Experimental Modeling of CO2 Sorption/Desorption Cycle with MDEA/PZ Blend: Kinetics and Regeneration Temperature
Abstract
:1. Introduction
General Kinetic Mechanism
2. Materials and Methods
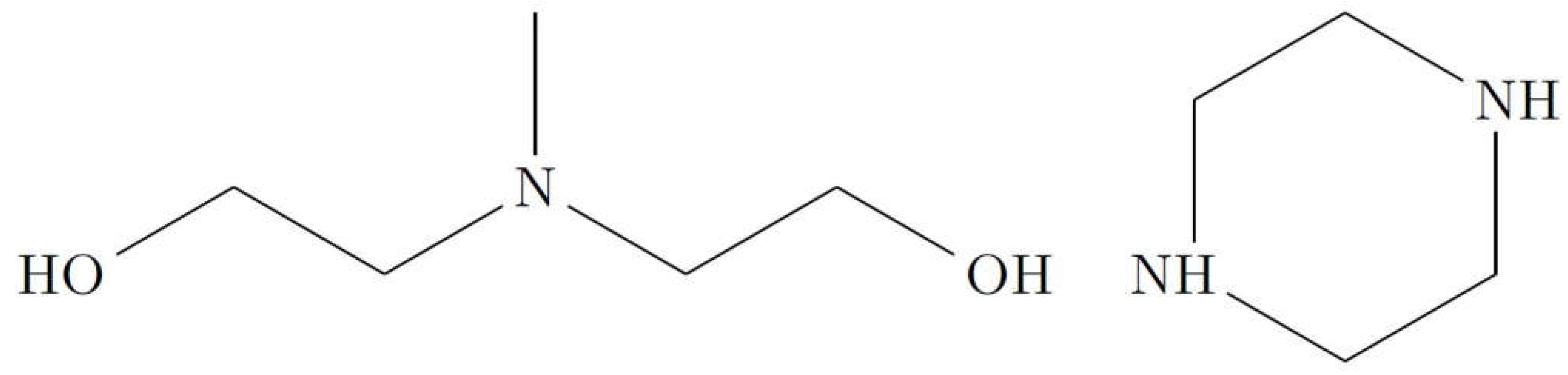
2.1. Chemical Reagents
2.2. Laboratory Equipment
2.3. Experimental Procedure
2.3.1. Blank Experiments
2.3.2. Sorption Mode Protocol
2.3.3. Desorption Mode Protocol
2.3.4. Data treatment
3. Results
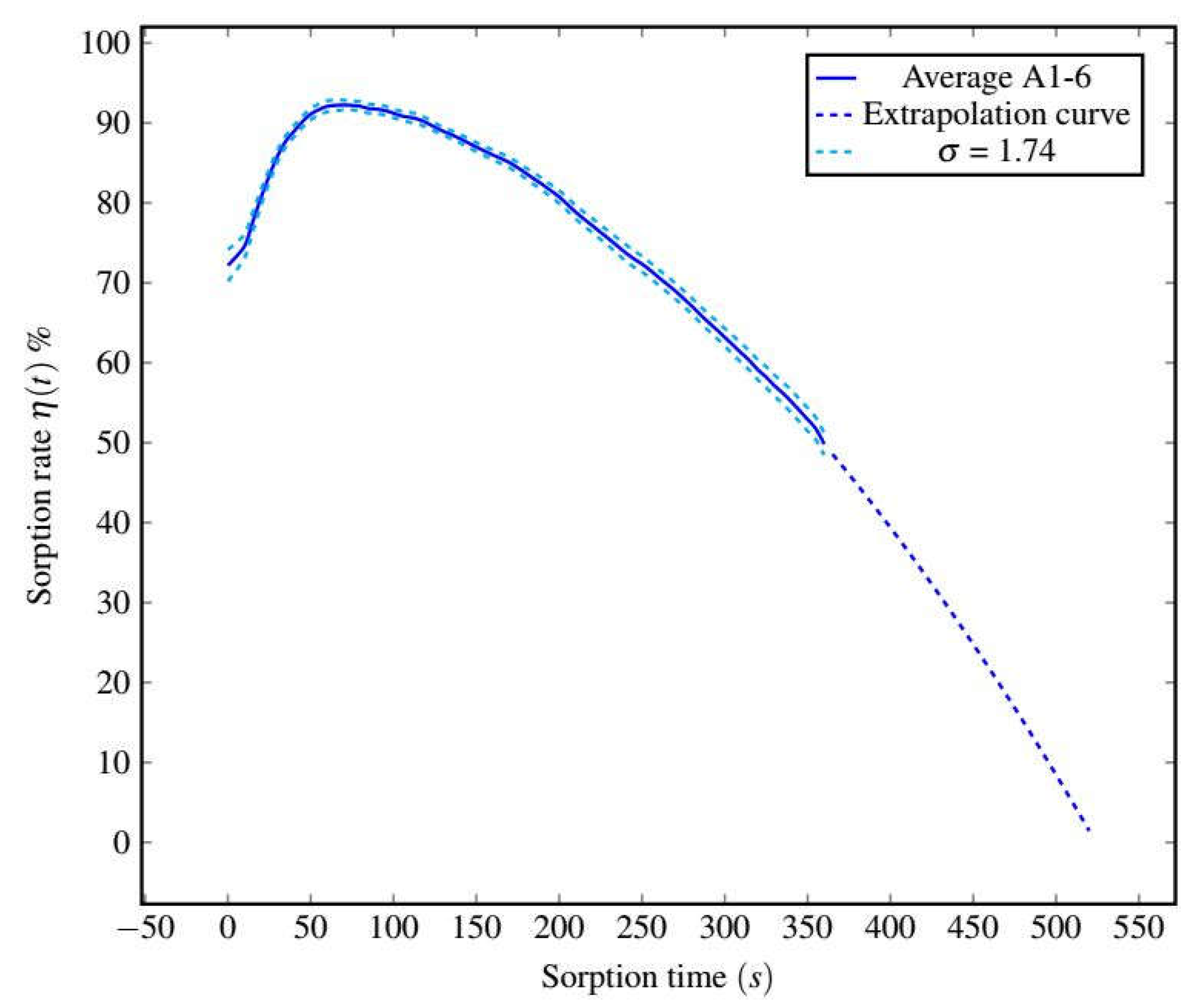
3.1. Sorption–Desorption Cycles’ General Characterization
3.2. Kinetics
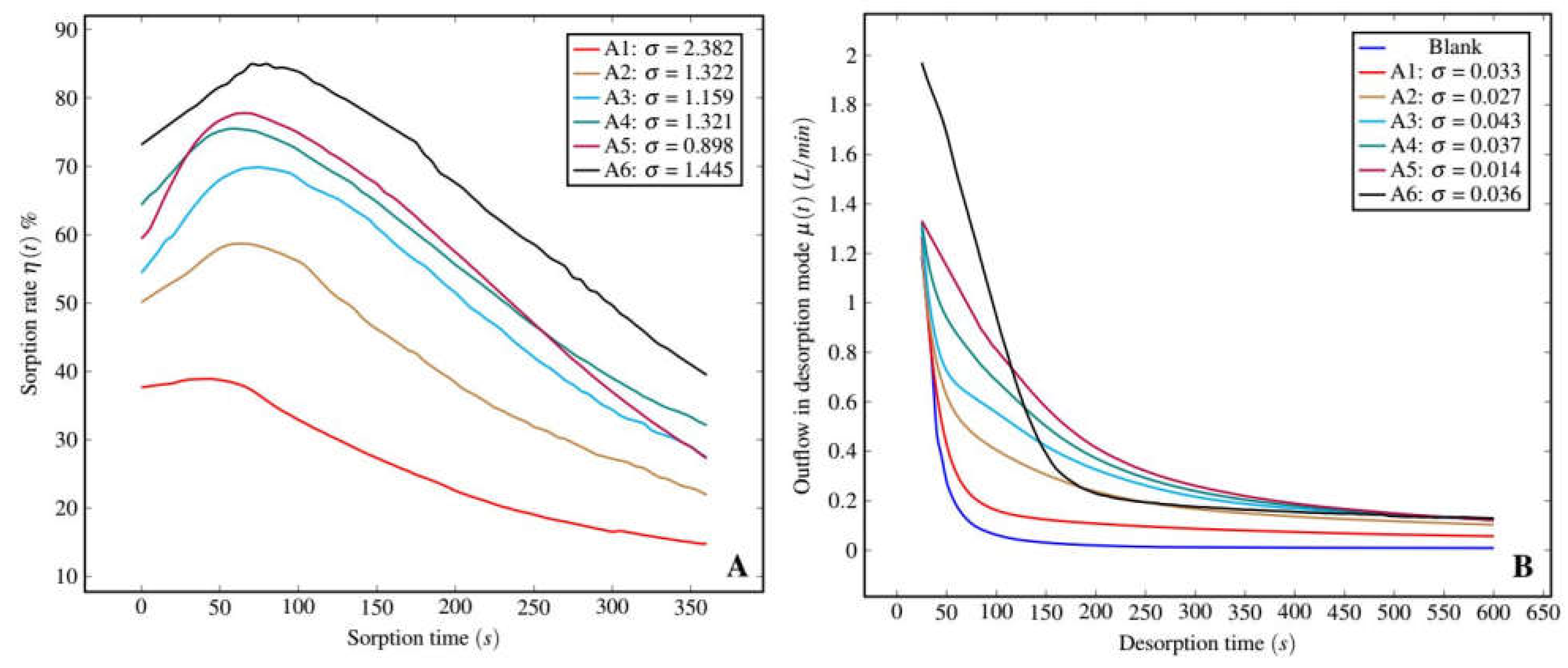
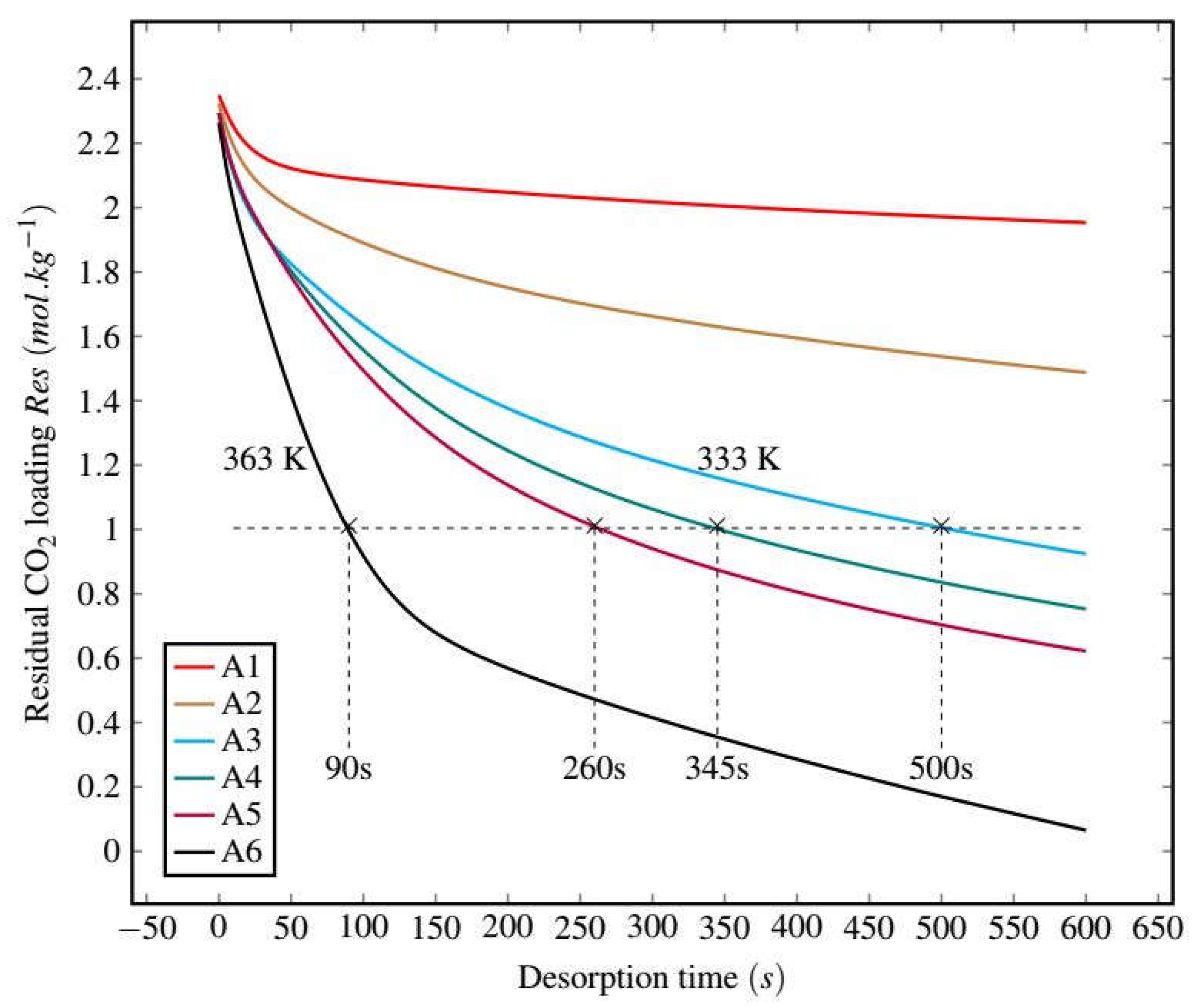
3.2.1. Experimental Results
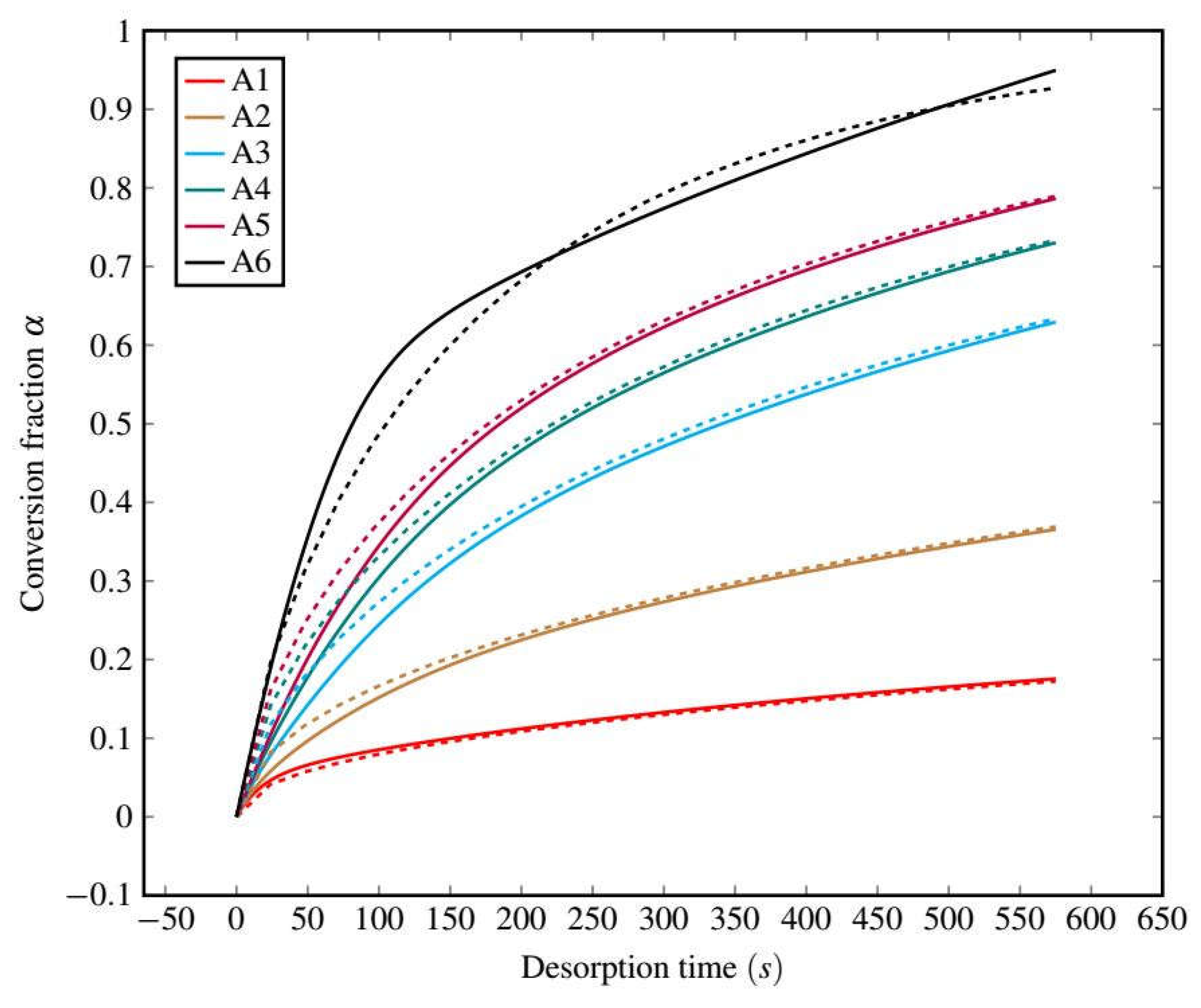
3.2.2. Desorption Kinetics Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Reversible carbon dioxide capture by aqueous and non- aqueous amine-based absorbents: A comparative analysis carried out by 13C NMR spectroscopy. Appl. Energy 2018, 220, 208–219. [Google Scholar] [CrossRef]
- Khalid, M.; Dharaskar, S.A.; Sillanpaa, M.; Siddiqui, H. (Eds.) Emerging Carbon Capture Technologies: Towards a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Closmann, F.; Nguyen, T.; Rochelle, G.T. MDEA/Piperazine as a solvent for CO2 capture. Energy Procedia 2009, 1, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Dugas, R.; Rochelle, G. Absorption and desorption rates of carbon dioxide with mo- noethanolamine and piperazine. Energy Procedia 2009, 1, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Svensson, H.; Hulteberg, C.; Karlsson, H.T. Heat of absorption of CO2 in aqueous solutions of N-methyldiethanolamine and piperazine. Int. J. Greenh. Gas Control 2013, 17, 89–98. [Google Scholar] [CrossRef]
- Artanto, Y.; Jansen, J.; Pearson, P.; Do, T.; Cottrell, A.; Meuleman, E.; Feron, P. Performance of MEA and amine-blends in the CSIRO PCC pilot plant at Loy Yang Power in Australia. Fuel 2012, 101, 264–275. [Google Scholar] [CrossRef]
- Yan, S.; Fang, M.; Luo, Z.; Cen, K. Regeneration of CO2 from CO2-rich alkanolamines solution by using reduced thickness and vacuum technology: Regeneration feasibility and characteristic of thin-layer solvent. Chem. Eng. Process. Process Intensif. 2009, 48, 515–523. [Google Scholar] [CrossRef]
- Fredriksen, S.; Jens, K.-J. Oxidative degradation of aqueous amine solutions of MEA, AMP, MDEA, Pz: A review. Energy Proc. 2013, 37, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.; Sun, Y.; Cakstins, J.; Sun, C.; Snape, C.E. Parametric study on the regeneration heat requirement of an amine-based solid adsorbent process for post-combustion carbon capture. Appl. Energy 2016, 168, 394–405. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Barifcani, A. Comprehensive evaluation and sensitivity analysis of regeneration energy for acid gas removal plant using single and activated-methyl diethanolamine solvents. Chin. J. Chem. Eng. 2020, 28, 1684–1693. [Google Scholar] [CrossRef]
- Li, F.; Hemmati, A.; Rashidi, H. Industrial CO2 absorption into methyldiethanolamine/piperazine in place of monoethanolamine in the absorption column. Process Saf. Environ. Prot. 2020, 142, 83–91. [Google Scholar] [CrossRef]
- Hemmati, A.; Farahzad, R.; Surendar, A.; Aminahmadi, B. Validation of mass transfer and liquid holdup correlations for CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator. Process Saf. Environ. Prot. 2019, 126, 214–222. [Google Scholar] [CrossRef]
- Esmaeili, A.; Tamuzi, A.; Borhani, T.N.; Xiang, Y.; Shao, L. Modeling of carbon dioxide absorption by solution of piperazine and methyldiethanolamine in a rotating packed bed. Chem. Eng. Sci. 2022, 248, 117118. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Continuous cycles of CO2 absorption and amine regeneration with aqueous alkanolamines: A comparison of the efficiency between pure and blended DEA, MDEA and AMP solutions by 13C NMR spectroscopy. Energy Env. Ment. Sci. 2010, 3, 772–779. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, Z.; Xu, G.; Zhang, K. Desorption kinetics and mechanisms of CO2 on amine-based mesoporous silica materials. Energies 2017, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, C.-F.; Qin, S.-J.; Zheng, Z.-S. A kinetics study on the absorption of carbon dioxide into a mixed aqueous solution of methyldiethanolamine and piperazine. Ind. Eng. Chem. Res. 2001, 40, 3785–3791. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Puxty, G.; Rowland, R. Modeling CO2 mass transfer in amine mixtures: PZ-AMP and PZ-MDEA. Environ. Sci. Technol. 2011, 45, 2398–2405. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, S.; Row, K.H.; Ahn, W.-S. Amine–silica composites for CO2 capture: A short review. J. Energy Chem. 2017, 26, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Fan, M.; Argyle, M. Desorption kinetics of the monoethanolamine/macroporous TiO2-based CO2 separation process. Energy Fuels 2011, 25, 2988–2996. [Google Scholar] [CrossRef]
- Saleh, T.A.; Elsharif, A.M.; Bin-Dahman, O.A. Synthesis of amine functionalization carbon nanotube-low symmetry porphyrin derivatives conjugates toward dye and metal ions removal. J. Mol. Liq. 2021, 340, 117024. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, Y.; Agar, D.W. Intensification of low temperature thermomorphic bipha- sic amine solvent regeneration for CO2 capture. Chem. Eng. Res. Des. 2012, 90, 743–749. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Shen, X.; Qiao, X.; Jia, L.; Xiang, J.; Jin, Y. Study on CO2 capture characteristics and kinetics of modified potassium-based adsorbents. Materials 2020, 13, 877. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Shi, J.; Zheng, S.; Tao, M.; He, Y.; Shi, Y. Kinetics studies of CO2 adsorption/desorption on amine-functionalized multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2014, 53, 11677–11683. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem. Eng. J. 2010, 161, 173–181. [Google Scholar] [CrossRef]
- Shunji, K.; Xizhou, S.; Wenze, Y. Investigation of CO2 desorption kinetics in MDEA and MDEA+ DEA rich amine solutions with thermo-gravimetric analysis method. Int. J. Greenh. Gas Control 2020, 95, 102947. [Google Scholar] [CrossRef]




| Sorption | Desorption | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiments Number | Initial | Mixing Velocity | Cycles | Time | Temp. | CO2 Flow | Time | Temp. | Pressure | |
