Brief Overview of Refuse-Derived Fuel Production and Energetic Valorization: Applied Technology and Main Challenges
Abstract
:1. Introduction
2. RDF Production in Portugal
3. RDF Quality Requirements
4. RDF Pretreatment Technologies
4.1. Physical, Chemical, and Thermochemical Pretreatment of RDF
4.1.1. Sorting
4.1.2. Particle Size Reduction and Pelletizing
4.1.3. Thermochemical Pretreatments
Torrefaction and Pyrolysis
Hydrothermal Carbonization (HTC)
Leaching for Chlorine Removal
5. Energy Recovery from RDF
5.1. Incineration
5.2. Gasification
5.3. Waste Incineration vs. Waste Gasification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyedotun, T.D.T.; Moonsammy, S.; Oyedotun, T.D.; Nedd, G.A.; Lawrence, R.N. Evaluation of waste dynamics at the local level: The search for a new paradigm in national waste management. Environ. Chall. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Directive 2018/851/UE, Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018. Off. J. Eur. Union 2018, 61, 109–140. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX%3A32018L0851 (accessed on 8 October 2021).
- Valorsul. Quanto Custa Depositar? 2021. Available online: https://www.valorsul.pt/pt/cliente/quanto-custa-depositar/ (accessed on 8 October 2021).
- Rezaei, H.; Panah, F.Y.; Lim, C.J.; Sokhansanj, S. Pelletization of Refuse-Derived Fuel with Varying Compositions of Plastic, Paper, Organic and Wood. Sustainability 2020, 12, 4645. [Google Scholar] [CrossRef]
- Rezaei, H.; Yazdanpanah, F.; Lim, C.J.; Sokhansanj, S. Pelletization properties of refuse-derived fuel—Effects of particle size and moisture content. Fuel Process. Technol. 2020, 205, 106437. [Google Scholar] [CrossRef]
- Alves, L.; Silva, S.; Soares, I. Waste management in insular areas: A Pay-As-You-Throw system in Funchal. Energy Rep. 2020, 6, 31–36. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Doña-Grimaldi, V.; Palma, A.; Montoya, M.R.; Morales, E.; Díaz, M. Energetic valorization of MSW compost valorization by selecting the maturity conditions. J. Environ. Manag. 2019, 238, 153–158. [Google Scholar] [CrossRef]
- Worldbank. Waste Composition. 16–21. Available online: worldbank.org (accessed on 8 October 2022).
- Kulkarni, B.N.; Anantharama, V. Repercussions of COVID-19 pandemic on municipal solid waste management: Challenges and opportunities. Sci. Total. Environ. 2020, 743, 140693. [Google Scholar] [CrossRef]
- APA. Relatório Anual Resíduos Urbanos 2020; Agência Portuguesa do Ambiente: Amadora, Portugal, 2021; Available online: https://apambiente.pt/sites/default/files/_Residuos/Producao_Gest%C3%A3o_Residuos/Dados%20RU/RARU%202018.pdf (accessed on 23 May 2022).
- Eurostat. Municipal Waste by Waste Management Operations. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASMUN/default/table?lang=en (accessed on 8 September 2021).
- Eurostat. Management of Waste by Waste Management Operations and Type of Material. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASSD__custom_1181470/default/table?lang=en (accessed on 8 October 2021).
- Arena, U.; Di Gregorio, F.; De Troia, G.; Saponaro, A. A techno-economic evaluation of a small-scale fluidized bed gasifier for solid recovered fuel. Fuel Process. Technol. 2015, 131, 69–77. [Google Scholar] [CrossRef]
- IEA Bioenergy. Trends in the Use of Solid Recovered Fuels. 2020. Available online: https://www.ieabioenergy.com/wp-content/uploads/2020/05/Trends-in-use-of-solid-recovered-fuels-Main-Report-Task36.pdf (accessed on 22 July 2022).
- Nobre, C. Thermochemical Upgrading of Refuse Derived Fuel; Faculdade de Ciências e Tecnologias da Universidade Nova de Lisboa: Caparica, Portugal, 2019; Available online: https://run.unl.pt/bitstream/10362/77043/1/Nobre_2019.pdf (accessed on 22 July 2022).
- Vaz, A.S.; Silva, F.; Bacalhau, R.; Mesquita, A.; Teixeira, C.A.; Borges, C.; Mil-Homens, F.; Pássaro, M. PERSU2020+ Reflexão Estratégica e Ajustamentos às Medidas do PERSU 2020. 2020, pp. 1–165. Available online: https://participa.pt/contents/consultationdocument/PERSU2020+.pdf (accessed on 22 July 2022).
- Kaur, A.; Bharti, R.; Sharma, R. Municipal solid waste as a source of energy. Mater. Today Proc. 2021, 81, 904–915. [Google Scholar] [CrossRef]
- Khosasaeng, T.; Suntivarakorn, R. Effect of Equivalence Ratio on an Efficiency of Single Throat Downdraft Gasifier Using RDF from Municipal solid waste. Energy Procedia 2017, 138, 784–788. [Google Scholar] [CrossRef]
- Montejo, C.; Tonini, D.; Márquez, M.C.; Astrup, T.F. Mechanical–biological treatment: Performance and potentials. An LCA of 8 MBT plants including waste characterization. J. Environ. Manag. 2013, 128, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Sample preparation for thermo-gravimetric determination and thermo-gravimetric characterization of refuse derived fuel. Waste Manag. 2016, 48, 265–274. [Google Scholar] [CrossRef]
- APA. Relatório Anual Resíduos Urbanos 2019. 2020. Available online: https://apambiente.pt/sites/default/files/_Residuos/Producao_Gest%C3%A3o_Residuos/Dados%20RU/RARU%202019.pdf (accessed on 2 August 2022).
