Chemical Recycling of Used Motor Oil by Catalytic Cracking with Metal-Doped Aluminum Silicate Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Cracking Process
2.3. Catalysts Synthesis
2.4. Characterization Methods
3. Results
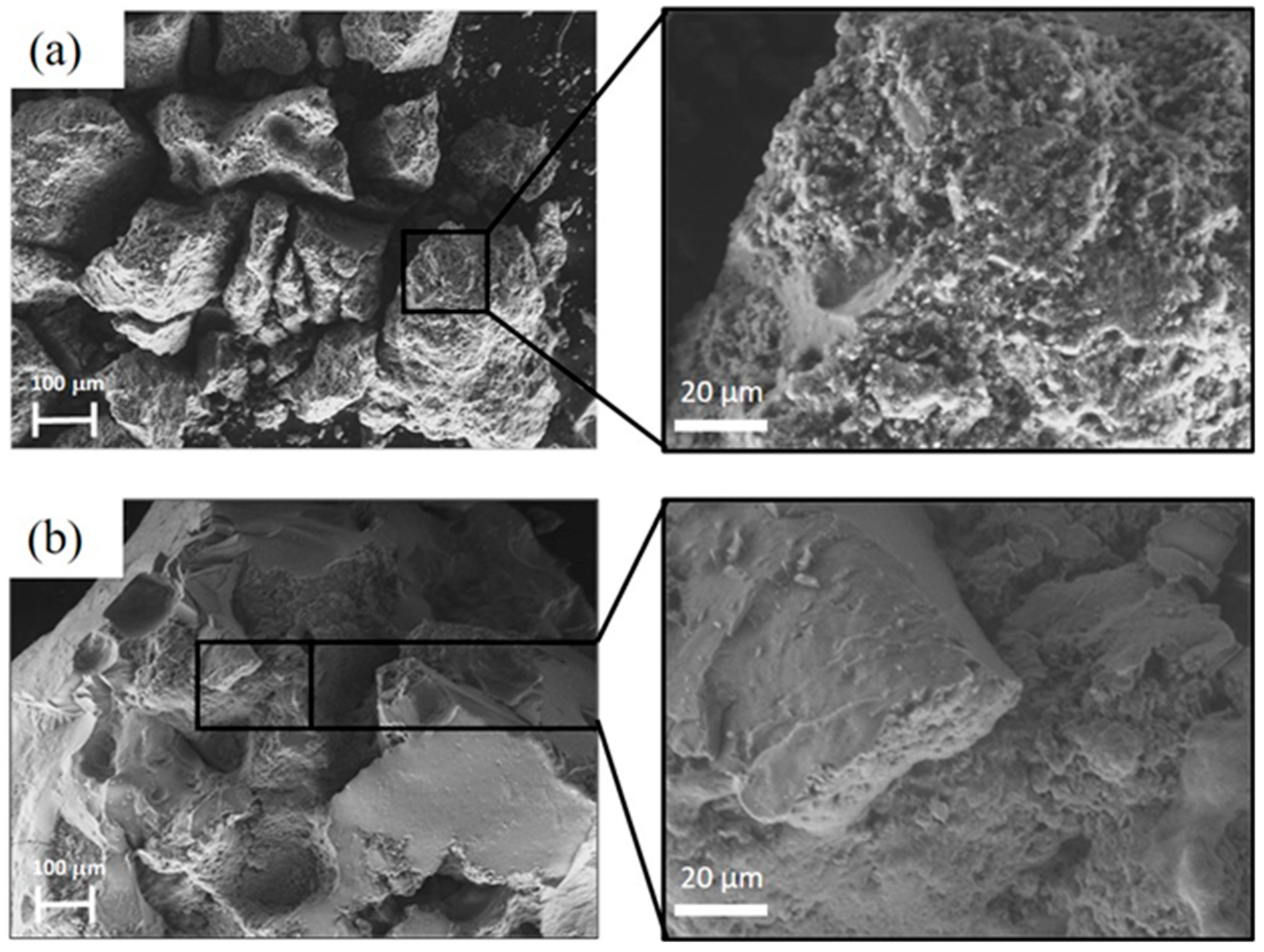
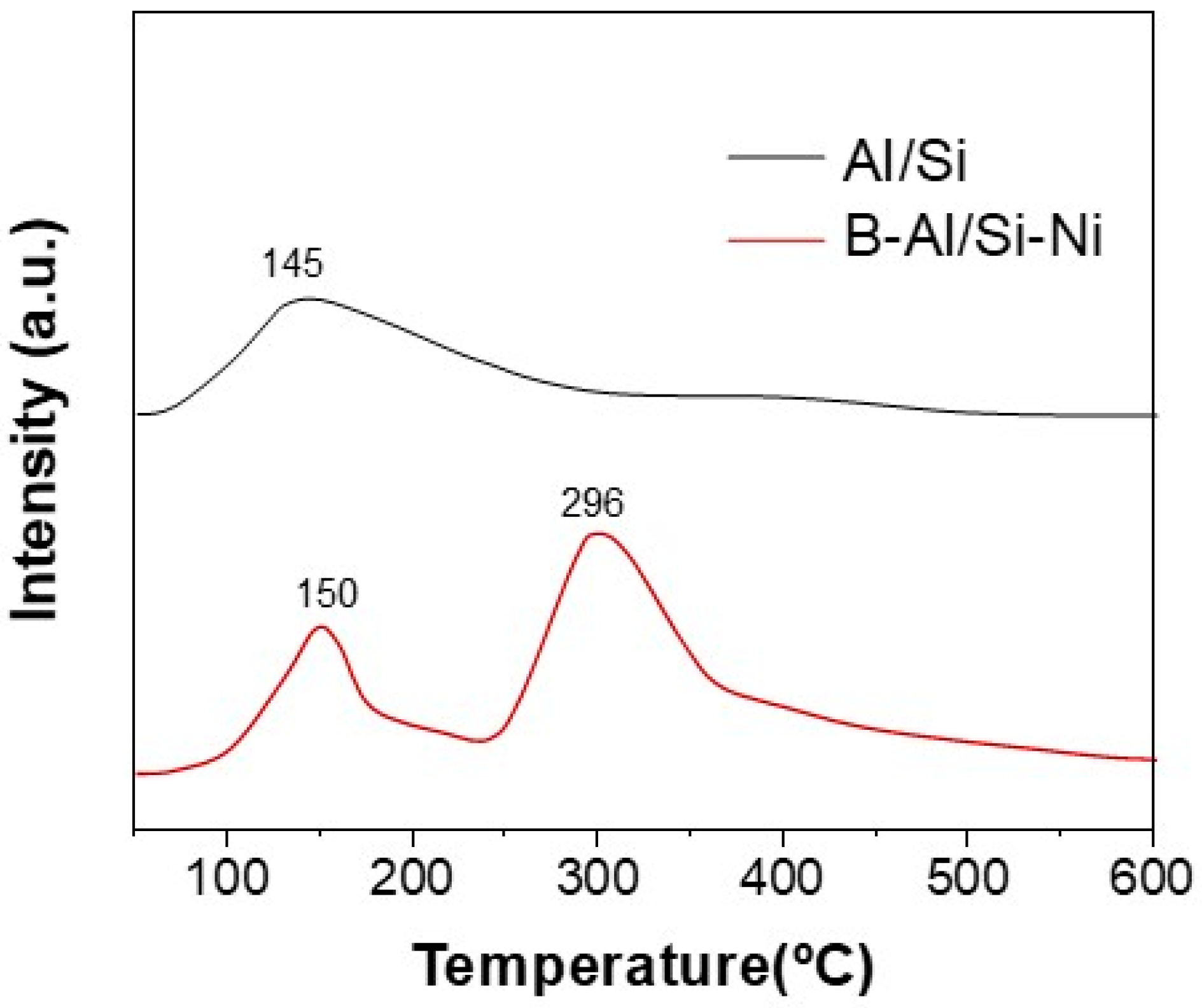
3.1. Catalysts Characterization
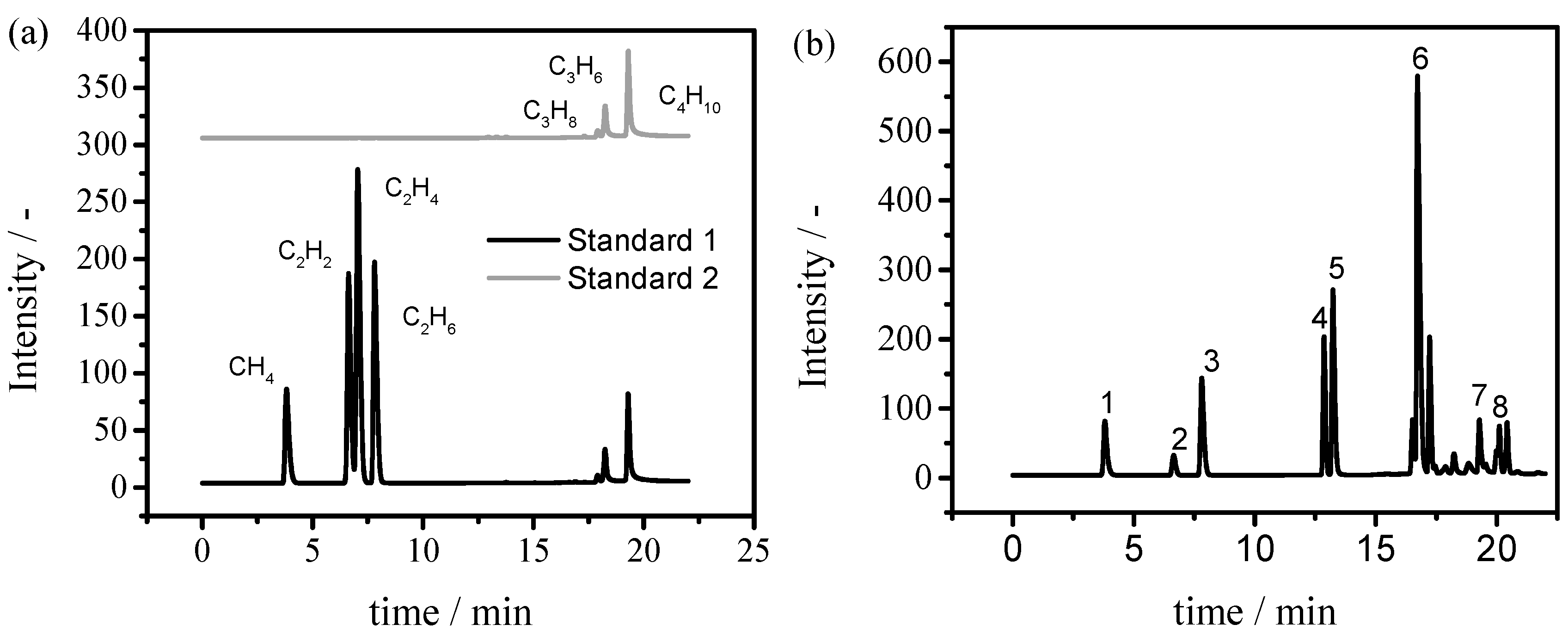