| Mass (g) | (rpm) | (Count) | (s) | (K) | (L/min) | (s) | (K) | mbar | ||
| A | 1 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 308 | 150 ± 20 |
| 2 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 323 | 150 ± 20 | |
| 3 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 333 | 150 ± 20 | |
| 4 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 343 | 150 ± 20 | |
| 5 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 353 | 150 ± 20 | |
| 6 | 100 | 120 | 10 | 360 | 308 | 1 | 600 | 363 | 150 ± 20 | |
| B | 1 | 100 | 120 | 2 | 360 | 308 | 1 | 600 | 323 | atm |
| 2 | 100 | 120 | 2 | 360 | 308 | 1 | 600 | 333 | atm | |
| 3 | 100 | 120 | 2 | 360 | 308 | 1 | 600 | 343 | atm | |
| 4 | 100 | 120 | 2 | 360 | 308 | 1 | 600 | 353 | atm | |
| 5 | 100 | 120 | 2 | 360 | 308 | 1 | 600 | 363 | atm | |
| Experiment | A1 | A2 | A3 | A4 | A5 | A6 | |
|---|---|---|---|---|---|---|---|
| CO2 sor | (L·cycle−1) | 0.98 | 1.86 | 2.31 | 2.77 | 3.08 | 3.28 |
| CO2 des | (L·cycle−1) | 0.81 | 1.74 | 2.25 | 2.75 | 3.07 | 3.27 |
| CO2 sor/CO2 des | 1.21 | 1.07 | 1.03 | 1.01 | 1.00 | 1.00 | |
| (t ≤ 150) | (L·min−1) | 0.12 | 0.26 | 0.33 | 0.39 | 0.54 | 0.82 |
| diff | (wt.%) | 0 | 0 | 0 | 0.35 | 0.8 | 47.4 |
| Experiments | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|
