Abstract
Most mercury supplies nowadays are limited due to their toxicity and difficulty in treatment. If mercury is stored inappropriately, it will not only contaminate the environment but also pass along the food chain and eventually to humans. Therefore, addressing mercury waste is crucial for the environment and human health. This study aims to stabilize waste mercury using sulfur powder and generate mercury production through a ball mill and heat treatment. To begin with, sulfur powder, waste mercury (98%) from chemicals, and milling balls will be mixed in this step. The parameters in this process were milling temperature, milling time, ball/material ratio, and milling speed. Under the optimal parameters of 35 °C for milling temperature, 12 h for milling time, 46% for the ball/material ratio, and 300 rpm for milling speed, β-HgS was obtained, and α-HgS was subsequently acquired through dry distillation in a tubular furnace at 600 °C for 3 h. On the other hand, high-purity mercury (99.5%) could be recovered under the circumstances of heating α-HgS with oxygen at 600 °C for 3 h. In a nutshell, waste mercury (98%) could be treated appropriately under the state of α-HgS, and high-purity mercury (99.5%) could be produced and reused for other industries through this research. Both contribute to environmental remediation and resource recovery goals.
1. Introduction
According to available information, the knowledge of α-HgS (cinnabar) and mercury can be traced back to the Mediterranean region around the fifth century B.C. During the Neolithic Age in Italy and Spain, α-HgS was documented as being used as a preservative for human bones. In addition, the vivid red color (vermillion) of α-HgS made it a highly sought-after pigment in ancient times. In China, α-HgS ink was used as early as 1100 B.C. for making rouges and decorating tombs or palaces [1]. Additionally, mercury was also commonly employed for treating syphilis in India and Europe [2]. In recent centuries, mercury found application in various fields, including thermometers, fluorescent lamps, dental fillings, antiparasitic treatments, antisyphilis medications, antiitch creams, preservatives, anti-inflammatory drugs, and so on [3,4]. Furthermore, inorganic mercury, such as C2H5Hg+ (ethylmercury) and aminosalicylic acid, was used in vaccines, pharmaceuticals, and cosmetics [2]. Nevertheless, on account of the toxicity of mercury and α-HgS, most mercury-containing products, such as medicine and other daily utilizations, have been restricted. Thus, there has been a decreasing trend in the application scope of mercury and mercury sulfide, primarily due to concerns over their adverse effects.
As previously mentioned, specific mercury utilizations have been phased out over time due to their toxicity. Nonetheless, improper storage of waste mercury has presented challenges. In the environment, mercury exists primarily in the form of elemental mercury, inorganic mercury, and organic mercury (a combination of bivalent mercury and alkyl). Among the above kinds, exposure to elemental mercury vapor is the most common cause of poisoning. Occupational exposure, use of mercury-containing medications, and accidents involving spilled mercury during production activities can lead to chronic mercury poisoning. In China, a total of 3596 cases of mercury exposure were reported by ATSC in 1997 [2]. Occupational mercury exposure is currently recognized in over 60 industries, including manufacturing glass thermometers, batteries, barometers, paper, paint, jewelry, fungicides, chlorine, caustic soda, dental clinics, and so on [4]. Inhalation of excessive amounts of mercury vapor can potentially cause idiopathic thrombocytopenic purpura in humans [2,5]. The primary source of environmental pollution is organomercury compounds, such as CH3HgCl, (CH3)2Hg, and CH3Hg+ [4], which pose a greater risk to human health than elemental mercury vapor. The main ways of human exposure to CH3Hg+ are food fish and marine mammals. Consequently, more than 3000 lakes in the United States have been closed to fishing due to mercury poisoning concerns [6]. Similar cases of organic mercury pollution can be found worldwide. In Japan, industrial discharge of CH3Hg+ into Minamata Bay and the Agana River led to the accumulation of toxic substances in fish, which then entered the food chain and affected humans [2,6,7]. The poisoning symptoms included cerebellar ataxia, dysarthria, visual-field constriction, sensory disturbances in the extremities, and hearing loss. These incidents were also responsible for the development of Minamata disease (CH3Hg+ poisoning) [3,8,9,10,11]. Furthermore, several studies have explored the relationship between child health, autism spectrum disorder (ASD), and heavy metals such as mercury. The findings suggest that exposure to various sources of contamination, including polluted air, water, soil, and food, may contribute to the development of behavioral disorders in children. Moreover, exposure to toxic metals during pregnancy has been associated with an increased risk of ASD (Autism Spectrum Disorder) in children, either prenatally or postnatally [8,12,13,14]. Alongside that, some research investigated the connection between mercury concentration and diabetes. The results indicate that higher mercury exposure during young adulthood may elevate the risk of developing diabetes later in life [15,16].
Due to the hazardous nature of mercury, various countries have implemented regulations to address its risks. The Mercury-Containing and Rechargeable Battery Management Act was passed in the United States in 1996. This legislation aims to eliminate mercury in batteries and establish effective and economical methods to dispose of used batteries [17]. In the European Union, the Restriction of Hazardous Substances Directive (RoHS) has prohibited the use of mercury in certain electrical and electronic products since January 2003 [18]. The European Union also banned mercury from nonelectrical measuring devices such as thermometers and barometers starting in July 2007 [19]. On top of that, 140 countries agreed to restrict mercury emissions in the Minamata Convention on Mercury. This convention aims to limit mercury emissions and prohibits importing and exporting mercury and its derivatives since 2020 [20].
Given the abovementioned pollution issues and mercury regulations, the primary focus now is to reduce the production of mercury. Moreover, proper storage methods are crucial for eliminating mercury production to prevent spills and subsequent pollution. Otherwise, the mercury stored inappropriately might be spilled and bring about pollution. The common mercury treatments now can be classified into hydrometallurgical routes, vacuum distillation and retorting, amalgamation, complexation with sulfur, immobilization, and so on [21]. The hydrometallurgical process typically comprises two main steps: leaching and recovery. Leaching involves the dissolution of mercury using aqueous chemicals such as HCl, HNO3, and Na2S2O3. On the other hand, recovery focuses on the specific retrieval of the dissolved mercury from the leachate. This can be achieved through cementation, adsorption, ion exchange, solvent extraction, and precipitation. In the process of thermal treatment, mercury is volatilized by applying heat and reduced pressure, then condensed into liquid Hg0. The desorption of mercury at elevated temperatures is a convenient operation that effectively lowers the mercury concentration in byproducts to a level that meets regulatory requirements [21]. Amalgamation involves the physical treatment of mercury through its dissolution in another metal, such as copper, zinc, nickel, and tin, resulting in the formation of a solid and nonvolatile product. However, the risks of volatilization or leaching are not eliminated, so it still needs to be combined with other methods [21].
Among the above methods, complexation with sulfur is a cost-effective and straightforward method. In accordance with the consequence of some research, mercury sulfide exists in two crystal forms. One is a cubic system known as β-HgS, a naturally occurring metastable substance. The other is a trigonal system called α-HgS, which is considered the most stable and the most commonly found mineral in nature [21,22,23,24]. Therefore, the main objective of this study is to convert waste mercury into α-HgS, the most stable form suitable for storage. Despite mercury’s toxicity, it is still utilized in the chemical industry. As a result, this study also explores the process of converting α-HgS back into mercury. Additionally, the mercury used in this experiment was obtained from waste chemicals rather than through ground mining of α-HgS ore. This approach not only reduces the energy required for mining but also utilizes a recycling resource. In sum, our core goals were to stabilize the waste mercury, safely recycle high-purity mercury, and reuse the waste mercury through this process. The following sections will discuss various methods for transforming waste mercury into α-HgS.
The narrative above not only assessed the risks associated with mercury and organomercury compounds but also highlighted the stability of α-HgS. Now turning to the methods for stabilizing mercury, two common approaches are melting and chemical methods. In the former method, the sulfur powder is heated in a high-temperature furnace at 140 °C for 2 h to initiate the melting process. Mercury was then added to the molten sulfur powder and stirred every 10 min to ensure the reaction continued. However, the operator might be burned in the stirring step on account of the 140 °C high temperature. The latter method involves soaking mercury in an excess of nitric acid and stirring for 6 h to produce Hg(NO3)2. Hereafter, Hg(NO3)2 and Na2S would then be mixed to produce α-HgS and Na2NO3. Nevertheless, it should be noted that this process may generate H2S, which is toxic to humans. Considering the potential risks involved in the above two methods, a safer alternative is to use the ball-mill method and heat treatment to react mercury with sulfide. In this method, the mixture of mercury and sulfur powder was ground through a ball mill first, producingβ-HgS [23]. In the subsequent step, the β-HgS was heated without O2 to induce a crystal phase switch and transform it into α-HgS. In the last part of the procedure, α-HgS would be heated with O2 and condensed to transform into high-purity mercury [10]. To sum up, this study aims to stabilize waste mercury using sulfur powder and recycle waste mercury (with a purity level of 98% to 99.5%) through the ball-mill method and heat treatment, respectively. By completing the mercury–mercury sulfide cycle, waste mercury can be recovered as resources in the form of α-HgS and high-purity of mercury, making the impact of waste mercury minimal and creating additional value to achieve the goals of waste-mercury stabilization, resource recovery, and environmental remediation.
2. Materials and Methods
2.1. Reagents and Chemicals
In the mercury-β-HgS process, waste mercury (98%) was sourced from waste chemicals, while sulfur powder was acquired from Sigma-Aldrich (St. Louis, MO, USA). The mixture of mercury and sulfur powder was achieved using the ball-mill method to form β-HgS. Nitric acid (≥65%) was purchased from Sigma-Aldrich (St. Louis, MO, USA) to dissolve mercury for analysis. In the β-HgS to α-HgS procedure, N2 gas was procured from Yun Shan Gas Co. (Tainan City, Taiwan). In the α-HgS-mercury process, the gas contained 20% of O2 and 80% of N2 was from Yun Shan Gas Co. (Tainan City, Taiwan). In addition, the conductivity of the deionized water used in this experiment was 5.5 μS/m.
2.2. Apparatus
A ball mill is a type of grinder with a mixing effect to facilitate the mixing of mercury and sulfur powder in this research. The rotation of the ball mill generates shear force, centrifugal force, and gravity, contributing to the blending process within the fuselage. More detailed information about the ball mill will be described in the follow-up chapter. SiO2 milling balls of 1 mm diameter are used to stir the mercury and sulfur powder thoroughly. A ball mill is commonly used in mineral dressing processes, paints, pyrotechnics, and ceramics [25,26]. The screen mesh was 40 to separate the milling balls from the mixture, which contained sulfur powder and unreacted mercury. As for the two heating processes, the tubular furnace was the main equipment, which could heat at high temperatures with the gas the experiment needed. Last, a helical condenser was used to condensate the mercury vapor to mercury.
