversity of bacterial nitrifiers and denitrifiers and their functional genes in tannery wastewater treatment plants revealed by high-throughput sequencing. PLoS ONE 2014, 9, e113603. [Google Scholar] [CrossRef]
- Ma, Q.; Qu, Y.; Shen, W. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef]
- Angnes, G.; Nicoloso, R.S.; Da Silva, M.L.B.; De Oliveira, P.A.V.; Higarashi, M.M.; Mezzari, M.P.; Miller, P.R.M. Correlating denitrifying catabolic genes with N2O and N2 emissions from swine slurry composting. Bioresour. Technol. 2013, 140, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechichi, T.; Stackebrandt, E.; Gad’on, N.; Fuchs, G. Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp. nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp. nov. Arch. Microbiol. 2002, 178, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, Y.M.; Konde, P.D.; Markad, V.L.; Kulkarni, S.V.; Chaudhari, A.U.; Kodam, K.M. Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl. Microbiol. Biotechnol. 2013, 97, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, X.; Zhu, H.; Yan, B.; Shutes, B.; Xu, Y.; Fu, B.; Wen, H. Isolation and characterization of a salt-tolerant denitrifying bacterium Alishewanella sp. F2 from seawall muddy water. Sci. Rep. 2020, 10, 10002. [Google Scholar] [CrossRef]
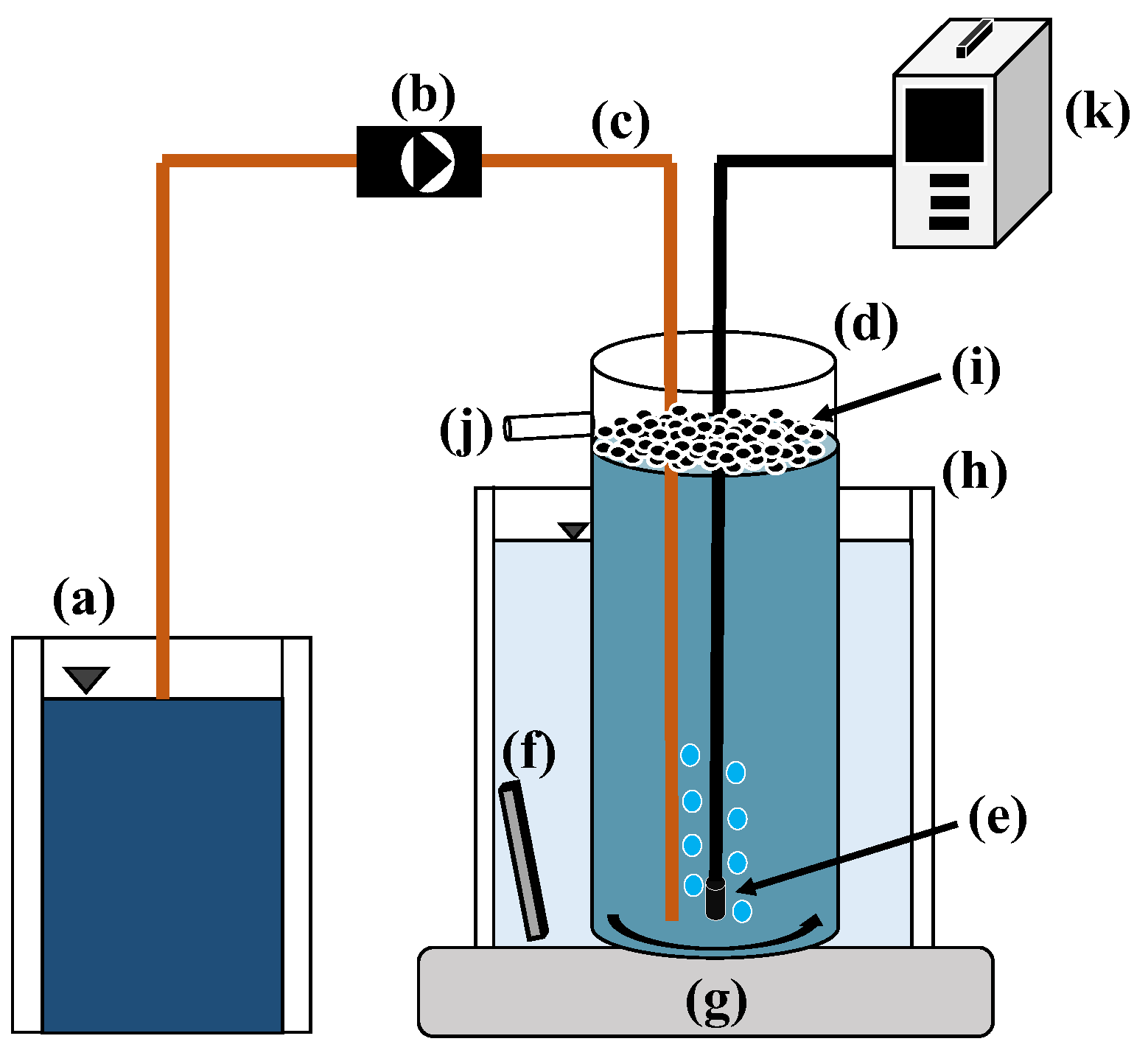


| Substrate | Concentration |
|---|---|
| NaNO3 | 0.486 g/L as 80 mg NO3-N/L |
| KH2PO4 | 0.055 g/L as 12.5 mg P |
| NaHCO3 | 1.0 g/L as an inorganic carbon source |
| CaCl2 | 0.021 g/L as 7.5 mg Ca |
| MgSO4·7H2O | 0.038 g/L as 3.75 mg Mg |
| Trace elements I a | 1 mL/L |
| Trace elements II b | 1 mL/L |
| Reactive Black 5 dye | 0.030 g/L as color of 30 mg/L |
| CH3COONa | 2.085 g/L as 1500 mg COD/L |
| Operating Day | No OM Addition | OM Addition | ||
|---|---|---|---|---|
| Day 0–20 RUN 1 | Day 21–40 RUN 2 | Day 41–60 RUN 3 | Day 61–80 RUN 4 | |