3.2. Characterization of Raw Material and Cracking Products
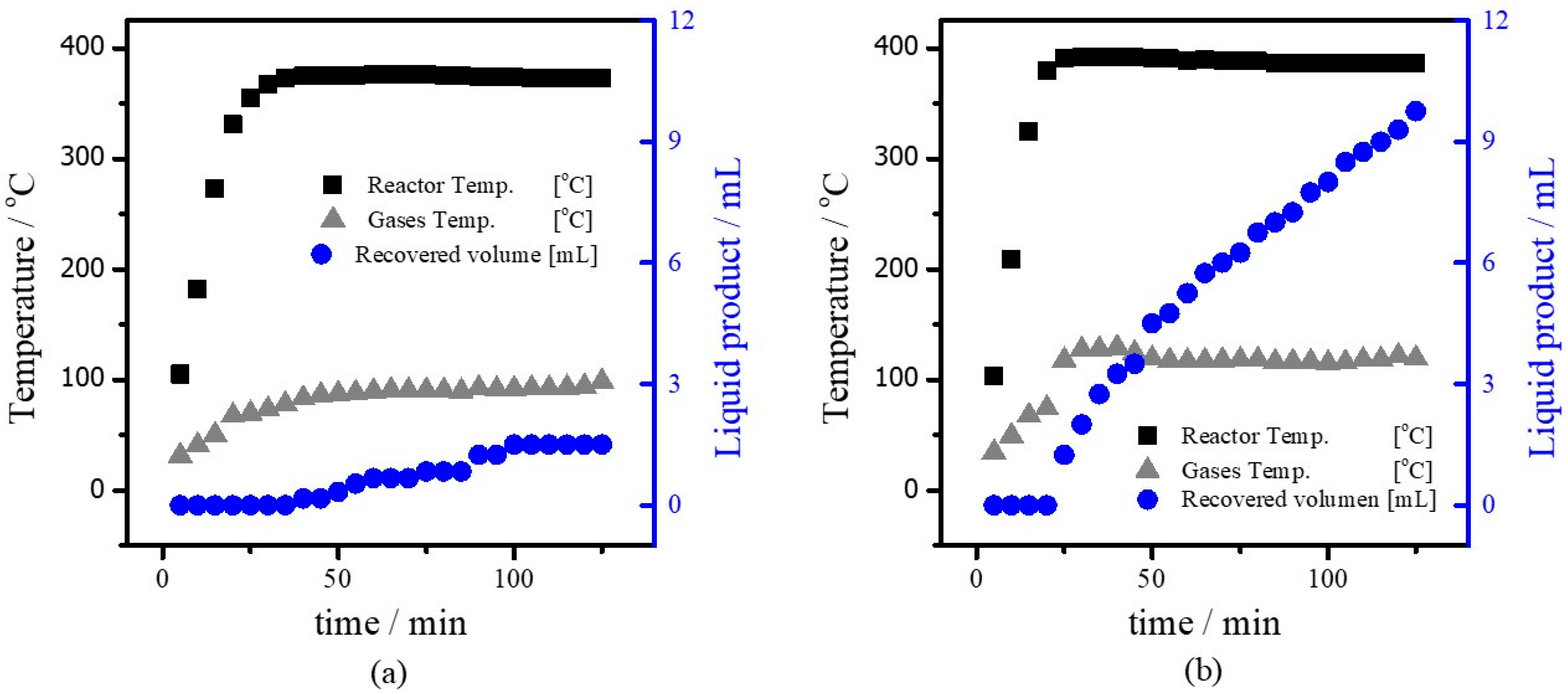
3.3. Cracking Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, M.; Biswas, D.; Sana, S.; Datta, S. Biodegradation of waste lubricants by a newly isolated Ochrobactrum sp. C1. 3Biotech 2015, 5, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.P.; Agamuthu, P.; Abdul Aziz, A.R. Biodegradation of Used Motor Oil in Soil Using Organic Waste Amendments. Biotechnol. Res. Int. 2012, 2012, 587041. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shadangi, K.P. Thermochemical conversion of waste engine oil (WEO) to gasoline-rich crude oil. J. Mater. Cycles Waste Manag. 2020, 22, 536–546. [Google Scholar] [CrossRef]
- Husaini, A.; Roslan, H.A.; Hii, K.S.Y.; Ang, C.H. Biodegradation of aliphatic hydrocarbon by indigenous fungi isolated from used motor oil contaminated sites. World J. Microbiol. Biotechnol. 2008, 24, 2789–2797. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- European Commision. Waste Oil. Available online: https://ec.europa.eu/environment/pdf/waste/studies/oil/waste_oil_xsum.pdf (accessed on 3 November 2022).