2.3. Analytical Methods
The β-HgS and α-HgS generated in this research were analyzed by X-ray diffraction (XRD, DX-2700, Dandong City, China) to investigate the mineralogical characteristics. The operational parameters of XRD are an operation voltage of 35 kV, operation current of 30 mA, scanning speed of 0.04° (2θ)/s, time per step of 1 s/step, and 2θ range of 15–80°. The endothermic process of β-HgS was analyzed by a Thermogravimetry/Differential Thermal Analysis Thermoanalyzer (TG-DTA, NETZSCH-409PC, Netzsh, Selb, Germany) to detect the mass change through temperature variations [27,28]. The temperature of TG-DTA analysis was from room temperature to 900 °C, and the heating rate was 5 °C/min.
2.4. Ball Mill
The primary functions of a ball mill include crushing, grinding, and mixing. When dealing with coarse materials, the main function is crushing, which is influenced by compressive stresses. For fine materials, the main function is grinding, which is affected by shearing stresses [27]. In this experiment, where mercury is in liquid form and sulfur powder is fine, the main functions of the ball mill are grinding and mixing. Additionally, the reaction can be effectively conducted by selecting appropriate milling balls, milling temperature, milling time, ball/material ratio, milling speed, and other parameters.
As mentioned above, the milling balls play a role in enhancing the mixing efficiency in addition to the function of the ball mill itself. Regarding the material of the milling balls, the hardness of the milling balls must be greater than the reactant, or the milling ball would be worn and result in impurified production. Moreover, the material of the milling balls should not react with the reactant to prevent any experimental errors or potential risks to the operators. The choice of suitable milling balls depends on the specific reactants and their respective properties, ensuring optimal efficiency [25,26].
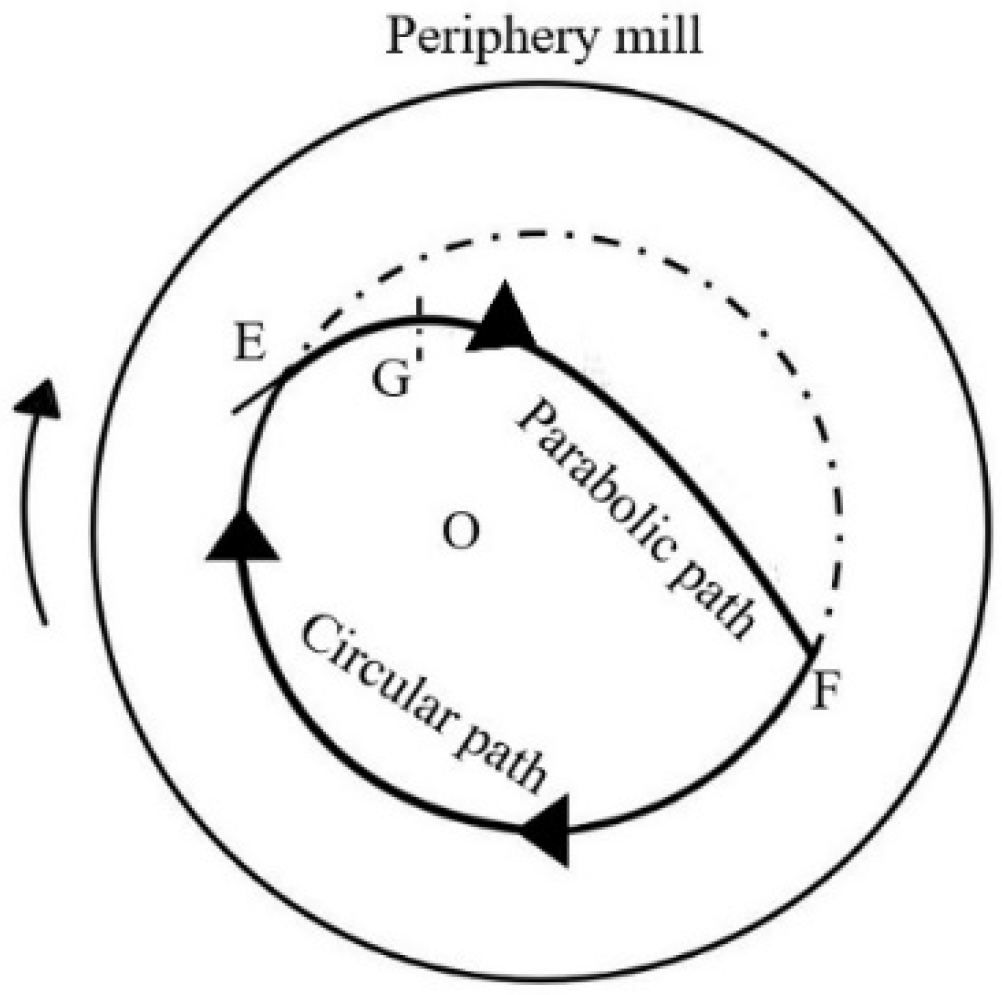
The movement path of the reactant and milling balls is illustrated in Figure 1 [26]. With the optimal parameters, including milling temperature, time, ball/material ratio, and speed, the mixture follows a circular path driven by kinetic energy and centrifugal force. Upon reaching point E, the effect of gravity would affect the reactants and then the route would be connected to the parabolic path. When the reactants and milling balls achieved the top point of the parabolic path (point G), they would descend under the influence of gravity, colliding with the milling-tube wall at point F. Afterward, the mixture continues along the circular and parabolic paths, repeatedly striking each other and the wall of the milling tube. In this study, ball milling effectively combines various forces to achieve grinding, mixing, and reaction. Ultimately, the optimal parameters result in the highest yield.
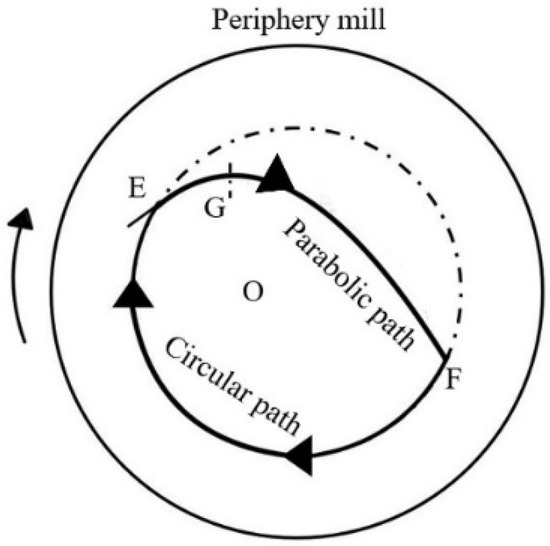
Figure 1.
The movement path of the reactant and milling balls in the ball mill [26].
The following steps describe the conversion of mercury to β-HgS. Initially, an excess of sulfur powder and 1 g of mercury are mixed in a ball mill to ensure a complete reaction of the mercury. The parameters are as follows: milling temperatures from 25 °C to 80 °C, milling time from 3 h to 12 h, ball/material ratio from 40% to 49%, and milling speeds from 200 rpm to 400 rpm. To dissolve any unreacted mercury, 10 mL of nitric acid were added (because mercury could dissolve in nitric acid but β-HgS could not). The mixture is then stirred using a magnetic stirrer at 240 rpm and 40 °C for 6 h. Eventually, after separating the solvent and solute (β-HgS), the yield of mercury transferred to β-HgS and the XRD analysis of β-HgS could be acquired.
2.5. Heat without O2 (β-HgS to α-HgS)
In order to induce a transformation of the crystal from the cubic system to the trigonal system, a sample of 10 g β-HgS was subjected to heating at 600 °C inside a silica tube using a tubular furnace, without the presence of O2 [28,29,30]. It should be noted that heating β-HgS in the presence of O2 would produce HgO and mercury vapor, which would escape into the air. However, the formation of HgO and mercury vapor was not the desired outcome in this particular phase of the experiment. The temperature was fixed at 600 °C, and a heating time of 2 h was maintained. To explore the relationship between yield and soaking time, the soaking times were adjusted as 1 h, 1.5 h, 2 h, 2.5 h, and 3 h.