- Veolia. Combustível Derivado de Resíduos. 2022. Available online: https://www.veolia.pt/solucoes/combustivel-derivado-de-residuos#no-back (accessed on 22 July 2022).
- Caputo, A.C.; Pelagagge, P.M. RDF production plants: I Design and costs. Appl. Therm. Eng. 2002, 22, 423–437. [Google Scholar] [CrossRef]
- BS EN ISO 21640:2021; Standards Publication Solid Recovered Fuels—Specifications and Classes. CEN: Geneva, Switzerland, 2021.
- CEN/TC 343; Solid Recovered Fuels. CEN—European Committee for Standardization: Brussels, Belgium, 2021. Available online: https://standards.iteh.ai/catalog/tc/cen/ea946eb8-b158-4ab3-87b3-83415b1f48a9/cen-tc-343 (accessed on 25 August 2022).
- ISO. When Waste Becomes Worthwhile: New Standards for Solid Recovered Fuels Just Published. Junho de 2021. 2021. Available online: https://www.iso.org/news/ref2690.html (accessed on 26 August 2022).
- Flamme, S.; Geiping, J. Quality standards and requirements for solid recovered fuels: A review. Waste Manag. Res. J. A Sustain. Circ. Econ. 2012, 30, 335–353. [Google Scholar] [CrossRef]
- Frankenhaeuser, M. European Standardisation of Solid Recovered Fuels; VTT Symposium (Valtion Teknillinen Tutkimuskeskus): Dublin, Ireland, 2011; pp. 283–285. [Google Scholar]
- ERFO. International Workshop on Solid Recovered Fuel: SRF Market Views in Europe. 2010. Available online: https://www.yumpu.com/en/document/read/5066076/international-workshop-on-solid-recovered-fuel-helsinki-31-erfo (accessed on 29 July 2022).
- Yang, Y.; Liew, R.K.; Tamothran, A.M.; Foong, S.Y.; Yek, P.N.Y.; Chia, P.W.; Van Tran, T.; Peng, W.; Lam, S.S. Gasification of refuse-derived fuel from municipal solid waste for energy production: A review. Environ. Chem. Lett. 2021, 19, 2127–2140. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Longo, A.; Vilarinho, C.; Gonçalves, M. Torrefaction and carbonization of refuse derived fuel: Char characterization and evaluation of gaseous and liquid emissions. Bioresour. Technol. 2019, 285, 121325. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Dziok, T.; Magdziarz, A.; Nowak, W. Composition and properties of fly ash collected from a multifuel fluidized bed boiler co-firing refuse derived fuel (RDF) and hard coal. Energy 2021, 234, 121229. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product—Carbonized Refuse Derived Fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef]
- Manyà, J.J.; García-Ceballos, F.; Azuara, M.; Latorre, N.; Royo, C. Pyrolysis and char reactivity of a poor-quality refuse-derived fuel (RDF) from municipal solid waste. Fuel Process. Technol. 2015, 140, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Alves, O.; Nobre, C.; Durão, L.; Monteiro, E.; Brito, P.; Gonçalves, M. Effects of dry and hydrothermal carbonisation on the properties of solid recovered fuels from construction and municipal solid wastes. Energy Convers. Manag. 2021, 237, 114101. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.B.; Tarelho, L.A.; Cardoso, J.S.; Eusébio, D. Snapshot review of refuse-derived fuels. Util. Policy 2022, 74, 101316. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of refuse derived fuel through torrefaction and carbonization: Evaluation of RDF char fuel properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Azam, M.; Jahromy, S.S.; Raza, W.; Raza, N.; Lee, S.S.; Kim, K.-H.; Winter, F. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ. Int. 2020, 134, 105291. [Google Scholar] [CrossRef]
- Jewiarz, M.; Mudryk, K.; Wróbel, M.; Frączek, J.; Dziedzic, K. Parameters Affecting RDF-Based Pellet Quality. Energies 2020, 13, 910. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Sharma, B.; Goswami, Y.; Sharma, S.; Shekhar, S. Inherent roadmap of conversion of plastic waste into energy and its life cycle assessment: A frontrunner compendium. Renew. Sustain. Energy Rev. 2021, 146, 111070. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kurniawan, W.; Aziz, M. Technological review on thermochemical conversion of COVID-19-related medical wastes. Resour. Conserv. Recycl. 2021, 167, 105429. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Ayoub, N. Comparative study of MSW heat treatment processes and electricity generation. J. Energy Inst. 2018, 91, 481–488. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Kobayashi, N.; Zhao, D.; Xing, S. Study of Fuel Properties of Torrefied Municipal Solid Waste. Energy Fuels 2015, 29, 4976–4980. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.-W.; Zhang, X. Pyrolysis Analysis of RDF by TG-FTIR Techniques. Environ. Sci. Technol. 2008, 31, 29–32. [Google Scholar]
- Veses, A.; Sanahuja-Parejo, O.; Callén, M.S.; Murillo, R.; García, T. A combined two-stage process of pyrolysis and catalytic cracking of municipal solid waste for the production of syngas and solid refuse-derived fuels. Waste Manag. 2020, 101, 171–179. [Google Scholar] [CrossRef]
- Whyte, H.E.; Loubar, K.; Awad, S.; Tazerout, M. Pyrolytic oil production by catalytic pyrolysis of refuse-derived fuels: Investigation of low cost catalysts. Fuel Process. Technol. 2015, 140, 32–38. [Google Scholar] [CrossRef]
- Gandidi, I.M.; Susila, M.D.; Mustofa, A.; Pambudi, N.A. Thermal—Catalytic cracking of real MSW into Bio-Crude Oil. J. Energy Inst. 2018, 91, 304–310. [Google Scholar] [CrossRef]
