A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products
Abstract
1. Introduction
2. Materials and Manufacturing Methods
2.1. Materials and Procurement for the 3D-Printed Pots
2.2. Methods for Producing the 3D-Printed Pots
3. Greenhouse Performance Study
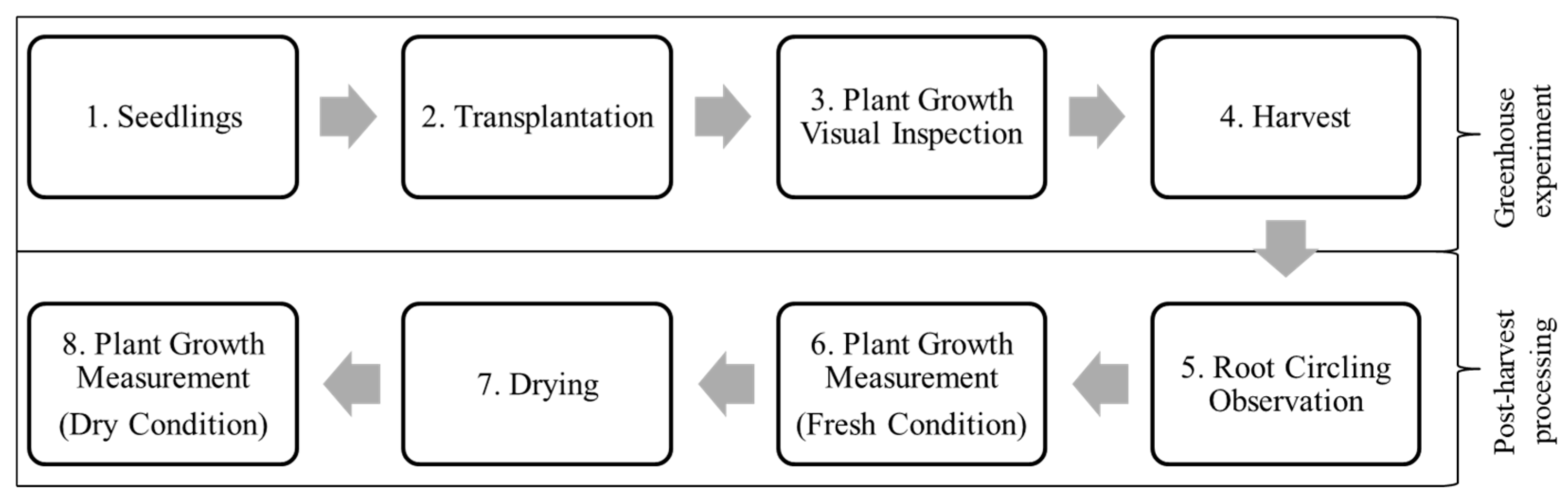
3.1. Overview of Greenhouse Experiment
3.2. Post-Harvest Processing
3.3. Overview of Statistical Analysis
3.4. Greenhouse Experiment Results
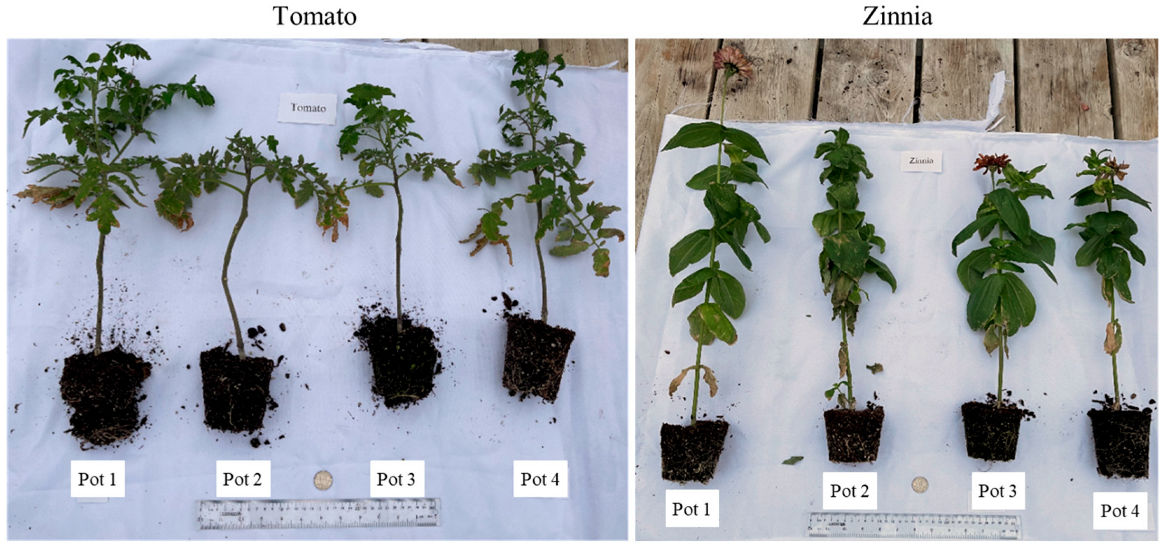
3.4.1. Plant Growth Measurements: Tomato
- In terms of plant height, tomato plants grown in non-biodegradable plastic Pot 4 outperformed those grown in 3D-printed bioplastic pots. However, for plant width, tomato plants grown in soy-based bioplastic Pot 2 showed comparable results to those grown in the control Pot 4.
- For fresh weight, the tomato shoot performed better when grown in Pot 4, while the root weight is comparable to the control Pot 4 when grown in soy-based bioplastic Pot 2. The higher fresh weight