| Initial concentrations | ||||
| NO3-N (mgN/L) | 80 | 80 | 80 | 80 |
| RB-5 dye (mg/L) | 30 | 30 | 30 | 30 |
| COD (mg/L) | - | 5000 | 3000 | 1500 |
| H2 supply conditions | Continuous supply at 50 mL/min | |||
| Sludge Samples | Bacterial Relative Abundance (%) | ||||
|---|---|---|---|---|---|
| Initial Sludge | RUN 1 | RUN 2 | RUN 3 | RUN 4 | |
| Bacterial communities | |||||
| Thauera spp. | 51.6 | 15.5 | 4.2 | 28.3 | 28.9 |
| Xanthomonadaceae | 0.6 | 16.3 | - | - | - |
| Rhodocyclaceae | 4.6 | 46.0 | 0.1 | 1.1 | 0.2 |
| Azoarcus | - | - | 48.6 | 9.7 | 11.8 |
| Erysipelothrix | - | - | 18.6 | 10.5 | 10.5 |
| Alishewanella | - | - | 0.7 | 15.4 | 9.2 |
| Alkaliphilus | - | - | 0.4 | 7.5 | 6.8 |
| Parameters | Treatment efficiency (%) | ||||
| Nitrogen | 86 ± 9 | 90 ± 3 | 95 ± 4 | 99 ± 2 | |
| Decolorization | 25 ± 14 | 50 ± 18 | 58 ± 17 | 51 ± 9 | |
| COD | - | 47 ± 446 | 43 ± 434 | 50 ± 359 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhopon, T.; Rujakom, S.; Shinoda, K.; Kamei, T.; Kazama, F. Application of Hydrogen-Based Denitrification: Simultaneous Removal of Nitrate and Reactive Black 5 Dye from Textile Wastewater Containing Organic Matter. Sustainability 2023, 15, 10324. https://doi.org/10.3390/su151310324
Singhopon T, Rujakom S, Shinoda K, Kamei T, Kazama F. Application of Hydrogen-Based Denitrification: Simultaneous Removal of Nitrate and Reactive Black 5 Dye from Textile Wastewater Containing Organic Matter. Sustainability. 2023; 15(13):10324. https://doi.org/10.3390/su151310324
Chicago/Turabian StyleSinghopon, Tippawan, Suphatchai Rujakom, Kenta Shinoda, Tatsuru Kamei, and Futaba Kazama. 2023. "Application of Hydrogen-Based Denitrification: Simultaneous Removal of Nitrate and Reactive Black 5 Dye from Textile Wastewater Containing Organic Matter" Sustainability 15, no. 13: 10324. https://doi.org/10.3390/su151310324