- Neuwahl, F.; Cusano, G.; Benavides, J.G.; Holbrook, S.; Serge, R. Best Available Techniques (BAT) Reference Document for Waste Treatment Industries; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Yan, W.; Perez, S.; Sheng, K. Upgrading fuel quality of moso bamboo via low temperature thermochemical treatments: Dry torrefaction and hydrothermal carbonization. Fuel 2017, 196, 473–480. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, C.; Qin, S.; Wang, B.; Zhao, P.; Cui, X. Effects of process water recirculation on yields and quality of hydrochar from hydrothermal carbonization process of rice husk. J. Anal. Appl. Pyrolysis 2022, 166, 105618. [Google Scholar] [CrossRef]
- Motavaf, B.; Dean, R.A.; Nicolas, J.; Savage, P.E. Hydrothermal carbonization of simulated food waste for recovery of fatty acids and nutrients. Bioresour. Technol. 2021, 341, 125872. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Bao, S.; Liu, J.; Zhu, L.; Tan, W. Evaluating the aqueous phase obtained from hydrothermal carbonization of municipal sludge as possible liquid fertilizer for plant growth: An analysis of heavy metals and their molecular composition. J. Clean. Prod. 2023, 404, 136989. [Google Scholar] [CrossRef]
- Wilk, M.; Czerwińska, K.; Śliz, M.; Imbierowicz, M. Hydrothermal carbonization of sewage sludge: Hydrochar properties and processing water treatment by distillation and wet oxidation. Energy Rep. 2023, 9, 39–58. [Google Scholar] [CrossRef]
- González-Arias, J.; de la Rubia, M.; Sánchez, M.; Gómez, X.; Cara-Jiménez, J.; Martínez, E. Treatment of hydrothermal carbonization process water by electrochemical oxidation: Assessment of process performance. Environ. Res. 2023, 216, 114773. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of hydrochar and process water from the hydrothermal carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Reddy, V.M.; Nagarajan, R. Ultrasonic coal washing to leach alkali elements from coals. Ultrason. Sonochem. 2015, 27, 235–240. [Google Scholar] [CrossRef]
- Lee, J.; Ghiasi, B.; Lau, A.; Sokhansanj, S. Chlorine and ash removal from salt-laden woody biomass by washing and pressing. Biomass- Bioenergy 2021, 155, 106272. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Zhao, W.; Yang, L. Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 2018, 217, 427–433. [Google Scholar] [CrossRef]
- Rajendran, N.; Gurunathan, B.; Han, J.; Krishna, S.; Ananth, A.; Venugopal, K.; Priyanka, R.S. Recent advances in valorization of organic municipal waste into energy using biorefinery approach, environment and economic analysis. Bioresour. Technol. 2021, 337, 125498. [Google Scholar] [CrossRef]
- Goli, V.S.N.S.; Singh, D.N.; Baser, T. A critical review on thermal treatment technologies of combustible fractions from mechanical biological treatment plants. J. Environ. Chem. Eng. 2021, 9, 105643. [Google Scholar] [CrossRef]
- Istrate, I.-R.; Medina-Martos, E.; Galvez-Martos, J.-L.; Dufour, J. Assessment of the energy recovery potential of municipal solid waste under future scenarios. Appl. Energy 2021, 293, 116915. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. The efficient use of energy: Tracing the global flow of energy from fuel to service. Energy Policy 2010, 38, 75–81. [Google Scholar] [CrossRef]
- Kirkels, A.F.; Verbong, G.P. Biomass gasification: Still promising? A 30-year global overview. Renew. Sustain. Energy Rev. 2011, 15, 471–481. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Du, M.A.; Huang, I.-T.; Liu, I.-H.; Chang, E.-E.; Chiang, P.-C. Strategies on implementation of waste-to-energy (WTE) supply chain for circular economy system: A review. J. Clean. Prod. 2015, 108, 409–421. [Google Scholar] [CrossRef]
- Bosmans, A.; Helsen, L. Energy From Waste: Review of Thermochemical Technologies for Refuse Derived Fuel (RDF) Treatment. In Proceedings of the Third International Symposium on Energy from Biomass and Waste, Venice, Italy, 8–11 November 2010; pp. 8–11. [Google Scholar]
- Pio, D.; Tarelho, L.; Tavares, A.; Matos, M.; Silva, V. Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fluidized bed reactor. Energy Convers. Manag. 2020, 206, 112476. [Google Scholar] [CrossRef]
- Giraud, R.J.; Taylor, P.H.; Huang, C.-P. Combustion operating conditions for municipal Waste-to-Energy facilities in the U.S. Waste Manag. 2021, 132, 124–132. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M.; Zhou, Z. Comparison of waste-to-energy technologies of gasification and incineration using life cycle assessment: Case studies in Finland, France and China. J. Clean. Prod. 2018, 203, 287–300. [Google Scholar] [CrossRef]
- Brożek, P.; Złoczowska, E.; Staude, M.; Baszak, K.; Sosnowski, M.; Bryll, K. Study of the Combustion Process for Two Refuse-Derived Fuel (RDF) Streams Using Statistical Methods and Heat Recovery Simulation. Energies 2022, 15, 9560. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M. Life cycle assessment of pyrolysis, gasification and incineration waste-to-energy technologies: Theoretical analysis and case study of commercial plants. Sci. Total. Environ. 2018, 626, 744–753. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2018, 235, 346–355. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Hameed, Z.; Aslam, M.; Khan, Z.; Maqsood, K.; Atabani, A.; Ghauri, M.; Khurram, M.S.; Rehan, M.; Nizami, A.-S. Gasification of municipal solid waste blends with biomass for energy production and resources recovery: Current status, hybrid technologies and innovative prospects. Renew. Sustain. Energy Rev. 2020, 136, 110375. [Google Scholar] [CrossRef]