of a plant indicates that the plant has a greater amount of water or other fluids in its tissues, which can be an indication of greater nutrient (e.g., nitrogen) uptake or efficient photosynthesis [39].
- In terms of dry weight, tomato plants grown in soy-based bioplastic Pots 1 and 2 showed better shoot dry weight compared to the other pots. The root dry weight of tomato plants grown in bioplastic Pot 2 is also comparable to that of the control Pot 4. A higher dry weight of a plant can indicate that the plant has accumulated more nitrogen from pots [39,40].
3.4.2. Plant Growth Measurements: Zinnia
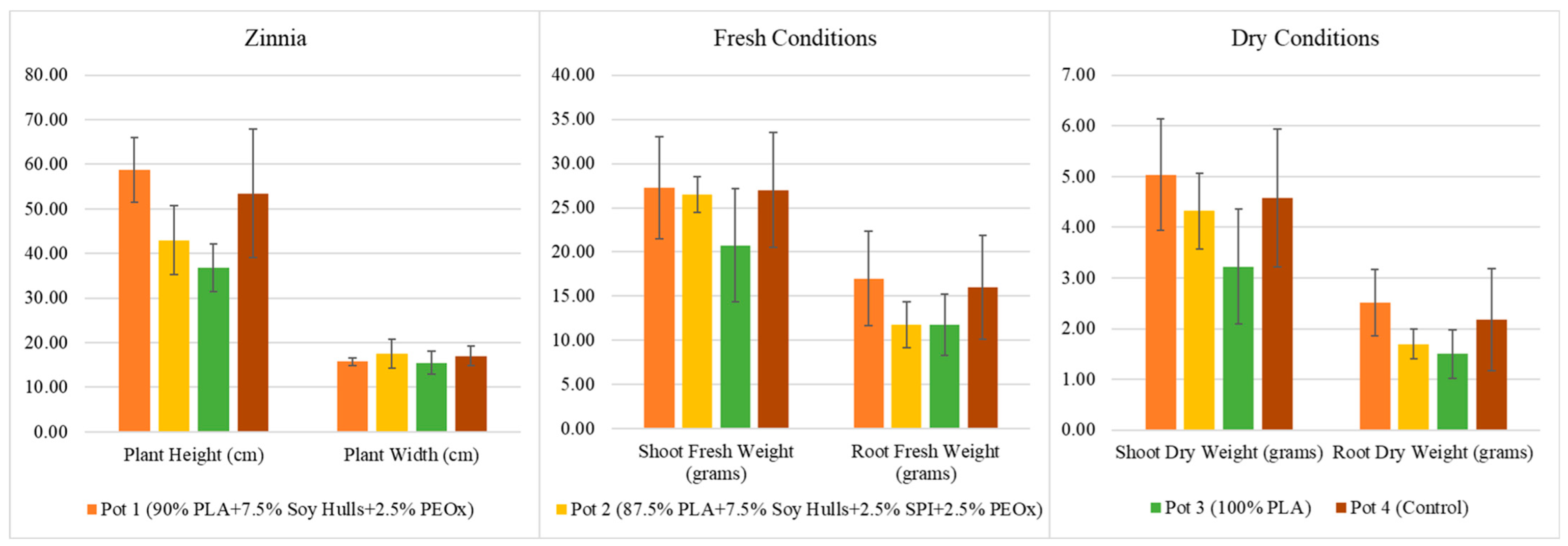
- Zinnia plants grown in soy-based biodegradable plastic Pot 1 showed superior plant height compared to other bioplastic pots (Pot 2 and Pot 3) and non-biodegradable control Pot 4. Soy-based bioplastic Pot 2 exhibited plant width results comparable to the control Pot 4, which is also observed for the tomato plant width detailed in the previous section.