| m | 0.469 | 0.529 | 0.655 | 0.679 | 0.686 | 0.781 |
| k (min−1) | 5.01·10−5 | 4.02·10−4 | 1.75·10−3 | 2.62·10−3 | 3.32·10−3 | 5.97·10−3 |
| R2 | 0.9997 | 0.9998 | 0.9997 | 0.9998 | 0.9998 | 0.9728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehrung, Q.; Destefanis, E.; Caviglia, C.; Bernasconi, D.; Pastero, L.; Bruno, M.; Bernasconi, A.; Magnetti Vernai, A.; Di Rienzo, A.; Pavese, A. Experimental Modeling of CO2 Sorption/Desorption Cycle with MDEA/PZ Blend: Kinetics and Regeneration Temperature. Sustainability 2023, 15, 10334. https://doi.org/10.3390/su151310334
Wehrung Q, Destefanis E, Caviglia C, Bernasconi D, Pastero L, Bruno M, Bernasconi A, Magnetti Vernai A, Di Rienzo A, Pavese A. Experimental Modeling of CO2 Sorption/Desorption Cycle with MDEA/PZ Blend: Kinetics and Regeneration Temperature. Sustainability. 2023; 15(13):10334. https://doi.org/10.3390/su151310334
Chicago/Turabian StyleWehrung, Quentin, Enrico Destefanis, Caterina Caviglia, Davide Bernasconi, Linda Pastero, Marco Bruno, Andrea Bernasconi, Alex Magnetti Vernai, Alice Di Rienzo, and Alessandro Pavese. 2023. "Experimental Modeling of CO2 Sorption/Desorption Cycle with MDEA/PZ Blend: Kinetics and Regeneration Temperature" Sustainability 15, no. 13: 10334. https://doi.org/10.3390/su151310334