- Safar, K.M.; Bux, M.R.; Faria, U.; Pervez, S. Integrated model of municipal solid waste management for energy recovery in Pakistan. Energy 2021, 219, 119632. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Na, J.I.; Park, S.J.; Kim, Y.K.; Lee, J.G.; Kim, J.H. Characteristics of oxygen-blown gasification for combustible waste in a fixed-bed gasifier. Appl. Energy 2003, 75, 275–285. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.; Khan, M.; Oo, A.M. Performance assessment of gasification reactors for sustainable management of municipal solid waste. J. Environ. Manag. 2021, 291, 112661. [Google Scholar] [CrossRef] [PubMed]
- Rajca, P. Innovative Technologies in the Development of Alternative RDF Fuel. Eng. Prot. Environ. 2018, 21, 251–260. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, J.; Zhao, P.; Wang, Z.; Yang, M.; Cui, X.; Tian, H.; Guo, Q. Gasification of real MSW-derived hydrochar under various atmosphere and temperature. Thermochim. Acta 2020, 683, 178470. [Google Scholar] [CrossRef]
- Haydary, J.; Šuhaj, P.; Šoral, M. Semi-Batch Gasification of Refuse-Derived Fuel (RDF). Processes 2021, 9, 343. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, L.; Jin, H. Catalytic supercritical water gasification mechanism of coal. Int. J. Hydrogen Energy 2020, 45, 9504–9511. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Kuang, D.; Gasem, K.A.; Adidharma, H.; Ding, D.; Fan, M. Kinetics and mechanism of CO2 gasification of coal catalyzed by Na2CO3, FeCO3 and Na2CO3–FeCO3. J. Energy Inst. 2020, 93, 922–933. [Google Scholar] [CrossRef]
- Lazzarotto, I.; Ferreira, S.; Junges, J.; Bassanesi, G.; Manera, C.; Perondi, D.; Godinho, M. The role of CaO in the steam gasification of plastic wastes recovered from the municipal solid waste in a fluidized bed reactor. Process. Saf. Environ. Prot. 2020, 140, 60–67. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Perdikaris, N.; Boukis, I. Gasification improvement of a poor quality solid recovered fuel (SRF). Effect of using natural minerals and biomass wastes blends. Fuel 2014, 117, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Li, A.; Zhang, L.; Ji, G.; Gao, Y.; Khushk, S. Hydrogen-rich syngas from wet municipal solid waste gasification using Ni/Waste marble powder catalyst promoted by transition metals. Waste Manag. 2021, 132, 96–104. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D. Techno-economic analysis of a biomass gasification power plant dealing with forestry residues blends for electricity production in Portugal. J. Clean. Prod. 2019, 212, 741–753. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Thermodynamic Evaluation of Portuguese municipal solid waste gasification. J. Clean. Prod. 2016, 139, 622–635. [Google Scholar] [CrossRef]
- Foster, W.; Azimov, U.; Gauthier-Maradei, P.; Molano, L.C.; Combrinck, M.; Munoz, J.; Esteves, J.J.; Patino, L. Waste-to-energy conversion technologies in the UK: Processes and barriers—A review. Renew. Sustain. Energy Rev. 2020, 135, 110226. [Google Scholar] [CrossRef]
- Arafat, H.A.; Jijakli, K. Modeling and comparative assessment of municipal solid waste gasification for energy production. Waste Manag. 2013, 33, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Liu, Y.; Xia, B.; Jiang, X.; Skitmore, M. Overview of public-private partnerships in the waste-to-energy incineration industry in China: Status, opportunities, and challenges. Energy Strat. Rev. 2020, 32, 100584. [Google Scholar] [CrossRef]
- Directive (UE) 2018/850, Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. 2018. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX%3A32018L0850. (accessed on 28 February 2022).
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef]
- CEWEP. Waste-to-Energy Plants in Europe in 2019; Confederation of European Waste-to-Energy Plants: Düsseldorf, Germany, 2019; Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2019/ (accessed on 22 July 2022).
- ETIP Bioenergy. Production Facilities. European Technology and Innovation Platform Bioenergy. 2022. Available online: https://www.etipbioenergy.eu/databases/production-facilities (accessed on 22 July 2022).
- GOV.UK. Task 2 Report: Review of Current Status of Advanced Gasification Technologies. 2021. Available online: https://www.gov.uk/government/publications/advanced-gasification-technologies-review-and-benchmarking (accessed on 22 July 2022).
- Aracil, C.; Haro, P.; Fuentes-Cano, D.; Gómez-Barea, A. Implementation of waste-to-energy options in landfill-dominated countries: Economic evaluation and GHG impact. Waste Manag. 2018, 76, 443–456. [Google Scholar] [CrossRef]
- WtERT. Silla 2 Waste-to-Energy Plant, Milano, Italy. WtERT Germany GmbH. 2022. Available online: https://www.wtert.net/bestpractice/8/Silla-2-Waste-to-Energy-Plant-Milano-Italy.html (accessed on 22 July 2022).
- SIERRA ENERGY. FastOx® Gasification is the Future. Available online: https://sierraenergy.com/ (accessed on 14 July 2022).
- Kauno Kogeneracinė Jėgainė. About the Power Plant Kauno CHP. 2018. Available online: https://kkj.lt/apie-mus/apie-jegaine/16 (accessed on 22 July 2022).
- Power Technology. Amager Bakke Waste-to-Energy Plant. 2022. Available online: https://www.power-technology.com/projects/amager-bakke-waste-energy-plant/ (accessed on 2 May 2022).