- For fresh weight, the shoot of zinnia plants performed better when grown in soy-based bioplastic Pots 1 and 2, which is comparable to the control Pot 4. However, zinnia root performed better in soy-based Pot 1 and Pot 4 than in Pot 2 or Pot 3.
- Regarding dry weight, zinnia plants grown in soy-based bioplastic Pot 1 showed better shoot dry weight compared to the other pots. Moreover, the root dry weight of zinnia plants grown in bioplastic Pot 1 is comparable to that of the control Pot 4.
4. Data Analysis and Discussion
4.1. Statistical Analysis Results
4.2. Discussion and Future Work towards Sustainable Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- North, E.J.; Halden, R.U. Plastics and environmental health: The road ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Harding, K.; Dennis, J.; Von Blottnitz, H.; Harrison, S. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, S. Biodegradable Polymer Synthesis from Renewable Sources. 2021. Available online: https://urn.fi/URN:NBN:fi:amk-2021060113024 (accessed on 27 April 2023).
- Dey, A.; Rahman, M.M.; Yodo, N.; Grewell, D. Development of Biocomposite Filament for Fused Filament Fabrication from Soy Hulls and Soy Protein Isolate. Mater. Today Commun. 2023, 34, 105316. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dey, A.; Yodo, N.; Lee, C.W.; Grewell, D. Soybean By-Products Bioplastic (Polylactic Acid)-Based Plant Containers: Sustainable Development and Performance Study. Sustainability 2023, 15, 5373. [Google Scholar] [CrossRef]
- Suriyamongkol, P.; Weselake, R.; Narine, S.; Moloney, M.; Shah, S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—A review. Biotechnol. Adv. 2007, 25, 148–175. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Schrader, J.A.; McCabe, K.G.; Srinivasan, G.; Grewell, D.; Graves, W.R. Performance and biodegradation in soil of novel horticulture containers made from bioplastics and biocomposites. HortTechnology 2015, 25, 119–131. [Google Scholar] [CrossRef]
- Sondhi, S. Applications of Bioplastic in Composting Bags and Planting Pots. In Handbook of Bioplastics and Biocomposites Engineering Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 605–617. [Google Scholar]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Tomadoni, B.; Merino, D.; Casalongué, C.; Alvarez, V. Biodegradable materials for planting pots. Adv. Appl. Bio-Degrad. Green Compos. 2020, 68, 85. [Google Scholar]
- Sun, E.; Liao, G.; Zhang, Q.; Qu, P.; Wu, G.; Huang, H. Biodegradable copolymer-based composites made from straw fiber for biocomposite flowerpots application. Compos. Part B Eng. 2019, 165, 193–198. [Google Scholar] [CrossRef]
- Liew, K.C.; Khor, L.K. Effect of different ratios of bioplastic to newspaper pulp fibres on the weight loss of bioplastic pot. J. King Saud Univ.-Eng. Sci. 2015, 27, 137–141. [Google Scholar] [CrossRef]
- Castronuovo, D.; Picuno, P.; Manera, C.; Scopa, A.; Sofo, A.; Candido, V. Biodegradable pots for poinsettia cultivation: Agronomic and technical traits. Sci. Hortic. 2015, 197, 150–156. [Google Scholar] [CrossRef]
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Mugnozza, G.S.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Ellison, B.; Kirwan, B.; Nepal, A. Consumers’ Willingness to Pay for Bioplastic Plant Containers: An Experimental Auction Approach. 2015. Available online: https://econpapers.repec.org/paper/agsaaea15/205670.htm (accessed on 27 April 2023).