2.6. Calcination and Condensation (α-HgS to Mercury)
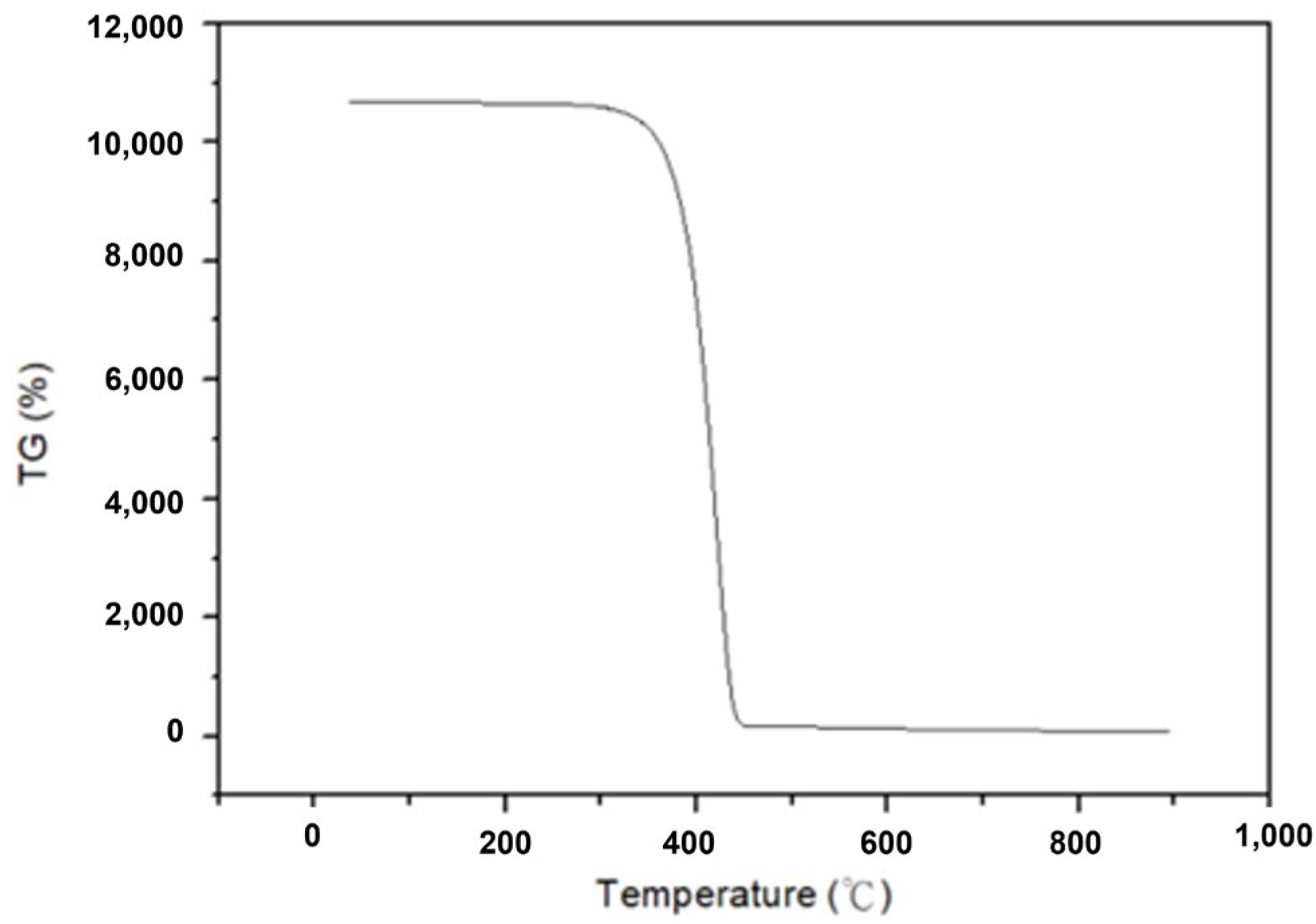
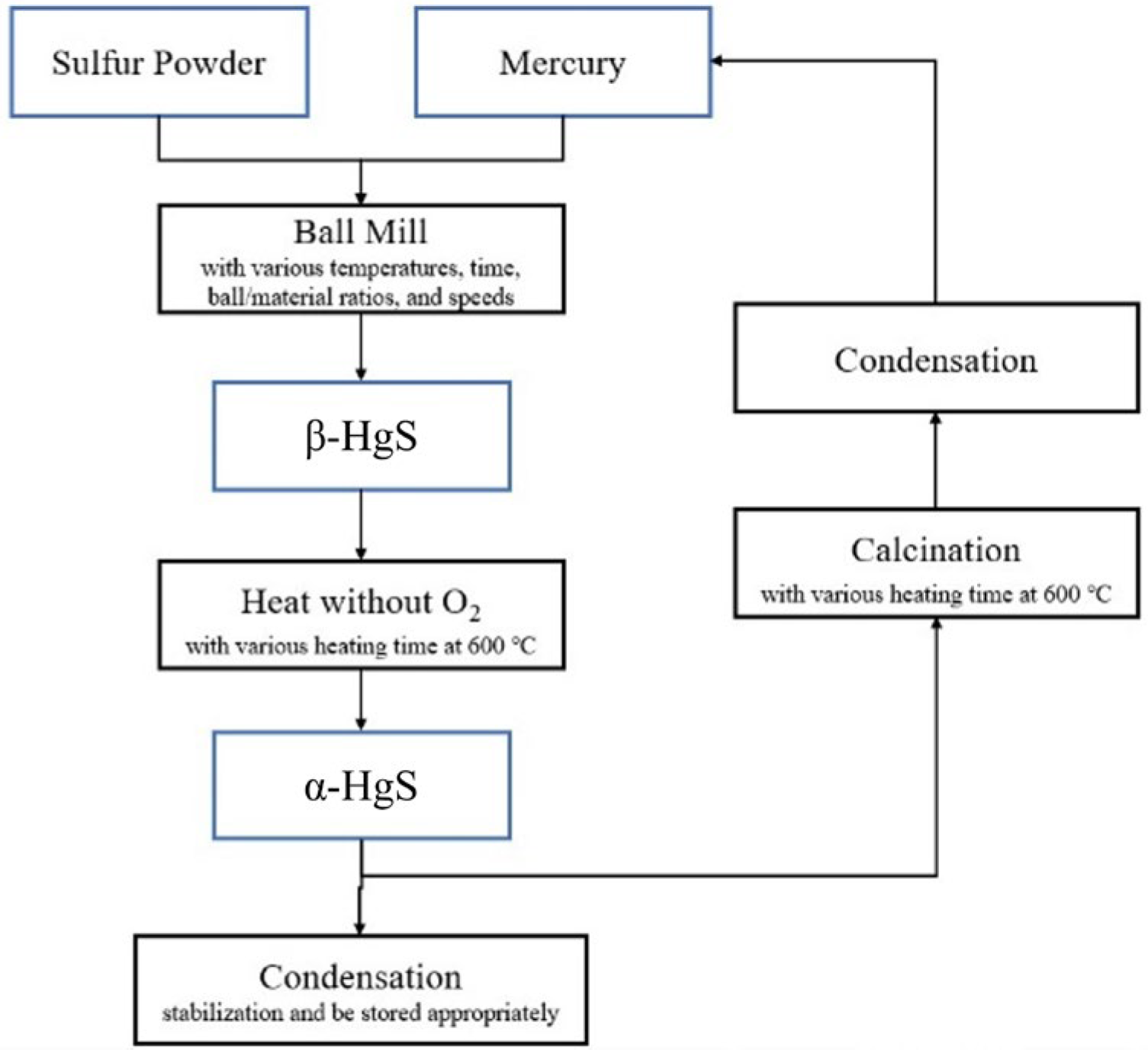
To determine the optimal temperature parameter for converting α-HgS into mercury, a TG/DTA analysis of α-HgS was initially conducted. Figure 2 demonstrates the analysis of TG/DTA, and it displays that the mass of α-HgS would drop dramatically at 450 °C. To ensure the experiment could be conducted completely, the heating temperature was set up at 600 °C. In the beginning, 3 g of α-HgS were calcinated in a silica tube by the tubular furnace [31]. The heating rate was set up as 5 °C per minute, and the heating-up time was 2 h to 600 °C. The experimental parameters for this step included soaking times of 1 h, 1.5 h, 2 h, 2.5 h, 3 h, and 3.5 h. This process resulted in the transformation of α-HgS into mercury vapor and SO2, as shown in Equation (1). After condensation, the mercury vapor was collected as the target product, achieving high-purity mercury, while the SO2 was passed through a condenser tube and dissolved in deionized water. Figure 3 provides a visual representation of the experimental flowchart, outlining all the steps conducted in this study.
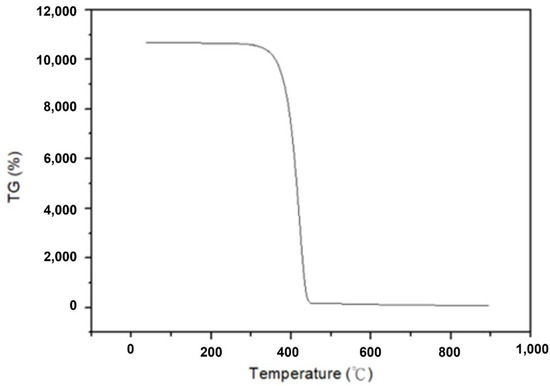
Figure 2.
TG/DTA analysis of α-HgS.
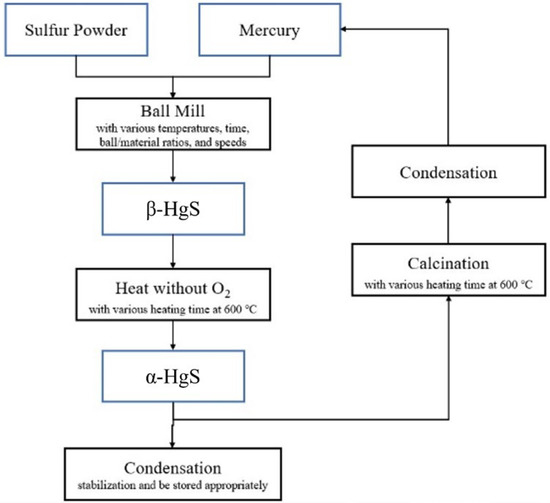
Figure 3.
The experiment flowchart.
3. Results and Discussion
3.1. Ball Mill
3.1.1. Milling Temperature of Mercury Transfers to β-HgS
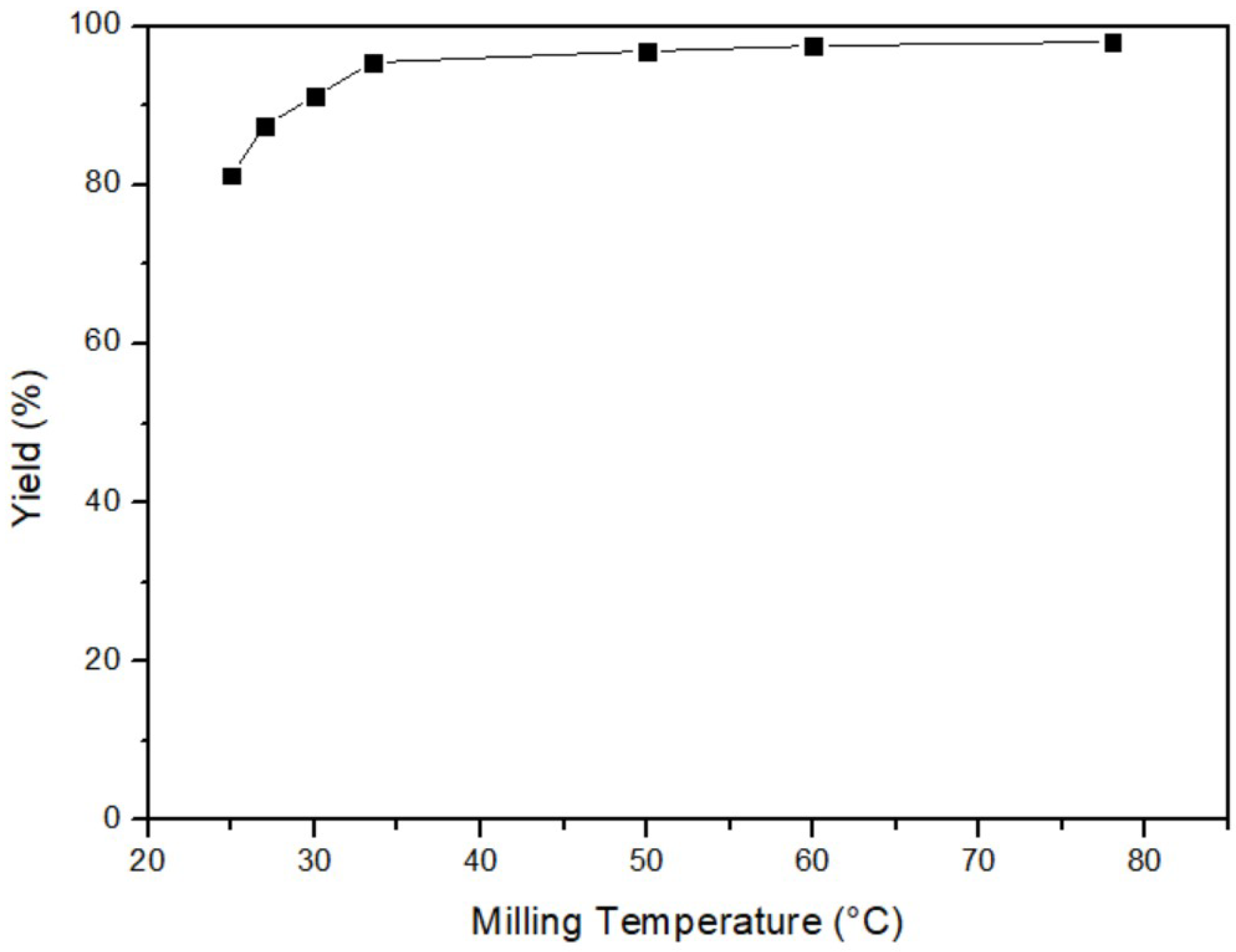
This study investigated the effect of milling temperature on yield at 25 °C, 28 °C, 30 °C, 35 °C, 50 °C, 60 °C, and 80 °C, initially. Other fixed parameters were 3 h of milling time, 40% of ball/material ratio, and 200 rpm of milling speed. Figure 4 depicts that the yield increased when the temperature rose, indicating a positive correlation between temperature and efficiency. Therefore, the optimal milling temperature was determined to be 80 °C. According to the ideal gas law and the formula of kinetic energy, as shown in Equation (2), an increase in temperature resulted in higher average molecular kinetic energy, providing more energy for the reaction and promoting its completeness [32]. However, considering cost and energy consumption, a milling temperature of 35 °C was selected for the subsequent experiments, as it offered a balance between efficiency and cost.