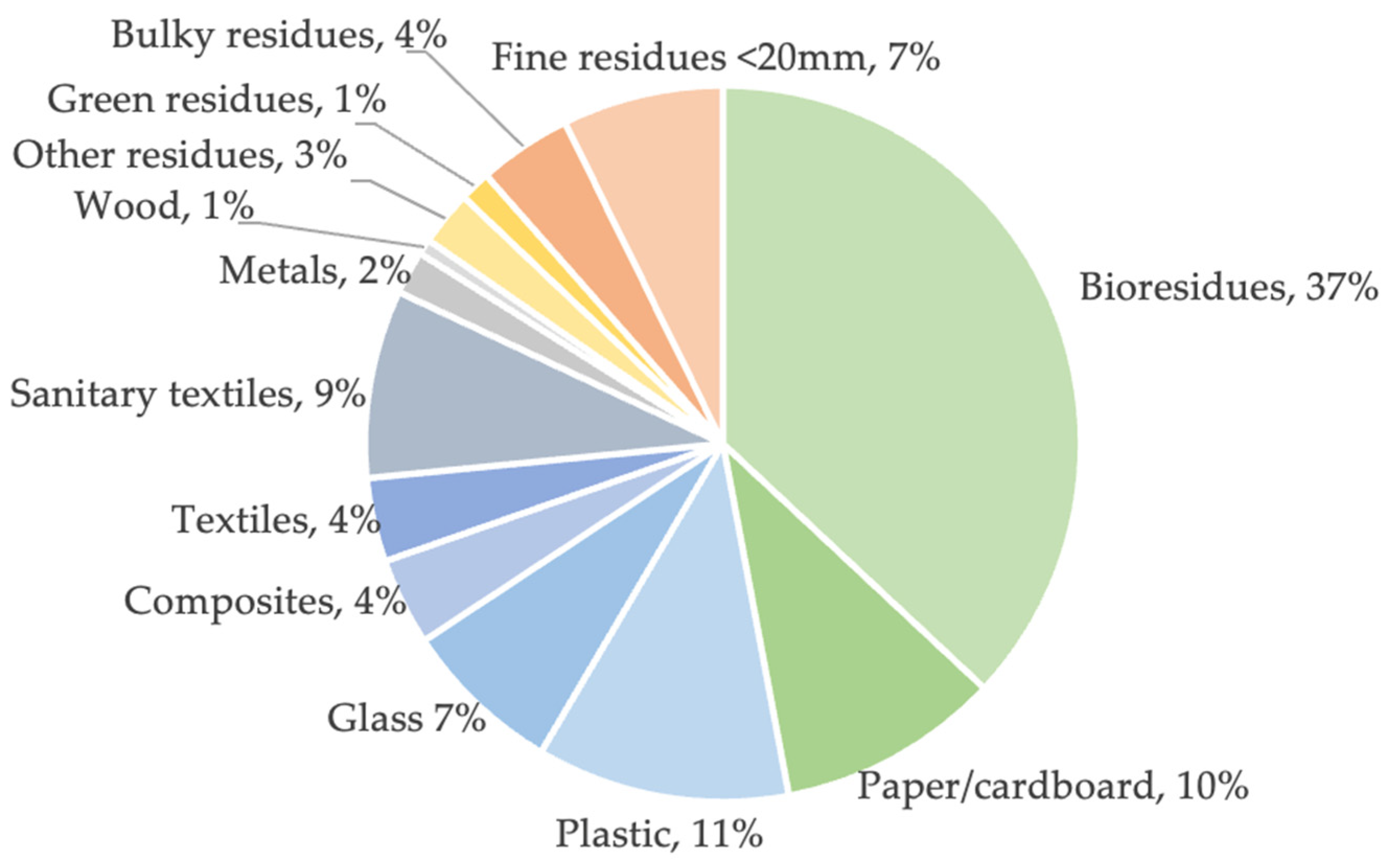
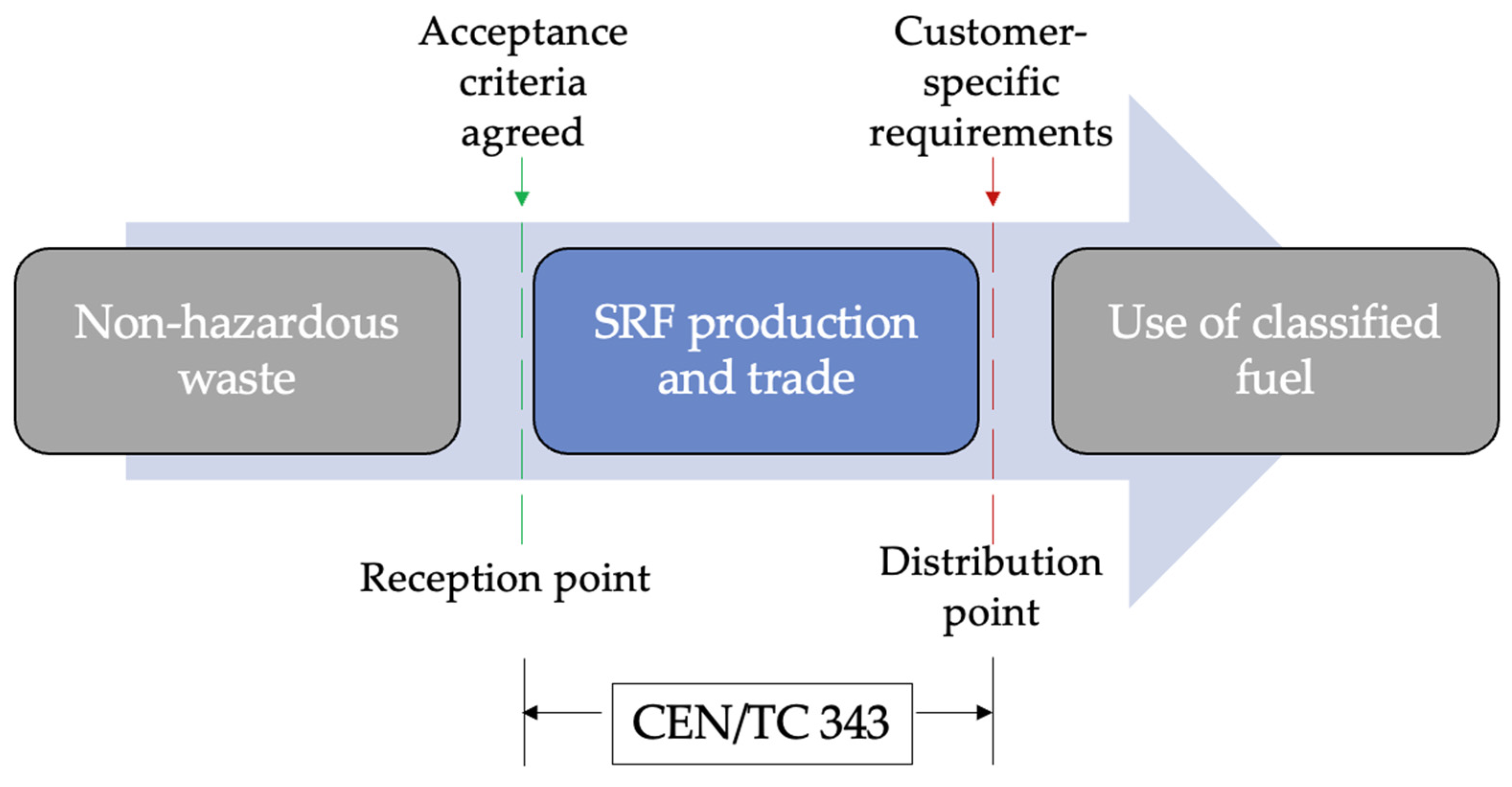
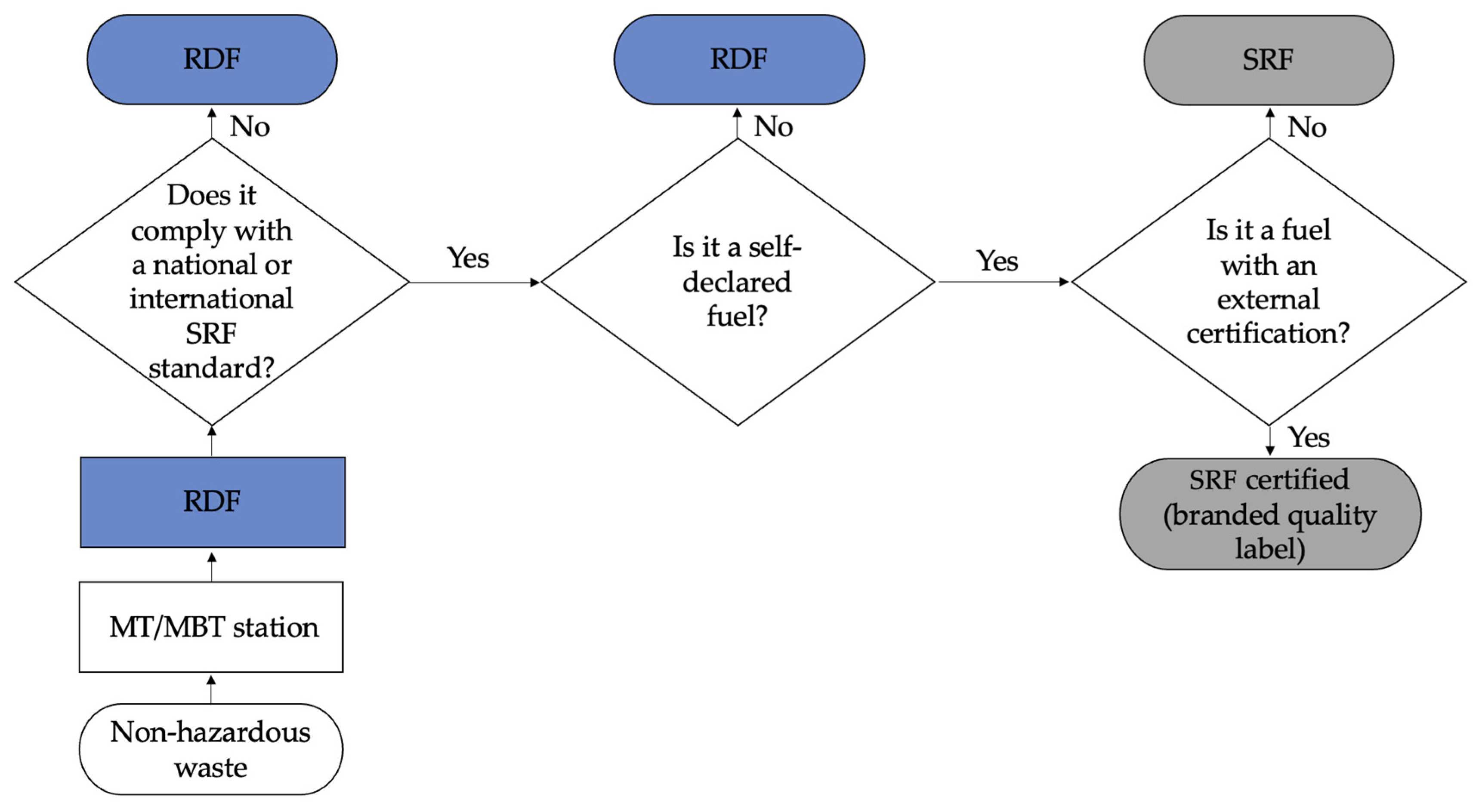

| Region | Unit | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| EU | 103 t | 218,028 | 220,957 | 221,610 | 223,956 | 225,732 |
| Portugal | 4640 | 4745 | 4945 | 5007 | 5014 | |
| EU | kg/hab.day−1 | 1.34 | 1.36 | 1.6 | 1.37 | 1.38 |
| Portugal | 1.29 | 1.33 | 1.38 | 1.40 | 1.40 |
| Region | Landfill | Energy Recovery | Organic Recovery | Multi-Material Recovery and Other Recoveries |
|---|---|---|---|---|
| EU (%) | 37.7 | 5.7 | n.m. * | 56.6 |
| Portugal (%) | 64.2 | 17.4 | 7.2 | 11.1 |
| RDF Production Installations | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Sorting station | 1308 | n.d. * | n.d. | 15 | 7 |
| MT | 33,750 | 21,042 | n.d. | n.d. | n.d. |
| MBT | 72,564 | 467 | 379 | 385 | 677 |
| RDF production unit | 6943 | n.d. | n.d. | n.d. | n.d. |
| Total | 114,566 | 21,509 | 379 | 400 | 683 |
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| WMO | 28,896 | 309 | 1449 | 622 | 706 |
| Total | 29,476 | 749 | 1466 | 622 | 706 |
| Standard | Description |
|---|---|
| CEN/TC 343/WG1 | Terminology and quality assurance |
| EN ISO 21637:2020 | Vocabulary |
| CEN/TC 343/WG2 | Specifications and fuel classes |
| EN ISO 21640:2021 | Specifications and classes |
| CEN/TC 343/WG3 | Sampling, sample reduction, and supplementary testing methods |
| EN ISO 15443:2011 | Methods for the preparation of the laboratory sample |
| EN ISO 15590:2011 | Determination of the current rate of aerobic microbial activity using the real dynamic respiration index |
| CEN/TR 15591:2007 | Determination of the biomass content based on the 14C method |
| EN ISO 21644:2021 | Methods for the determination of biomass content |
| EN ISO 21645:2021 | Methods for sampling |
| CEN/TC 343/WG4 | Physical/mechanical testing |
| CEN/TS 15401:2010 | Determination of bulk density |
| CEN/TS 15405:2010 | Determination of density of pellets and briquettes |
| CEN/TS 15406:2010 | Determination of bridging properties of bulk material |
| CEN/TS 15414-1:2010 | Determination of moisture content using the oven dry method—Part 1: determination of total moisture by a reference method |
| CEN/TS 15414-2:2010 | Determination of moisture content using the oven dry method—Part 2: determination of total moisture content by a simplified method |
| EN ISO 15415-1:2011 | Determination of particle size distribution—Part 1: Screen method for small dimension particles |
| EN ISO 15415-2:2012 | Determination of particle size distribution—Part 2: maximum projected length method (manual) for large-dimension particles |
| EN ISO 15415-3:2012 | Determination of particle size distribution—Part 3: Method by image analysis for large-dimension particles |