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Liu, H.-M.; Li, H.-Y. Application and conversion of soybean hulls. In Soybean—The Basis of Yield, Biomass and Productivity; IntechOpen: London, UK, 2017. [Google Scholar]
- Nahashon, S.N.; Kilonzo-Nthenge, A.K. Advances in Soybean and Soybean by-products in monogastric nutrition and health. In Soybean and Nutrition; BoD—Books on Demand: Hamburg, Germany, 2011; pp. 125–156. [Google Scholar]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. Mechanical Performance of Soy-Hull-Reinforced Bioplastic Green Composites: A Comparison with Polypropylene Composites. Macromol. Mater. Eng. 2012, 297, 184–194. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Maigret, J.-E.; Perez-Puyana, V.; Romero, A.; Lourdin, D. Revaluation of a Soy Protein By-product in Eco-friendly Bioplastics by Extrusion. J. Polym. Environ. 2022, 30, 1587–1599. [Google Scholar] [CrossRef]
- Perez, K.B.; Anderson, D.S.; Wood, K.L. Crowdsourced design principles for leveraging the capabilities of additive manufacturing. In Proceedings of the International Conference of Engineerring Design, Politecnico Di Milano, Italy, 27–30 July 2015; pp. 1–10. [Google Scholar]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Dey, A.; Roan Eagle, I.N.; Yodo, N. A review on filament materials for fused filament fabrication. J. Manuf. Mater. Process. 2021, 5, 69. [Google Scholar] [CrossRef]
- De Clercq, M.; Vats, A.; Biel, A. Agriculture 4.0: The Future of Farming Technology. 2018. Available online: https://www.oliverwyman.com/content/dam/oliver-wyman/v2/publications/2021/apr/agriculture-4-0-the-future-of-farming-technology.pdf (accessed on 5 May 2023).
- Dey, A.; Yodo, N. A systematic survey of FDM process parameter optimization and their influence on part characteristics. J. Manuf. Mater. Process. 2019, 3, 64. [Google Scholar] [CrossRef]
- Pearce, J.M. Applications of open source 3-D printing on small farms. Org. Farming 2015, 1, 19–35. [Google Scholar] [CrossRef]
- Ratshoshi, B.K.; Farzad, S.; Görgens, J.F. Techno-economic assessment of polylactic acid and polybutylene succinate production in an integrated sugarcane biorefinery. Biofuels Bioprod. Biorefin. 2021, 15, 1871–1887. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Shi, W.; Li, L.; Tian, R.; Huang, J.; Lin, R.; Wang, B.; Zhou, B. Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting. Bioresour. Technol. 2020, 316, 123823. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.A.; Srinivasan, G.; Grewell, D.; McCabe, K.G.; Graves, W.R. Fertilizer effects of soy-plastic containers during crop production and transplant establishment. HortScience 2013, 48, 724–731. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sabapathy, S.; Bawa, A. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Dey, A.; Rahman, M.M.; Yodo, N. Soy-based Biocomposite Filament Development for Fused Filament Fabrication Process. In Proceedings of the IIE Annual Conference, Seattle, WA, USA, 21–24 May 2022. [Google Scholar]
- Jeong, B.R.; Lee, C.W. Effect of NH4+, NO3+, and cl-ions on ion uptake and solution ph in hydroponic culture of ageratum and salvia. HortScience 1990, 25, 1171a-1171. [Google Scholar] [CrossRef]
- Choi, J.M.; Pak, C.H.; Lee, C.W. Micro nutrient toxicity in French marigold. J. Plant Nutr. 1996, 19, 901–916. [Google Scholar] [CrossRef]
- Yodo, N.; Rafi, M. Statistics. In Mathematics in Cyber Research, 1st ed.; Goethals, P.L., Scala, N.M., Bennett, D.T., Eds.; Chapman and Hall/CRC: New York, NY, USA, 2022; Volume 1, p. 524. [Google Scholar]
- Gilman, E.F.; Harchick, C.; Paz, M. Effect of container type on root form and growth of red maple. J. Environ. Hortic. 2010, 28, 1. [Google Scholar]
- Bush, E.A. Botryosphaeria Canker and Dieback of Trees and Shrubs in the Landscape. Available online: https://digitalpubs.ext.vt.edu/vcedigitalpubs/5865835883187342/MobilePagedReplica.action?pm=2&folio=1#pg1 (accessed on 5 May 2023).