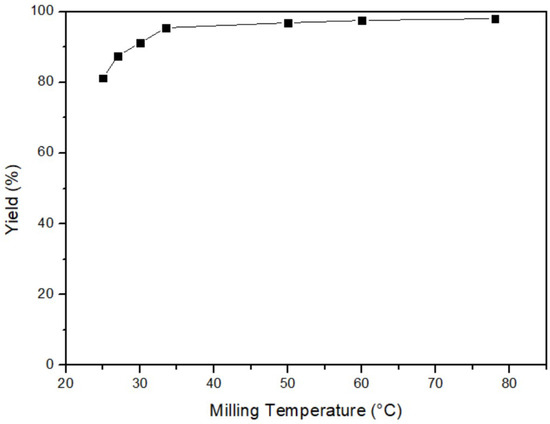
Figure 4.
Milling temperature of mercury transfers to β-HgS.
3.1.2. Milling Time of Mercury Transfers to β-HgS
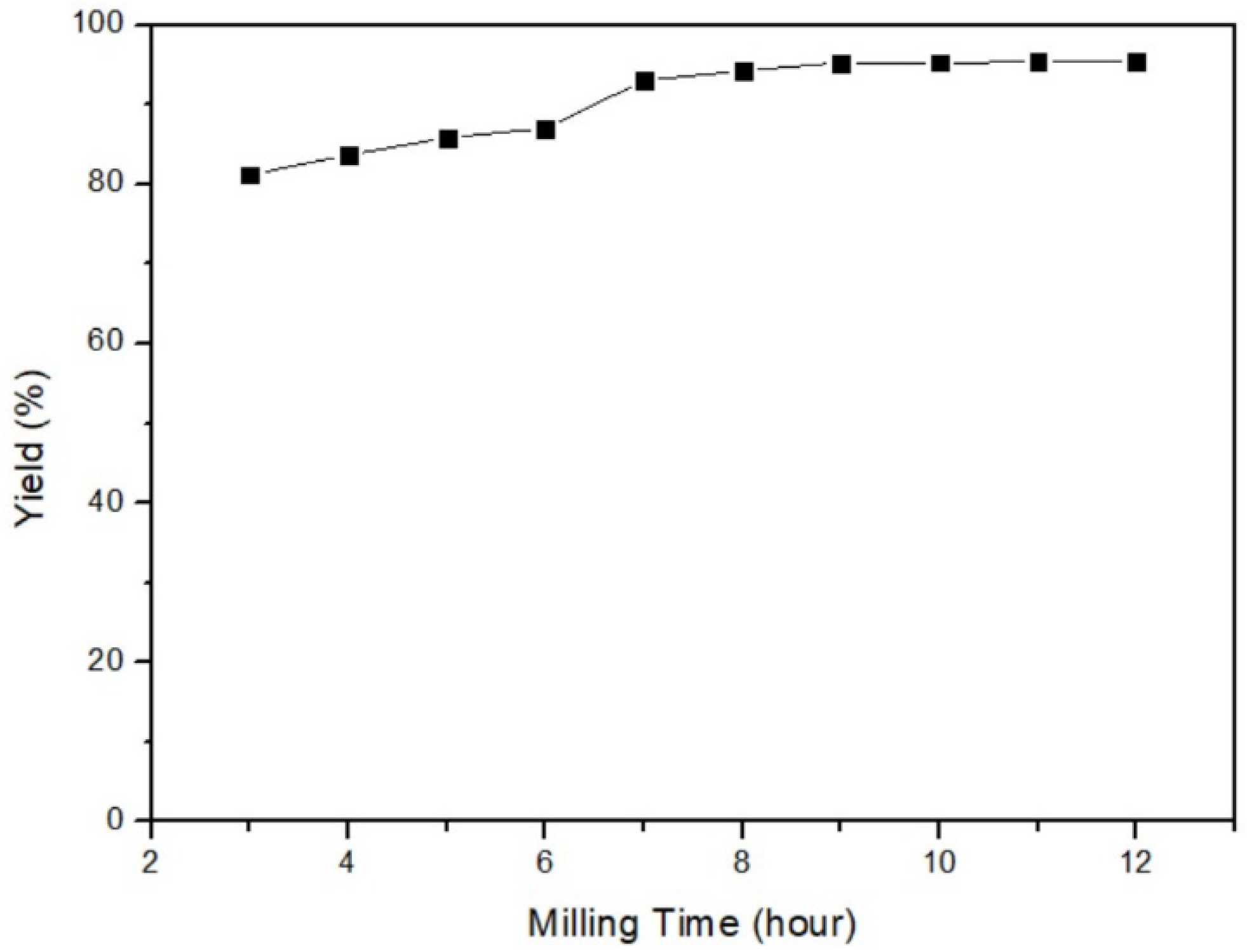
In this part, the relationship between yield and milling time was examined. The different parameters were 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 h, while the fixed parameters were 35 °C of milling temperature, 40% ball/material ratio, and 200 rpm milling speed. Figure 5 reveals that the longer grinding time could improve yield. As the milling time exceeded 7 h, the yield gradually reached a stable level. The optimal parameter for milling time was determined to be 12 h, as it provided sufficient time for the reaction to occur. Nevertheless, for practical reasons and time constraints, a milling time of 3 h was selected as the experimental parameter.
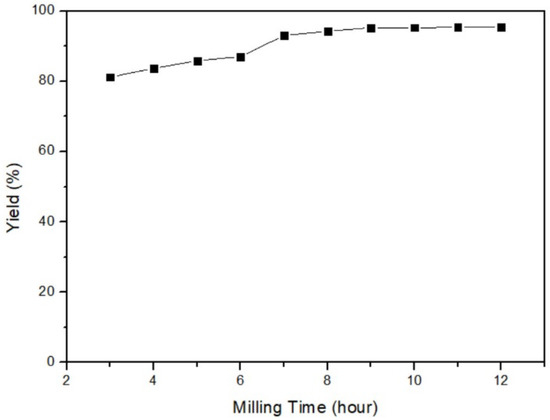
Figure 5.
Milling time of mercury transfers to β-HgS.