| CEN/TS 15639:2010 | Determination of mechanical durability of pellets |
| EN ISO 21654:2021 | Determination of calorific value |
| EN ISO 21656:2021 | Determination of ash content |
| EN ISO 21660-3:2021 | Determination of moisture content using the oven dry method—Part 3: moisture in general analysis sample |
| EN ISO 22167:2021 | Determination of content of volatile matter |
| CEN/TC 343/WG5 | Chemical testing |
| EN ISO 15408:2011 | Methods for the determination of sulfur (S), chlorine (Cl), fluorine (F), and bromine (Br) content |
| EN ISO 15410:2011 | Methods for the determination of the content of major elements (Al, Ca, Fe, K, Mg, Na, P, Si, and Ti) |
| EN ISO 15411:2011 | Methods for the determination of the content of trace elements (As, Ba, Be, Cd, Co, Cr, Cu, Hg, Mo, Mn, Ni, Pb, Sb, Se, Tl, V, and Zn) |
| CEN/TS 15412:2010 | Methods for the determination of metallic aluminum |
| EN ISO 15413:2011 | Methods for the preparation of the test sample from the laboratory sample |
| EN ISO 21663:2020 | Methods for the determination of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) by the instrumental method |
| Classification Properties | Statistical Measure | Unit | Classes | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Net calorific value | Average | MJ/kg, ar | ≥25 | ≥20 | ≥15 | ≥10 | ≥3 |
| Chlorine (Cl) | Average | %, db | ≤0.2 | ≤0.6 | ≤1.0 | ≤1.5 | ≤3 |
| Mercury (Hg) | Median | mg/MJ, ar | ≤0.02 | ≤0.03 | ≤0.08 | ≤0.15 | ≤0.5 |
| 80° percentile | ≤0.04 | ≤0.06 | ≤0.16 | ≤0.3 | ≤1.0 | ||
| Parameters | Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis |
|---|---|---|---|
| Operating temperature (°C) | up to 900 | 850–1250 | 1050–1300 |
| Heating rate (°C/s) | 0.1–1 | 10–200 | >1000 |
| Residence time (s) | 300–3600 | 0.5–10 | <0.5 |
| Air Flow (Nm3/h) | CO (%) | CH4 (%) | CO2 (%) | H2 (%) | Heating Value (MJ/Nm3) |
|---|---|---|---|---|---|
| 12 | 21.61 | 1.52 | 4.02 | 10.19 | 4.37 |
| 15 | 17.69 | 0.97 | 4.25 | 10.46 | 3.71 |
| 18 | 19.35 | 1.14 | 3.09 | 9.44 | 3.87 |
| S | W | O | T | |
|---|---|---|---|---|
| Strengths | Weaknesses | Opportunities | Threats | |
| Incineration | Ability to deal with a high degree of waste variety, easy setup, and fast treatment [92] Reduces waste volume by up to 80% [63] Produces more energy (the energy potential from MSW incineration can be higher) [93] | Requires a high production capacity and a waste heating value range of 10 to 12 MJ/kg [65] Low electricity efficiency up to about 22–25% [74] Need for flue gas cleaning devices (removal of acid gases, NOX, dioxins, and furans) [72] Production of large amounts of ash (MSW incineration produces about 25 to 30% bottom ash and 1 to 5% fly ash relative to the input