- Mishra, A.; Siddiqi, H.; Kumari, U.; Behera, I.D.; Mukherjee, S.; Meikap, B.C. Pyrolysis of waste lubricating oil/waste motor oil to generate high-grade fuel oil: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 150, 111446. [Google Scholar] [CrossRef]
- Nyashina, G.S.; Vershinina, K.Y.; Shlegel, N.E.; Strizhak, P.A. Effective incineration of fuel-waste slurries from several related industries. Environ. Res. 2019, 176, 108559. [Google Scholar] [CrossRef]
- Lesmana, D.; Wu, H.-S. Pyrolysis of waste oil in the presence of a spent catalyst. J. Environ. Chem. Eng. 2015, 3, 2522–2527. [Google Scholar] [CrossRef]
- Sınağ, A.; Gülbay, S.; Uskan, B.; Uçar, S.; Özgürler, S.B. Production and characterization of pyrolytic oils by pyrolysis of waste machinery oil. J. Hazard. Mater. 2010, 173, 420–426. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176–177, 601–617. [Google Scholar] [CrossRef]
- Siddiqi, H.; Kumari, U.; Biswas, S.; Mishra, A.; Meikap, B.C. A synergistic study of reaction kinetics and heat transfer with multi-component modelling approach for the pyrolysis of biomass waste. Energy 2020, 204, 117933. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Pablo Villegas, J.; de Barros Neto, E.L.; Ghislain, T.; Lavoie, J.-M. Waste surgical masks to fuels via thermochemical co-processing with waste motor oil and biomass. Bioresour. Technol. 2022, 348, 126798. [Google Scholar] [CrossRef] [PubMed]
- Tekin, K.; Ucar, S.; Karagöz, S. Influence of Co-Pyrolysis of Waste Tetra Pak with Waste Motor Oil on Product Distribution and Properties for Fuel Application. Energy Fuels 2019, 33, 11101–11112. [Google Scholar] [CrossRef]
- Rimez, B.; Breyer, S.; Vekemans, O.; Haut, B. Co-Pyrolysis of Low-Density Polyethylene and Motor Oil—Investigation of the Chemical Interactions between the Components. Recycling 2020, 5, 33. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Thermal Cracking and Coking; Chapter 6; Elsevier: Amsterdam, The Netherlands, 2010; pp. 123–152. ISBN 978-0-444-52785-1. [Google Scholar]
- Tamunaidu, P.; Bhatia, S. Catalytic cracking of palm oil for the production of biofuels: Optimization studies. Bioresour. Technol. 2007, 98, 3593–3601. [Google Scholar] [CrossRef]