3.1.3. Ball/Material Ratio of Mercury Transfers to β-HgS
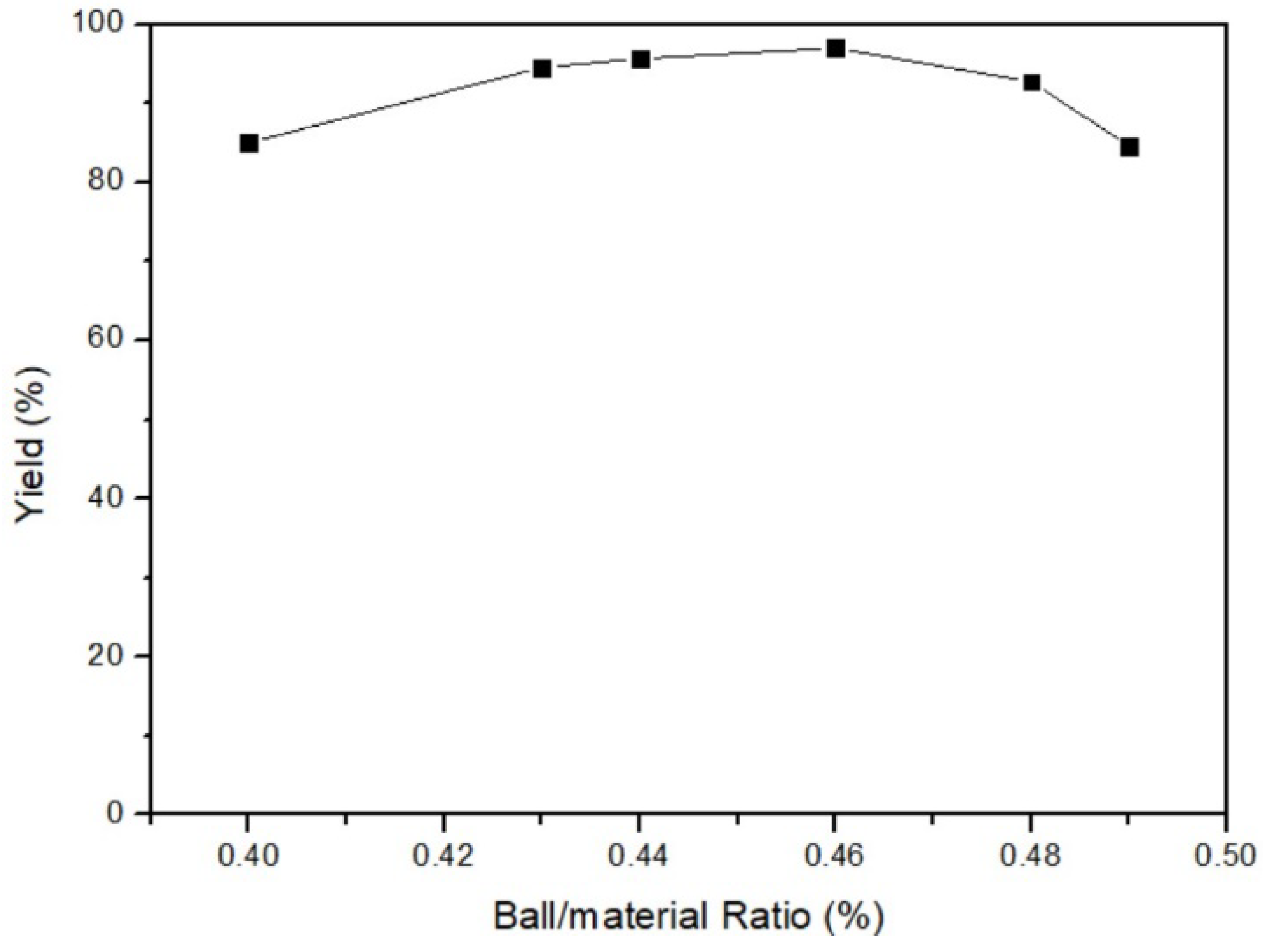
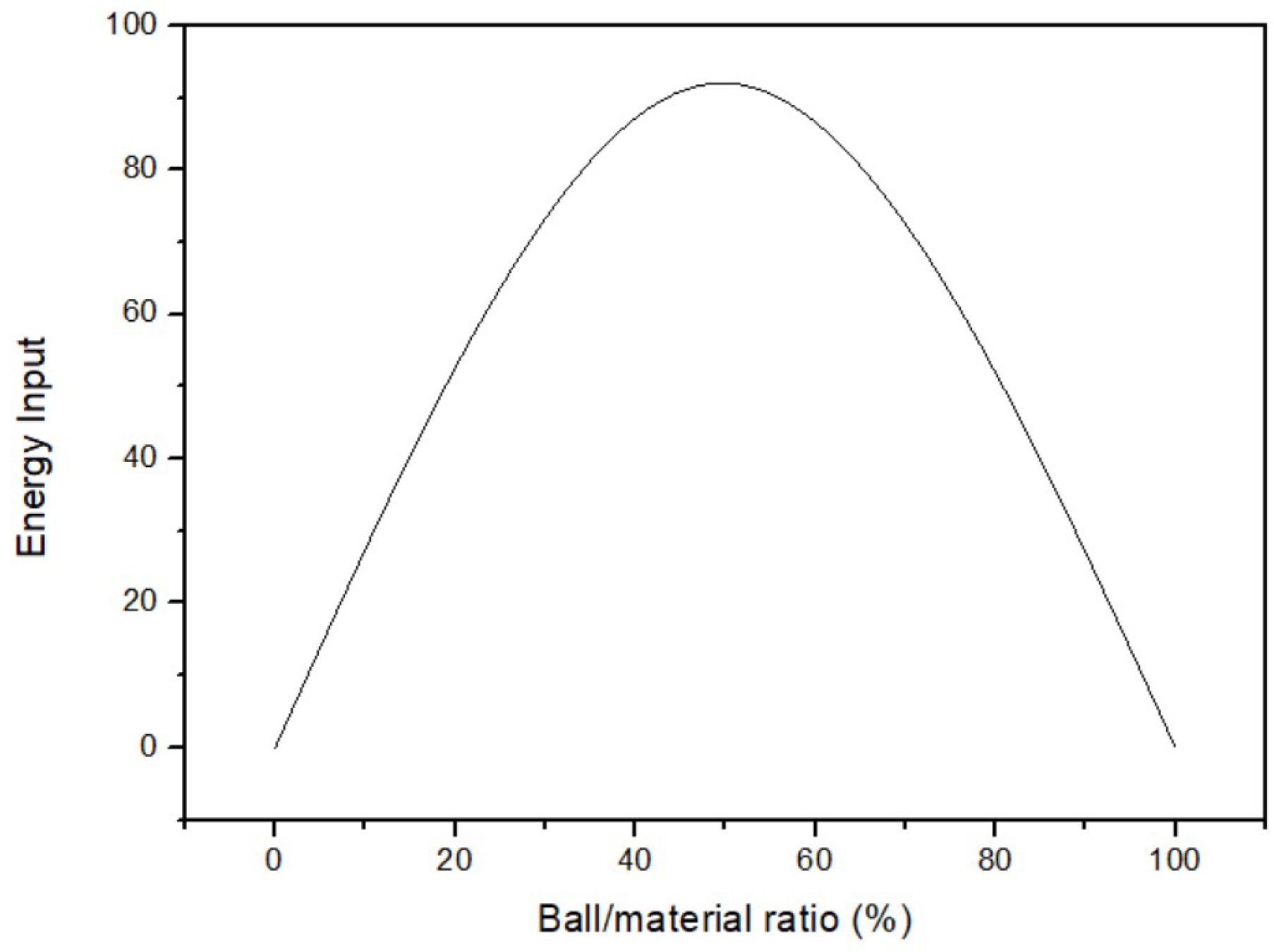
The effect of the ball/material ratio on yield was surveyed after obtaining optimal temperature and time. The fixed parameters were 35 °C of milling temperature, 3 h of milling time, and 200 rpm of milling speed. The ball/material ratio parameters were 40%, 43%, 44%, 46%, 48%, and 49%. Figure 6 displays that the yield of 46% ball/material ratio was the highest among all parameters. As a result, the optimal parameter of the ball/material ratio was 46%. According to the principle of milling, increasing the ball/material ratio initially enhances the milling power of the mill. However, once the ball/material ratio is above 50%, the yield starts to decrease significantly [26]. Figure 7 shows the link between yield and the ball/material ratio. It is widely recognized in the relevant literature that the optimal yield lies within the range of 40% to 60% for the ball/material ratio. Exceeding this range leads to an excessive number of milling balls, resulting in collisions among them. Consequently, yield decreases when the ball/material ratio is too high, indicating poor reaction performance due to an inappropriate ratio.
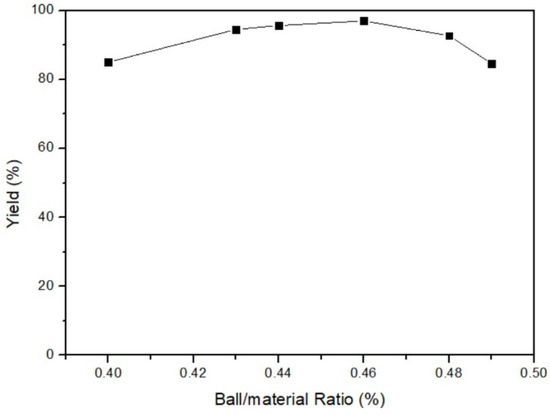
Figure 6.
Ball/material ratio of mercury transfers to β-HgS.
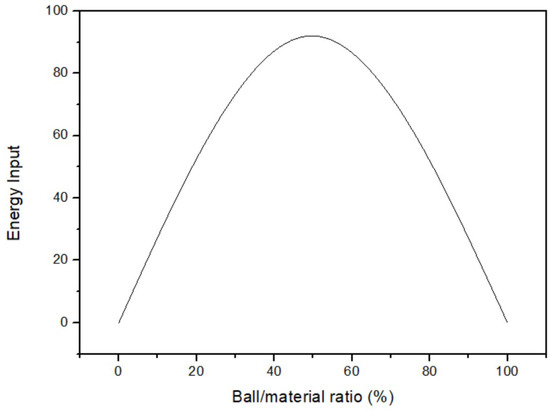
Figure 7.
The relation between the yield and the ball/material ratio [26].
3.1.4. Milling Speeds of Mercury Transfers to β-HgS
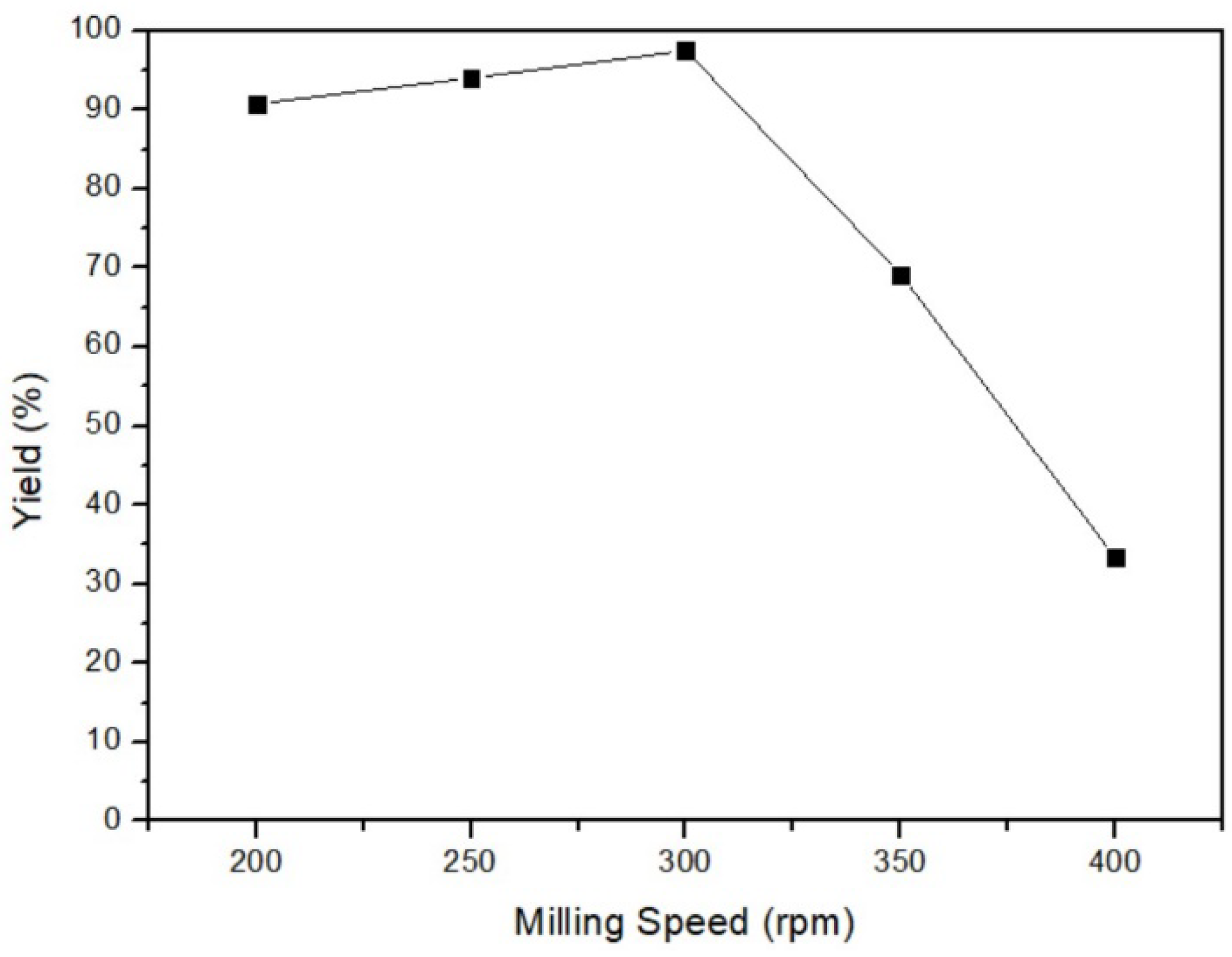
In this part, the reaction between the mercury and sulfur powder mixture was conducted under the parameters of 35 °C milling temperature, 3 h milling time, and 46% ball/material ratio, but at different milling speeds of 200 rpm, 250 rpm, 300 rpm, 350 rpm, and 400 rpm. The consequence is depicted in Figure 8, and the yield of 300 rpm of milling speed had the highest value, 97.5%. Based on the principle of milling, excessively high speed generates excessive centrifugal force, preventing the mixture from following the optimal circular path and hindering efficient production. In this experiment, the yield was the lowest at 400 rpm, indicating an incomplete reaction due to excessively high speed. Nonetheless, if the milling speed is too slow, the yield also could not be improved. The limited kinetic energy at low milling speeds fails to propel the reactants to the highest point, thereby impeding the utilization of gravity for effective collision. This incomplete trajectory results in insufficient reaction and could not acquire the optimal yield. In sum, the most favorable production occurred at a milling speed of 300 rpm, where the centripetal force and kinetic energy were well-balanced to rotate and mix the reactants efficiently.