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Sohail, H.; Afzal, M.; Ali, M.A.; Bie, Z.; Huang, Y. Genome-wide expression profiling of leaves and roots of watermelon in response to low nitrogen. BMC Genom. 2018, 19, 456. [Google Scholar] [CrossRef]
- Huett, D.; Dettmann, E. Effect of nitrogen on growth, fruit quality and nutrient uptake of tomatoes grown in sand culture. Aust. J. Exp. Agric. 1988, 28, 391–399. [Google Scholar] [CrossRef]
- Solomon, I.J.; Sevvel, P.; Gunasekaran, J. A review on the various processing parameters in FDM. Mater. Today Proc. 2021, 37, 509–514. [Google Scholar] [CrossRef]
- Flax, N.J.; Currey, C.J.; Schrader, J.A.; Grewell, D.; Graves, W.R. Herbaceous perennial producers can grow high-quality blanket flower in bioplastic-based plant containers. HortTechnology 2018, 28, 212–217. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Panahi, R.; Baghban-Salehi, M. Protein-based hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1561–1600. [Google Scholar]
- Choi, J.M.; Lee, C.W.; Chun, J.-P. Optimization of substrate formulation and mineral nutrition during the production of vegetable seedling grafts. Hortic. Environ. Biotechnol. 2012, 53, 212–221. [Google Scholar] [CrossRef]

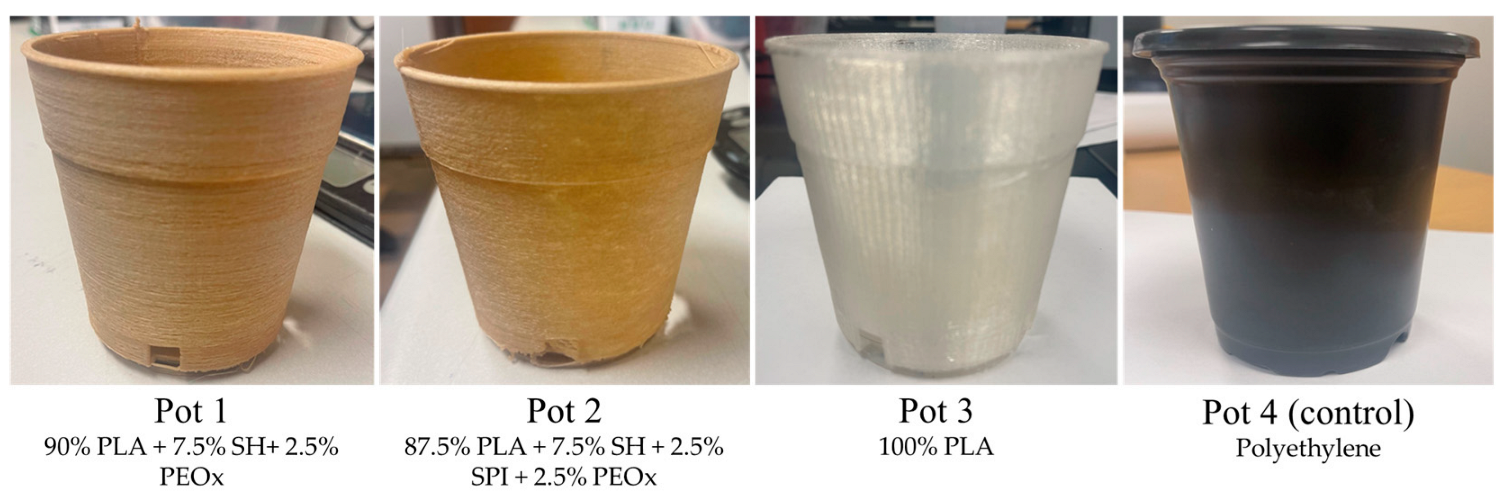





| Pot ID | Formulations * | Type of Pots | Manufacturing Methods |
|---|---|---|---|
| Pot 1 ** | 90% PLA + 7.5% SH + 2.5% PEOX | Biodegradable | 3D-printed (soy-based filament) |
| Pot 2 ** | 87.5% PLA + 7.5% SH + 2.5% SPI + 2.5% PEOX | Biodegradable | 3D-printed (soy-based filament) |
| Pot 3 ** | 100% PLA | Biodegradable | 3D-printed (PLA filament) |
| Pot 4 (control) *** | Polyethylene | Non-biodegradable | Injection molding |
| Pot ID | Samples | Plant Height (cm) | Plant Width (cm) | Shoot Fresh Weight (gm) | Root Fresh Weight (gm) | Shoot Dry Weight (gm) | Root Dry Weight (gm) |
|---|---|---|---|---|---|---|---|
| Pot 1 | S1 | 44.00 | 21.00 | 22.00 | 4.00 | 3.15 | 0.66 |
| S2 | 32.00 | 17.00 | 15.00 | 3.00 | 2.06 | 0.43 | |