- Ishihara, A.; Negura, H.; Hashimoto, T.; Nasu, H. Catalytic properties of amorphous silica-alumina prepared using malic acid as a matrix in catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2010, 388, 68–76. [Google Scholar] [CrossRef]
- Giraldo, L.F.; López, B.L.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous Silica Applications. Macromol. Symp. 2007, 258, 129–141. [Google Scholar] [CrossRef]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 452, 131319. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Huang, Y.; Yu, Y.; Julson, J. Catalytic cracking of camelina oil for hydrocarbon biofuel over ZSM-5-Zn catalyst. Fuel Process. Technol. 2015, 139, 117–126. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; Wu, Y.; Liu, X.; Li, X.; Zhang, Y.; Xia, H.; Wang, F. Catalytic Cracking of Fatty Acid Methyl Esters for the Production of Green Aromatics Using Zn-Modified HZSM-5 Catalysts. Energy Fuels 2022, 36, 6922–6938. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Hamdan, M.; Halawy, L.; Aramouni, N.A.K.; Ahmad, M.N.; Zeaiter, J. Analytical review of the catalytic cracking of methane. Fuel 2022, 324, 124455. [Google Scholar] [CrossRef]
- Vargas, D.C.; Alvarez, M.B.; Hidrobo Portilla, A.; Van Geem, K.M.; Almeida Streitwieser, D. Kinetic Study of the Thermal and Catalytic Cracking of Waste Motor Oil to Diesel-like Fuels. Energy Fuels 2016, 30, 9712–9720. [Google Scholar] [CrossRef]
- Maceiras, R.; Alfonsín, V.; Morales, F.J. Recycling of waste engine oil for diesel production. Waste Manag. 2017, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, K.R.; Breitzke, H.; Buntkowsky, G.; Reimhult, E.; Willinger, M.; Rennert, T. Synthesis of short-range ordered aluminosilicates at ambient conditions. Sci. Rep. 2021, 11, 4207. [Google Scholar] [CrossRef] [PubMed]
- Treto-Suárez, M.A.; Prieto-García, J.O.; Mollineda-Trujillo, Á.; Lamazares, E.; Hidalgo-Rosa, Y.; Mena-Ulecia, K. Kinetic study of removal heavy metal from aqueous solution using the synthetic aluminum silicate. Sci. Rep. 2020, 10, 10836. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Mokaya, R. Strongly acidic mesoporous aluminosilicates prepared via hydrothermal restructuring of a crystalline layered silicate. J. Mater. Chem. A 2015, 3, 7799–7809. [Google Scholar] [CrossRef]