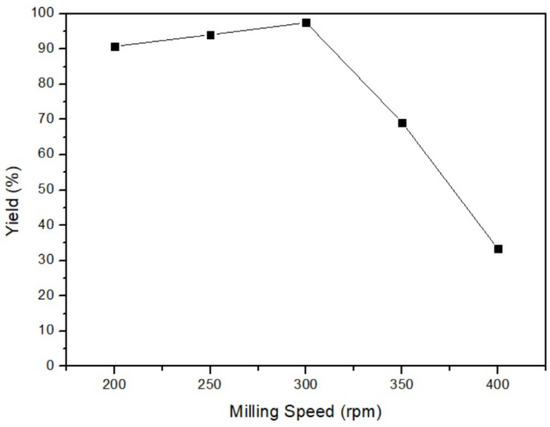
Figure 8.
Milling speed of mercury transfers to β-HgS.
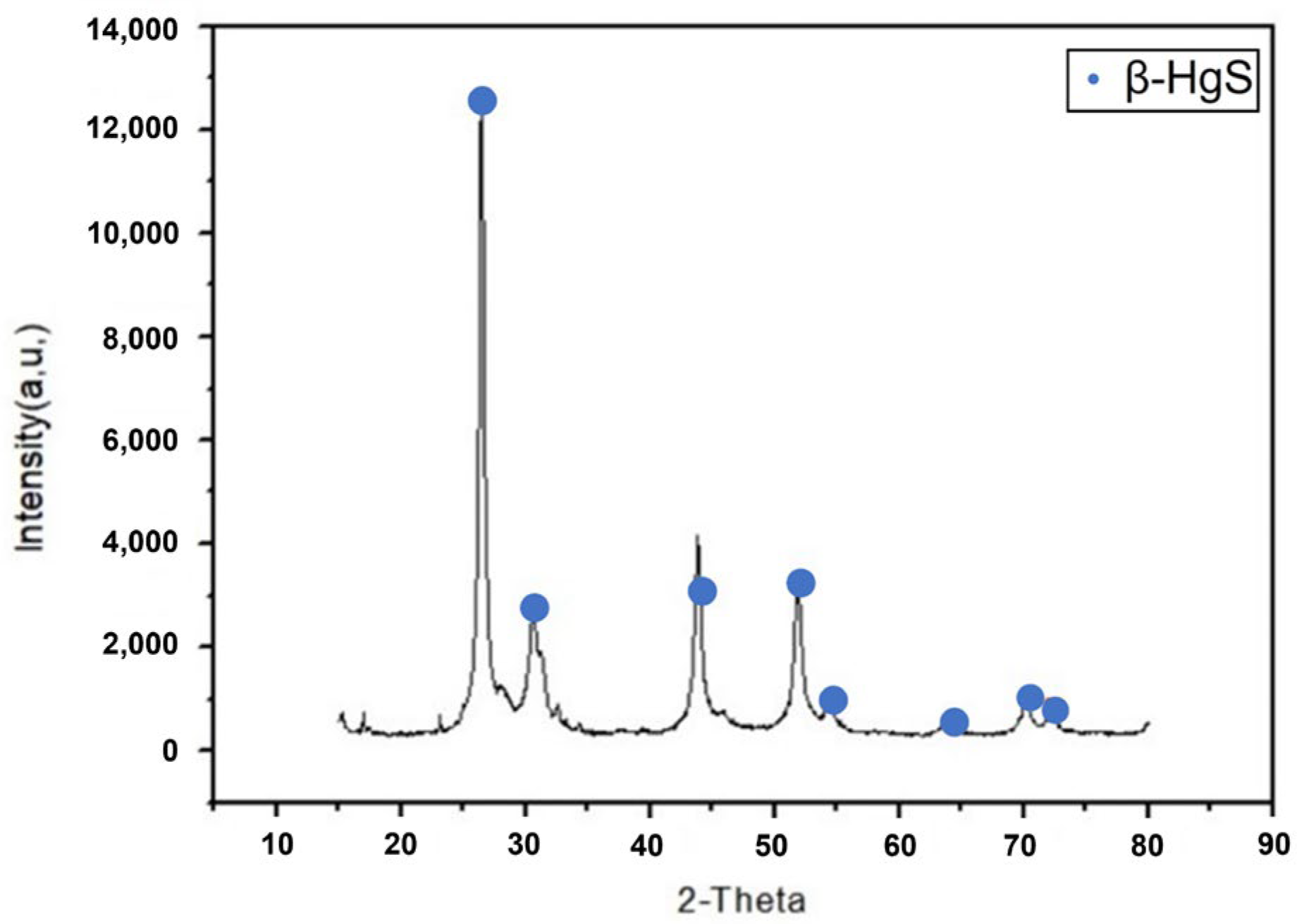
After the reaction, Figure 9 shows the XRD analysis of the β-HgS under the operational parameters. It could be found that the peak was consistent with β-HgS, confirming that the production in this study aligns with the intended target.
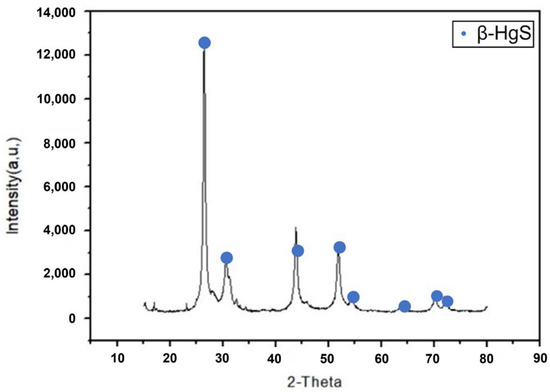
Figure 9.
XRD analysis of β-HgS.
3.2. Soaking Time of β-HgS Transfers to α-HgS
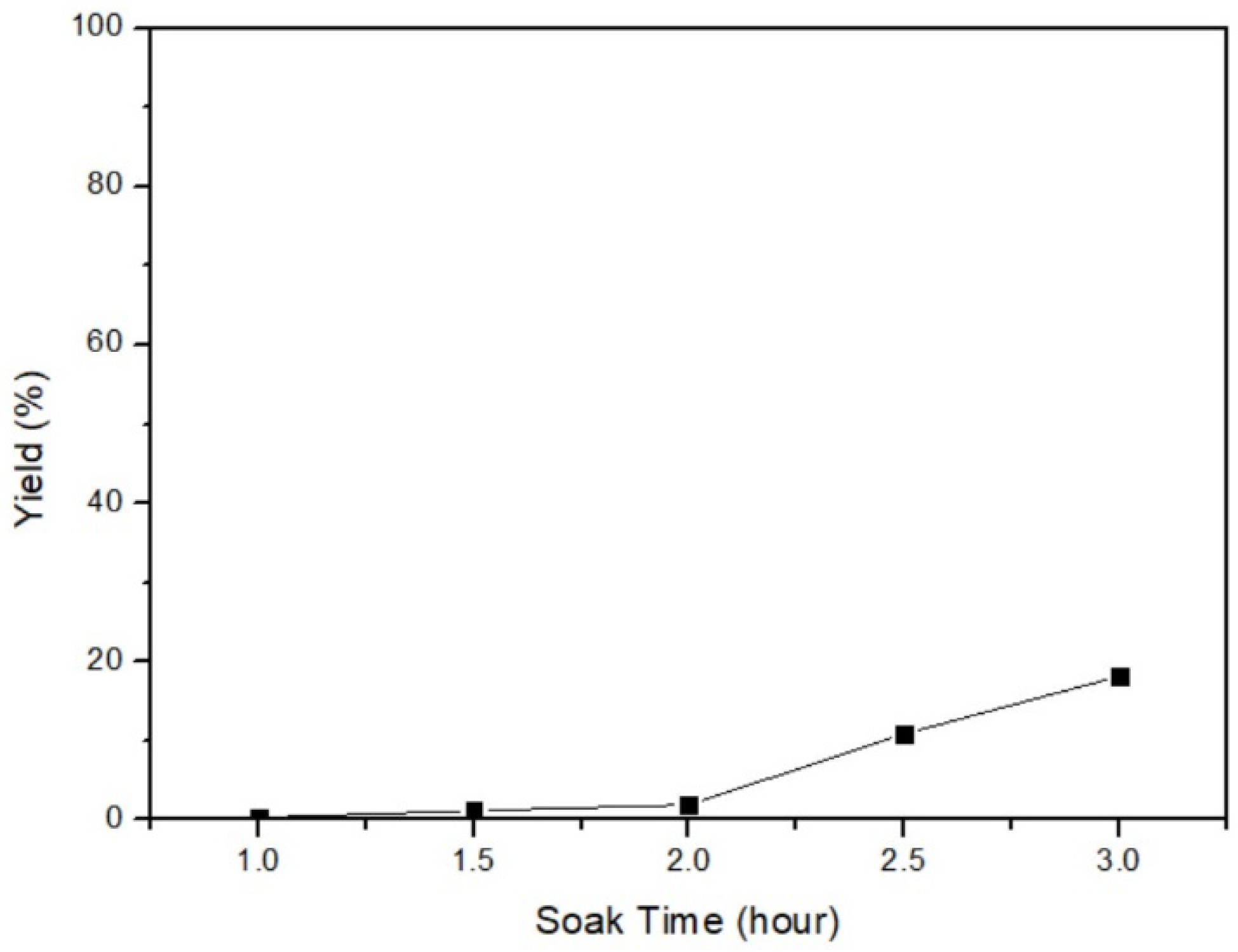
In this phase, the conversion of β-HgS to α-HgS was achieved by heating β-HgS in the absence of O2. The relationship between the yield and the soaking time was investigated to determine the optimal parameter. The fixed parameters were 10 g of β-HgS, 600 °C of the temperature, and 2 h of the heating-up time (5 °C/min). The soaking time parameters were set as 1 h, 1.5 h, 2 h, 2.5 h, and 3 h. Figure 10 illustrates an increasing yield with longer soaking times. This finding indicates that a longer soaking time allows greater energy to be added to the system, leading to a more complete process. As a consequence, the optimal parameter was 3 h of soaking time. After conducting under the optimal process, Figure 11 presents the XRD analysis of the obtained product, demonstrating a peak corresponding to α-HgS. This outcome confirms the feasibility of the experimental approach employed.