| S3 | 30.00 | 22.00 | 16.00 | 3.00 | 2.83 | 0.33 | |
| S4 * | - | - | - | - | - | - | |
| Avg. | 35.33 | 20.00 | 17.67 | 3.33 | 2.68 | 0.47 | |
| Pot 2 | S1 | 32.00 | 25.00 | 18.00 | 6.00 | 3.33 | 0.73 |
| S2 | 29.00 | 27.00 | 19.00 | 7.00 | 3.27 | 0.73 | |
| S3 | 27.50 | 12.00 | 6.00 | 1.00 | 0.57 | 0.09 | |
| S4 | 37.00 | 35.00 | 24.00 | 7.00 | 5.48 | 0.93 | |
| Avg. | 31.38 | 24.75 | 16.75 | 5.25 | 3.16 | 0.62 | |
| Pot 3 | S1 | 38.00 | 20.00 | 12.00 | 4.00 | 1.42 | 0.33 |
| S2 | 27.00 | 26.00 | 18.00 | 5.00 | 3.19 | 0.62 | |
| S3 | 24.00 | 11.60 | 6.00 | 2.00 | 0.85 | 0.17 | |
| S4 | 36.00 | 21.00 | 16.00 | 3.00 | 1.14 | 0.33 | |
| Avg. | 31.25 | 19.65 | 13.00 | 3.50 | 1.65 | 0.36 | |
| Pot 4 (Control) | S1 | 42.00 | 25.00 | 22.00 | 6.00 | 3.06 | 0.66 |
| S2 | 35.00 | 22.00 | 18.00 | 4.00 | 2.30 | 0.58 | |
| S3 | 35.00 | 23.00 | 14.00 | 3.00 | 2.21 | 0.35 | |
| S4 | 37.00 | 28.00 | 20.00 | 7.00 | 2.68 | 0.66 | |
| Avg. | 37.25 | 24.50 | 18.50 | 5.00 | 2.56 | 0.56 |
| Pot ID | Samples | Plant Height (cm) | Plant Width (cm) | Shoot Fresh Weight (gm) | Root Fresh Weight (gm) | Shoot Dry Weight (gm) | Root Dry Weight (gm) |
|---|---|---|---|---|---|---|---|
| Pot 1 | S1 | 67.00 | 17.00 | 36.00 | 26.00 | 6.50 | 3.38 |
| S2 | 65.00 | 16.00 | 29.00 | 16.00 | 4.60 | 2.08 | |
| S3 | 51.00 | 15.00 | 22.00 | 13.00 | 3.54 | 1.71 | |
| S4 | 52.00 | 15.00 | 22.00 | 13.00 | 5.51 | 2.87 | |
| Avg. | 58.75 | 15.75 | 27.25 | 17.00 | 5.04 | 2.51 | |
| Pot 2 | S1 | 53.00 | 13.00 | 29.00 | 13.00 | 5.29 | 1.84 |
| S2 | 35.00 | 16.00 | 28.00 | 15.00 | 4.42 | 1.96 | |
| S3 | 36.00 | 21.00 | 24.00 | 8.00 | 3.19 | 1.21 | |
| S4 | 48.00 | 20.00 | 25.00 | 11.00 | 4.38 | 1.79 | |
| Avg. | 43.00 | 17.50 | 26.50 | 11.75 | 4.32 | 1.70 | |
| Pot 3 | S1 | 44.00 | 18.00 | 31.00 | 16.00 | 4.77 | 1.90 |
| S2 | 38.00 | 12.00 | 17.00 | 7.00 | 2.38 | 0.96 | |
| S3 | 36.50 | 14.00 | 21.00 | 14.00 | 3.81 | 2.05 | |
| S4 | 29.00 | 18.00 | 14.00 | 10.00 | 1.94 | 1.08 | |