| Experiment | Denomination | Impregnated Metals | Percentage of Metal in Catalyst | Reaction Temperature (°C) |
|---|---|---|---|---|
| Thermal cracking | Thermal | - | - | 375–415 |
| Catalytic cracking using Al/Si under acidic and basic preparation | A-Al/Si/ B-Al/Si | - | - | 380–390 |
| Catalytic cracking using Al/Si acid preparation with metal-doped | A-Al/Si-Me | Zn-Mg-Cu-Ni | 1% | 380–390 |
| Catalytic cracking using Al/Si basic preparation with metal-doped | B-Al/Si-Me | Zn-Mg-Cu-Ni | 1% | 380–390 |
| Sample | BET (m2 g−1) | Volume Mesopore (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Al/Si | 310 | 1.15 | 12.9 |
| A-Al/Si | 490 | 0.71 | 5.81 |
| B-Al/Si | 190 | 0.67 | 12.82 |
| A-Al/Si-metal | |||
| Mg | 246 | 0.95 | 11.31 |
| Zn | 132 | 0.42 | 13.07 |
| Cu | 218 | 0.66 | 12.45 |
| Ni | 298 | 0.74 | 8.98 |
| B-Al/Si-metal | |||
| Mg | 394 | 0.6 | 6.12 |
| Zn | 104 | 0.49 | 20.79 |
| Cu | 122 | 0.36 | 13.41 |
| Ni | 642 | 0.24 | 2.53 |
| Property | Method | INEN 1489:2012 Diesel II | Raw Material UMO | Ther-mal | A-Al/Si | B-Al/Si | A-Al/Si-Co | B-Al/Si-Ni |
|---|---|---|---|---|---|---|---|---|
| Density at 15 °C (g ml−1) | ASTM D1298 | ~0.87 | 0.88 | 0.84 | 0.81 | 0.82 | 0.82 | 0.82 |
| Kin. Viscosity at 40 °C (cSt) | ASTM D2270 | 2.5–5.0 | 101.4 | 3.2 | 1.6 | 2.0 | 1.9 | 2.4 |
| API Degree at 15 °C | ASTM 1298 | ~33.00 | 28.82 | 37.12 | 43.09 | 41.07 | 41.86 | 41.09 |
| 90% Distillation Temp. (°C) | ASTM D86 | Less than 360 | - | 280 | 320 | 310 | 315 | 318 |
| Ignition Point (°C) | ASTM D56 | 51 | - | 66 | 51 | 46 | 41 | 51 |
| Reactor Temperature (°C) | Conversion (%) | Liquid Products Yield (%) | Gaseous Products Yield (%) | Liquid Products Selectivity (%) | Gaseous Products Selectivity (%) |
|---|---|---|---|---|---|
| Thermal cracking | |||||
| 380 | 5.89 | 2.62 | 3.26 | 45 | 55 |
| 385 | 8.44 | 5.81 | 2.63 | 69 | 31 |
| 390 | 15.50 | 11.50 | 3.99 | 74 | 26 |
| A-Al/Si- catalytic cracking | |||||
| 380 | 9.28 | 4.19 | 5.10 | 45 | 55 |
| 385 | 10.60 | 6.73 | 3.87 | 63 | 37 |
| 390 | 20.58 | 13.91 | 6.66 | 68 | 32 |
| B-Al/Si- catalytic cracking | |||||
| 380 | 4.13 | 1.29 | 2.84 | 31 | 69 |
| 385 | 8.08 | 4.91 | 3.17 | 61 | 39 |