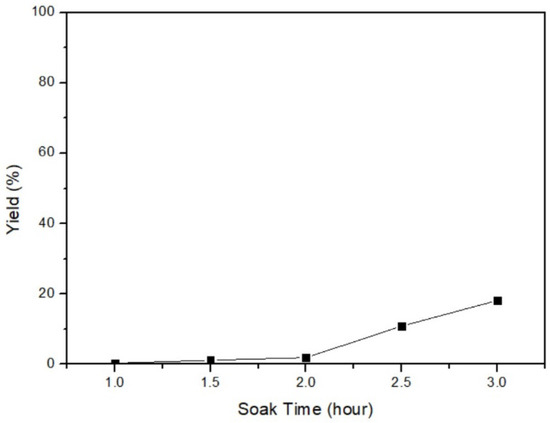
Figure 10.
Soaking time of β-HgS transfers to α-HgS.
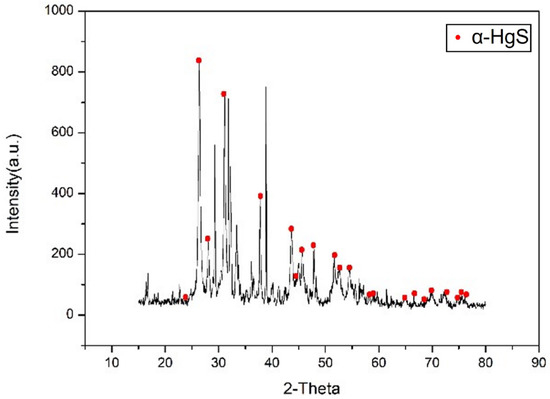
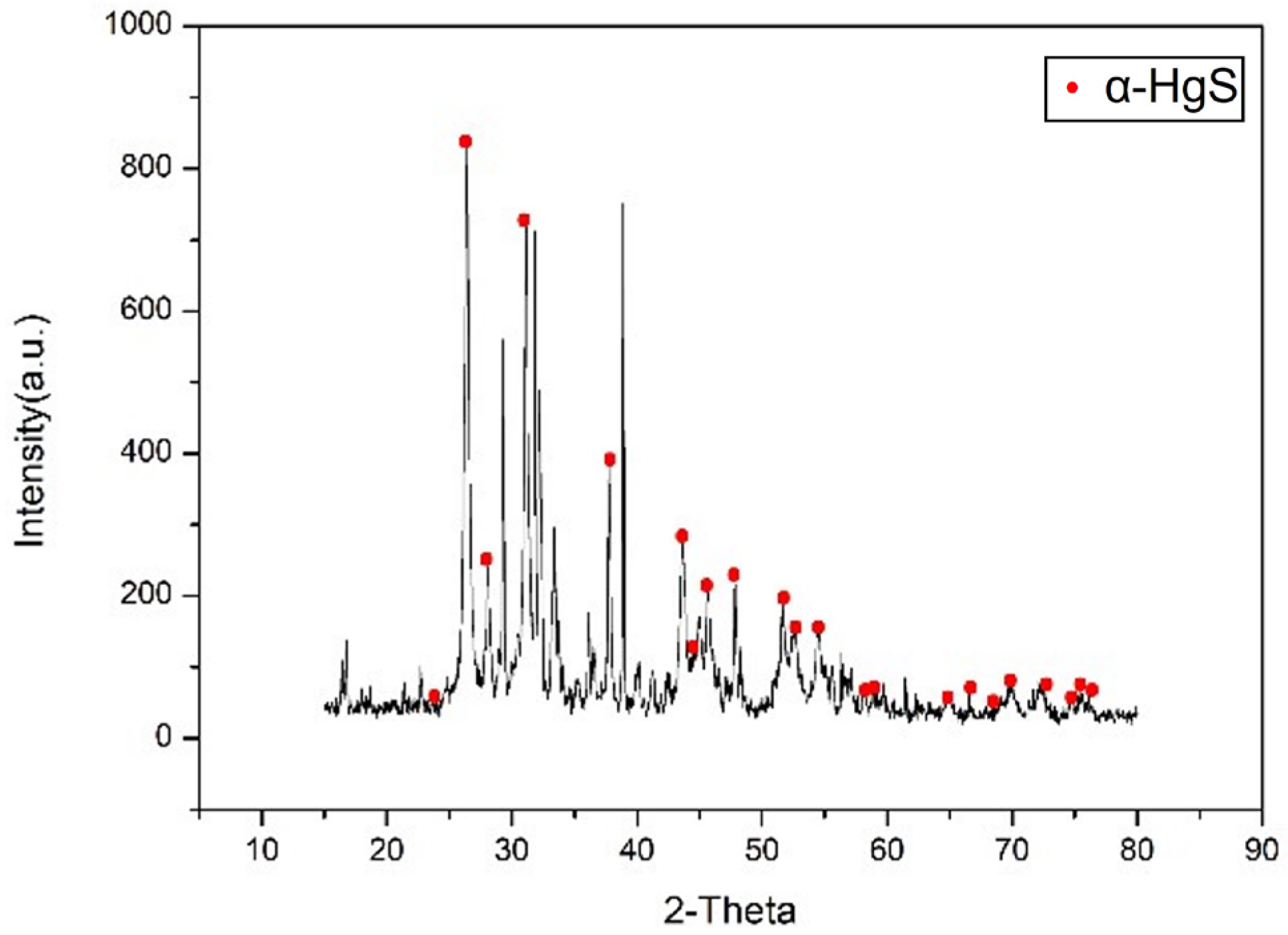
Figure 11.
XRD analysis of α-HgS.
3.3. Soaking Time of α-HgS Transfers to Mercury
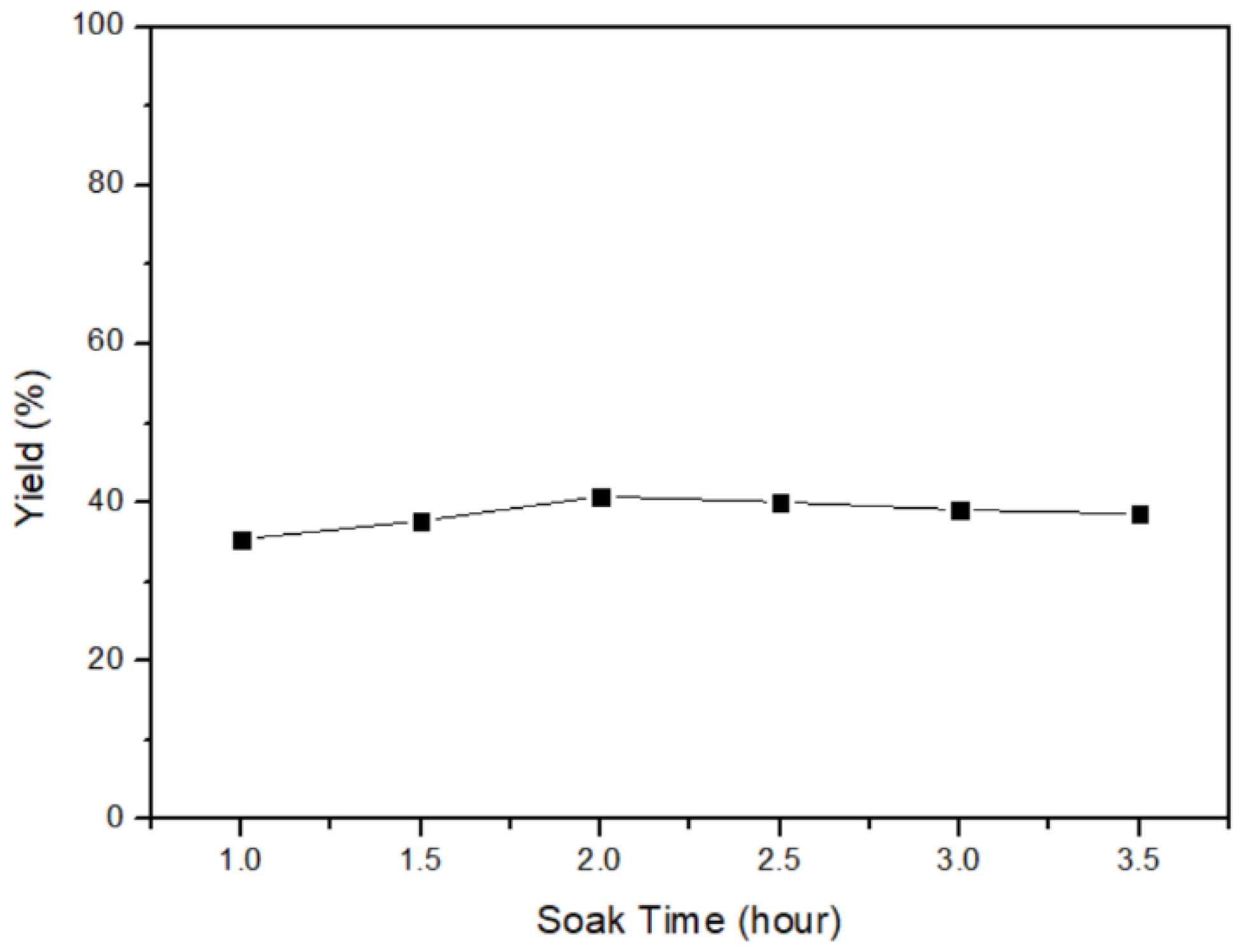
In this section, the yield of mercury, which transformed from α-HgS with different soaking times, is discussed. The heating process was conducted with parameters, including a temperature of 600 °C, a heating-up time of 2 h, and soaking times of 1 h, 1.5 h, 2 h, 2.5 h, 3 h, and 3.5 h. The results in Figure 12 indicate that the highest yield of 40.806% was achieved with a soaking time of 2 h. Subsequently, the product rate remained relatively stable beyond the 2-h mark. Therefore, the optimal parameter for obtaining the maximum yield of mercury was identified as 2 h of soaking time. Following the heating step, the mercury vapor would be produced, necessitating the condensation of the vapor in the final step. Hereafter, the high purity of metallic mercury (99.5% through ICP-OES and the mercury analyzer) could be acquired, and the mercury–mercury sulfide cycle was completed.