| Avg. | 36.88 | 15.50 | 20.75 | 11.75 | 3.23 | 1.50 | |
| Pot 4 (Control) | S1 | 46.50 | 16.00 | 24.00 | 10.00 | 3.96 | 1.08 |
| S2 | 40.50 | 15.00 | 21.00 | 14.00 | 3.31 | 1.95 | |
| S3 | 73.50 | 20.00 | 36.00 | 24.00 | 6.47 | 3.51 | |
| S4 * | - | - | - | - | - | - | |
| Avg. | 53.50 | 17.00 | 27.00 | 16.00 | 4.58 | 2.18 |
| Plant | ANOVA | Plant Height (cm) | Plant Width (cm) | Shoot Fresh Weight (gm) | Root Fresh Weight (gm) | Shoot Dry Weight (gm) | Root Dry Weight (gm) |
|---|---|---|---|---|---|---|---|
| Tomato | p-value * | 0.267 | 0.493 | 0.406 | 0.424 | 0.352 | 0.474 |
| Results | Accept Ho | Accept Ho | Accept Ho | Accept Ho | Accept Ho | Accept Ho | |
| Zinnia | p-value * | 0.045 | 0.703 | 0.445 | 0.445 | 0.260 | 0.308 |
| Results | Reject Ho ** | Accept Ho | Accept Ho | Accept Ho | Accept Ho | Accept Ho |
| Plant/Variable | Pot ID | Description | Mean (cm) | Grouping | |
|---|---|---|---|---|---|
| Zinnia/ plant height | Pot 1 | 3D-printed (soy-based) | 58.75 | A | - |
| Pot 2 | 3D-printed (soy-based) | 43.00 | A | B | |
| Pot 3 | 3D-printed (PLA) | 36.88 | - | B | |
| Pot 4 (control) | Injection molding | 53.30 | A | B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, A.; Rahman, M.M.; Gupta, A.; Yodo, N.; Lee, C.W. A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products. Sustainability 2023, 15, 10535. https://doi.org/10.3390/su151310535
Dey A, Rahman MM, Gupta A, Yodo N, Lee CW. A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products. Sustainability. 2023; 15(13):10535. https://doi.org/10.3390/su151310535
Chicago/Turabian StyleDey, Arup, Md Mahbubar Rahman, Anunay Gupta, Nita Yodo, and Chiwon W. Lee. 2023. "A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products" Sustainability 15, no. 13: 10535. https://doi.org/10.3390/su151310535
APA StyleDey, A., Rahman, M. M., Gupta, A., Yodo, N., & Lee, C. W. (2023). A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products. Sustainability, 15(13), 10535. https://doi.org/10.3390/su151310535