| 390 | 23.46 | 21.81 | 1.65 | 93 | 7 |
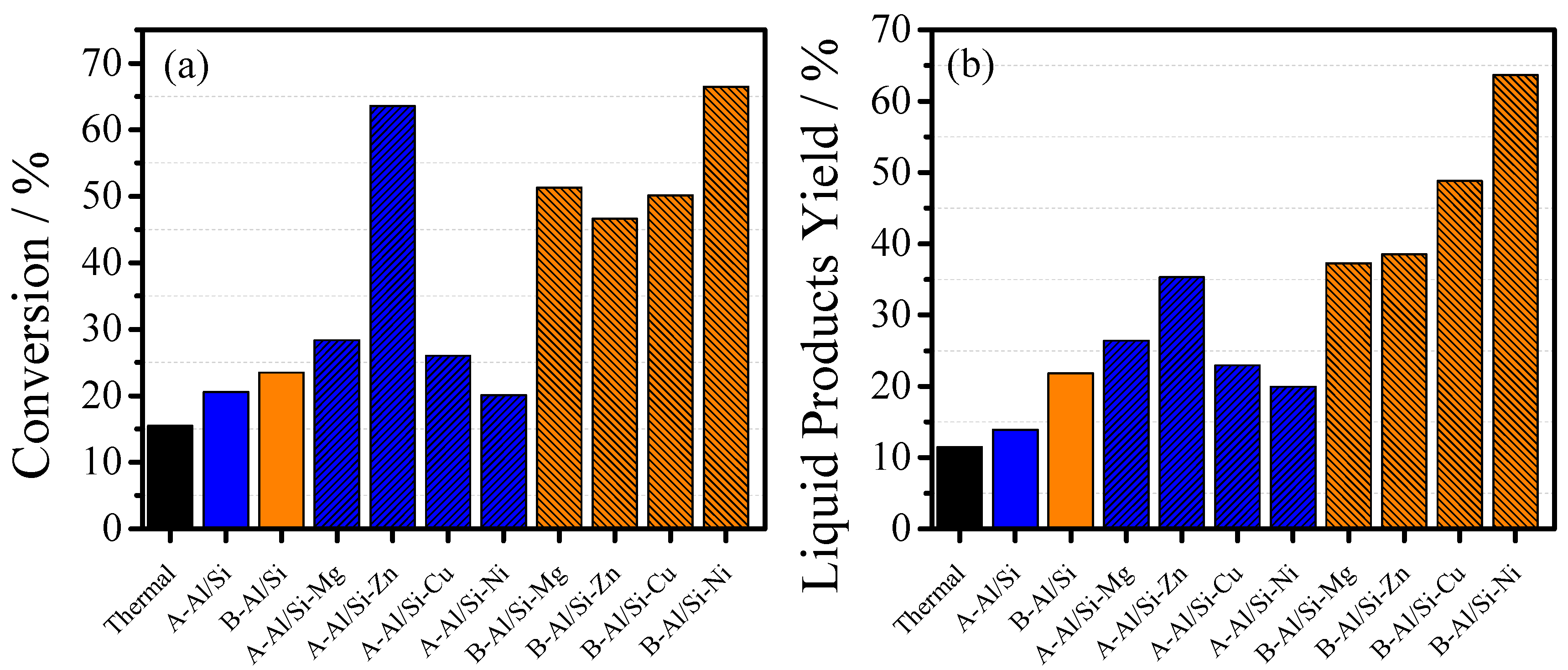
| Reactor Temperature (°C) | Metal | Conversion (%) | Liquid Products Yield (%) | Gaseous Products Yield (%) | Liquid Products Selectivity (%) | Gaseous Products Selectivity (%) |
|---|---|---|---|---|---|---|
| A-Al/Si-metal catalytic cracking | ||||||
| 385 | Mg | 17.82 | 15.63 | 2.19 | 88 | 12 |
| 390 | 28.36 | 26.39 | 1.97 | 93 | 7 | |
| 385 | Zn | 17.69 | 17.67 | 0.02 | 100 | 0 |
| 390 | 63.55 | 35.29 | 28.26 | 56 | 44 | |
| 385 | Cu | 13.61 | 12.45 | 1.16 | 91 | 9 |
| 390 | 26.00 | 22.95 | 3.05 | 88 | 12 | |
| 385 | Ni | 15.15 | 12.28 | 2.87 | 81 | 19 |
| 390 | 20.11 | 19.96 | 0.15 | 99 | 1 | |
| B-Al/Si-metal catalytic cracking | ||||||
| 385 | Mg | 12.19 | 11.23 | 0.96 | 92 | 8 |
| 390 | 51.30 | 37.28 | 14.02 | 73 | 27 | |
| 385 | Zn | 21.81 | 20.77 | 1.04 | 95 | 5 |
| 390 | 46.58 | 38.57 | 8.01 | 83 | 17 | |
| 385 | Cu | 16.05 | 12.89 | 3.16 | 80 | 20 |
| 390 | 50.12 | 48.83 | 1.29 | 97 | 3 | |
| 385 | Ni | 20.87 | 17.45 | 3.42 | 84 | 16 |
| 390 | 66.49 | 63.66 | 2.82 | 96 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida Streitwieser, D.; Arteaga, A.; Gallo-Cordova, A.; Hidrobo, A.; Ponce, S. Chemical Recycling of Used Motor Oil by Catalytic Cracking with Metal-Doped Aluminum Silicate Catalysts. Sustainability 2023, 15, 10522. https://doi.org/10.3390/su151310522
Almeida Streitwieser D, Arteaga A, Gallo-Cordova A, Hidrobo A, Ponce S. Chemical Recycling of Used Motor Oil by Catalytic Cracking with Metal-Doped Aluminum Silicate Catalysts. Sustainability. 2023; 15(13):10522. https://doi.org/10.3390/su151310522
Chicago/Turabian StyleAlmeida Streitwieser, Daniela, Arturo Arteaga, Alvaro Gallo-Cordova, Alexis Hidrobo, and Sebastian Ponce. 2023. "Chemical Recycling of Used Motor Oil by Catalytic Cracking with Metal-Doped Aluminum Silicate Catalysts" Sustainability 15, no. 13: 10522. https://doi.org/10.3390/su151310522
APA StyleAlmeida Streitwieser, D., Arteaga, A., Gallo-Cordova, A., Hidrobo, A., & Ponce, S. (2023). Chemical Recycling of Used Motor Oil by Catalytic Cracking with Metal-Doped Aluminum Silicate Catalysts. Sustainability, 15(13), 10522. https://doi.org/10.3390/su151310522