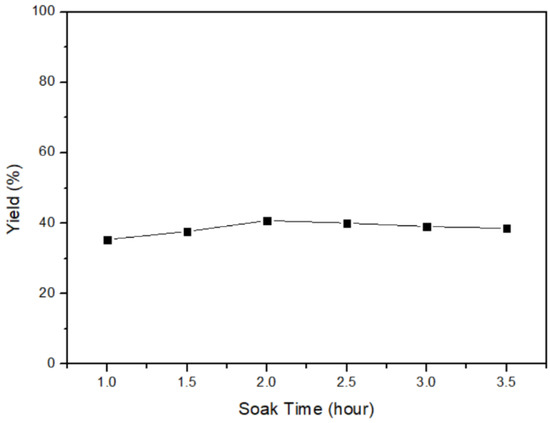
Figure 12.
Soaking time of α-HgS transfers to mercury.
After obtaining the optimal parameters of this research, this study is then compared with other common treatments of mercury. Table 1 reveals that the complexation with sulfur through a ball mill has the merits of being simple, safe, and requires low energy consumption. However, the recovery rate and the yield are not high enough. Therefore, the improvement would be conducted in future research.

Table 1.
Comparison of this study with other methods [21].
4. Conclusions
This study aims to address the issue of mercury toxicity and its environmental impact by stabilizing waste mercury through its conversion into α-HgS. Proper management of waste mercury is crucial to prevent pollution and associated health hazards such as Minamata disease. Conversely, transforming α-HgS, the most stable form of mercury sulfide back into reusable mercury offers a sustainable solution for industrial applications. Regarding the experiment steps, the main method of converting waste mercury (98%) to β-HgS was by the ball-mill method, which was operated by shear stresses, centripetal force, and gravitation to grind, mix, and caused a chemical reaction. It was chosen as a safer alternative to melting and chemical methods. By conducting experiments with varying parameters such as milling temperature, milling time, ball/material ratio, and milling speed, the optimal conditions were determined to be 35 °C for milling temperature, 12 h for milling time, 46% for the ball/material ratio, and 300 rpm for milling speed. Next, the conversion of β-HgS to α-HgS was accomplished through a heating process conducted in the absence of O2. The optimal parameter of soaking time was 3 h at 600 °C. Finally, to reclaim mercury for reuse, α-HgS was subjected to heating for under 2 h at 600 °C in the presence of O2, followed by condensation to obtain pure metallic mercury (99.5%). In a nutshell, this research completed the mercury–mercury sulfide cycle, which could not only store waste mercury appropriately to prevent poisoning by the toxicity of mercury but also reuses the mercury from waste (98% to 99.5%). Although the transformation yield from β-HgS to α-HgS was not ideal, the selected operational parameter could make waste mercury be recovered as resources in the form of α-HgS and high-purity of mercury to reach the goal and accord with the economic efficiency. Future research endeavors will focus on exploring alternative methods to enhance the translation process from β-HgS to α-HgS.
Author Contributions
Conceptualization, W.-S.C. and C.-C.C.; methodology, C.-C.C. and C.-H.L.; validation, W.-S.C., C.-C.C. and C.-H.L.; formal analysis, C.-C.C. and C.-H.L.; investigation, C.-C.C. and C.-H.L.; resources, W.-S.C.; data curation, C.-C.C. and C.-H.L.; writing—original draft preparation, C.-C.C.; writing—review and editing, W.-S.C., C.-C.C. and C.-H.L.; visualization, C.-C.C. and C.-H.L.; supervision, W.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Laboratory of Resource Circulation in the Dept. of Resources Engineering, National Cheng-Kung University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parsons, M.B.; Percival, J.B. A brief history of mercury and its environmental impact. In Mercury: Sources, Measurements, Cycles, and Effects; Mineralogical Association of Canada: Québec, QC, Canada, 2005; Volume 34, pp. 1–20. [Google Scholar]
- Zhao, M.; Li, Y.; Wang, Z. Mercury and mercury-containing preparations: History of use, clinical applications, pharmacology, toxicology, and pharmacokinetics in traditional Chinese medicine. Front. Pharmacol. 2022, 13, 510. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.Z.; Yu, L.M.; Goyer, R.A.; Waalkes, M.P. Mercury in traditional medicines: Is cinnabar toxicologically similar to common mercurials? Exp. Biol. Med. 2008, 233, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kosnett, M.J. The role of chelation in the treatment of arsenic and mercury poisoning. J. Med. Toxicol. 2013, 9, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Skaug, M.A.; Andersen, O.; Aaseth, J. Chelation therapy in intoxications with mercury, lead, and copper. J. Trace Elem. Med. Biol. 2015, 31, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C. Chronic neurological disease due to methylmercury poisoning. Can. J. Neurol. Sci. 2018, 45, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.G.; Snider, T.E.; Montiel, M.M. Toxicity of a family from vacuumed mercury. Am. J. Emerg. Med. 1992, 10, 258–261. [Google Scholar] [CrossRef]
- Langford, N.J.; Ferner, R.E. Toxicity of mercury. J. Hum. Hypertens. 1999, 13, 651–656. [Google Scholar] [CrossRef]
- Tsai, W.T. Multimedia Pollution Prevention of Mercury-Containing Waste and Articles: Case Study in Taiwan. Sustainability 2022, 14, 1557. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Shi, J.; Lu, Y. New insights and rethinking of cinnabar for chemical and its pharmacological dynamics. Bioengineered 2019, 10, 353–364. [Google Scholar] [CrossRef]
- Eto, K. Minamata disease. Neuropathology 2000, 20, 14–19. [Google Scholar] [CrossRef]
- Dufault, R.J.; Wolle, M.M.; Kingston, H.S.; Gilbert, S.G.; Murray, J.A. Connecting inorganic mercury and lead measurements in blood to dietary sources of exposure that may impact child development. World J. Methodol. 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Shah-Kulkarni, S.; Lee, S.; Jeong, K.S.; Hong, Y.-C.; Park, H.; Ha, M.; Kim, Y.; Ha, E.-H. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ. Res. 2020, 182, 109122. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Systematic review and meta-analysis links autism and toxic metals and highlight the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 340–368. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xun, P.; Liu, K.; Morris, S.; Reis, J.; Guallar, E. Mercury exposure in young adulthood and incidence of diabetes later in life: The CARDIA Trace Element Study. Diabetes Care 2013, 36, 1584–1589. [Google Scholar] [CrossRef]
- Tsai, T.L.; Kuo, C.C.; Pan, W.H.; Wu, T.N.; Lin, P.; Wang, S.L. Type 2 diabetes occurrence and mercury exposure–From the National Nutrition and Health Survey in Taiwan. Environ. Int. 2019, 126, 260–267. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Quality Assurance Guidance Document-Model Quality Assurance Project Plan for the PM Ambient Air; United States Environmental Protection Agency: Washington, DC, USA, 2001; Volume 2, p. 12.
- E.U. Directive. Restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS). Off. J. Eur. Commun. 2013, 46, 19–23. [Google Scholar]
- Jones, H. EU Bans Mercury in Barometers, and Thermometers. Reuters, 10 July 2007. [Google Scholar]
- Kessler, R. The Minamata Convention on Mercury: A first step toward protecting future generations. Environ. Health Perspect. 2013, 121, 10. [Google Scholar] [CrossRef]
- Chalkidis, A.; Jampaiah, D.; Aryana, A.; Wood, C.; Hartley, P.G.; Sabri, Y.M.; Bhargava, S.K. Mercury-bearing wastes: Sources, policies and treatment technologies for mercury recovery and safe disposal. J. Environ. Manag. 2020, 270, 110945. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.J.; Szuszkiewicz, W.; Orlowski, B.A.; He, Z.Q.; Ilver, L.; Kanski, J.; Nilsson, P.O. Photoemission study of β-HgS. J. Electron Spectrosc. Relat. Phenom. 1997, 85, 17–22. [Google Scholar] [CrossRef]
- Fukuda, N.; Takaoka, M.; Oshita, K.; Mizuno, T. Stabilizing conditions of metal mercury in mercury sulfurization using a planetary ball mill. J. Hazard. Mater. 2014, 276, 433–441. [Google Scholar] [CrossRef]
- Cardona, M.; Kremer, R.K.; Lauck, R.; Siegle, G.; Muñoz, A.; Romero, A.H. Electronic, vibrational, and thermodynamic properties of metacinnabar β-HgS, HgSe, and HgTe. Phys. Rev. B 2009, 80, 195204. [Google Scholar] [CrossRef]
- Lynch, A.J.; Rowland, C.A. The History of Grinding; SME: Littleton, CO, USA, 2005. [Google Scholar]
- Gaudin, A.M. Principles of Mineral Dressing (No. 622.7 GAU); McGraw Hill: New York, NY, USA, 1939. [Google Scholar]
- Menczel, J.D.; Prime, R.B. (Eds.) Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chen, W.S.; Chen, Y.C.; Lee, C.H. Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation. Processes 2022, 10, 748. [Google Scholar] [CrossRef]
- Rayner-Canham, G.W.; Rayner-Canham, G.; Overton, T. Descriptive Inorganic Chemistry; Macmillan: New York, NY, USA, 2003. [Google Scholar]
- Ballirano, P.; Botticelli, M.; Maras, A. Thermal behavior of cinnabar, α-HgS, and the kinetics of the β-HgS (metacinnabar)→ α-HgS conversion at room temperature. Eur. J. Mineral. 2013, 25, 957–965. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Laugier, A.; Garai, J. Derivation of the ideal gas law. J. Chem. Educ. 2007, 84, 1832. [Google Scholar] [CrossRef]
- Hagemann, S. Technologies for the Stabilization of Elemental Mercury and Mercury-Containing Wastes; Gesellschaft für Anlagen-und Reaktorsicherheit (GRS): Köln, Germany, 2009. [Google Scholar]
- Randall, P.; Chattopadhyay, S. Advances in encapsulation technologies for the management of mercury-contaminated hazardous wastes. J. Hazard. Mater. 2004, 114, 211–223. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).