material) [92] Small incinerators with a design capacity of less than 300 t/d are usually inefficient in terms of economy, technology, and environmental protection [94] | Restrictions on waste disposal in landfills [95] Increase in MSW production [12,13] The closure of coal-fired power plants opens the door for the use of waste as a raw material in these plants; Increased electrification energy demand [78] Technological innovations (e.g., grate furnaces) have eliminated a critical obstacle to the sustainable development of WtE incineration industry [94] | Opposition by the public owing to potential health risk, for example, dioxins [74,94] Legislation, such as Directive 2010/75/EU, Directive (EU) 2015/2193, and Directive 2012/27/EU, that regulates the emission limits with which they must comply and sets energy-efficiency requirements for cogeneration plants; Policy changes and government decision-making capacity [94] |
| S | W | O | T | |
|---|---|---|---|---|
| Strengths | Weaknesses | Opportunities | Threats | |
| Gasification | Well-established technology [96] Operates at lower temperatures [93] Overall thermal efficiency is more than 75% [92] Multiple applications of the product gas (directly combusted, electricity generation) [65] Lower NOX and dioxins and furans because of reducing atmosphere, reduced excess air and much easier emission control [72] Technology widely accepted by the public [78]. | Requires stricter feedstock pretreatment, feedstock must be finely granulated [74,92] The product gas requires improving the quality before it is further used; Tars and char in product gas; Cost and energy intensive [96] Are still in their infancy regarding commercial implementation as large-scale MSW management solutions [65] | Increase in MSW production and the need for more efficient and sustainable management [12,13] Alternative to mechanical recycling of plastics [96] Syngas as a feedstock for renewable chemicals and fuels [65] Possible hydrogen production from steam and hydrothermal gasification [96] Development of low-cost and efficient catalysts for the removal of tar from gas; Integration in hybrid technologies (e.g., gasification + fuel cell, anaerobic digestion + gasification) [77]. Public appreciation and approval of this technology can promote its application. | High competitiveness of fossil fuels, direct competitors; Low efficiency of MSW sorting and poor waste segregation impoverish the composition of MSW, SRF, or RDF and limit their use in gasification [4,25] Detour of waste to landfills or incineration [25] Technical challenge and high costs of gas cleaning and purification [96] |
| Gasification | Incineration/Combustion | References | |
|---|---|---|---|
| TRL | 6–8 (for advanced gasification technologies | 9 | [92,99] |
| Total costs (t waste/day) | USD 86–97 | USD 115 | [93] |
| Emissions associated | 285 kg CO2 eq./t MSW using grate gasifier with steam Rankine cycle | 331 kg CO2 eq./t MSW using grate combustor with steam Rankine cycle | [100] |
| Available facilities | Kymijärvi II-Lahti Energia (250 kt SRF/year, produces 50 MWe, 90 MWth, commercial-scale); | Silla 2 incineration plant, Milan, Italy (541 kt MSW/year, produces 378 GWhe, 403 GWhth; | [12,74,98,99,101,102,103,104] |
| Enerkem Alberta Biofuels LP-Edmonton Waste-to-Biofuels Project, (100 kt MSW/year, produces ethanol (30 kt/year) and methanol, TRL 8) | LIPOR, incinerator, Portugal (423 kt MSW/year, produces 199 GWh of energy, 89 kt/year of slag and 14 kt/year of ash); | ||
| Sierra Energy’s FastOX gasification- Fort Hunter Liggett project (capacity of 10 t MSW, produces electricity and fuels, demonstration plant at commercial scale); | VALORSUL incinerator, Portugal (577 kt MSW/year, produces 312 GWh of energy, 13 kt/year of slag, 35 kt/year of ash and 84 kt/year of aggregate); | ||
| Surrey Municipality-Surrey Biofuel facility (organic residues and waste streams, produces 240 t/year of SNG, TRL 8); | Kauno Cogeneration Power Plant, Lithuania (200 kt MSW/year, to produce 170 GWhe and 500 GWhth; | ||
| ThermoChem Recovery International (TRI)- Fully Integrated BioRefinery (4 t/day of organic residues and waste streams, produces 1 t/year of Fischer-Tropsch liquids, TRL 6–7. | Amager Bakke Waste-to-Energy Plant, Copenhagen, Denmark (443 kt MSW and biomass, producing 1259 GWh of total energy in 2018). | ||
| Sogama Waste-to-Energy Plant, Cerceda, Spain 500 kt/year RDF, 49 MWe capacity); |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.M.; Nobre, C.; Brito, P.; Gonçalves, M. Brief Overview of Refuse-Derived Fuel Production and Energetic Valorization: Applied Technology and Main Challenges. Sustainability 2023, 15, 10342. https://doi.org/10.3390/su151310342
Santos SM, Nobre C, Brito P, Gonçalves M. Brief Overview of Refuse-Derived Fuel Production and Energetic Valorization: Applied Technology and Main Challenges. Sustainability. 2023; 15(13):10342. https://doi.org/10.3390/su151310342
Chicago/Turabian StyleSantos, Santa Margarida, Catarina Nobre, Paulo Brito, and Margarida Gonçalves. 2023. "Brief Overview of Refuse-Derived Fuel Production and Energetic Valorization: Applied Technology and Main Challenges" Sustainability 15, no. 13: 10342. https://doi.org/10.3390/su151310342
APA StyleSantos, S. M., Nobre, C., Brito, P., & Gonçalves, M. (2023). Brief Overview of Refuse-Derived Fuel Production and Energetic Valorization: Applied Technology and Main Challenges. Sustainability, 15(13), 10342. https://doi.org/10.3390/su151310342









