Biogeochemical Characteristics of Sedimentary Organic Matter in Coastal Waters of a Mariculture Area: The Big Impact of Bay Scallop Farming
Abstract
:1. Introduction
2. Materials and Methods
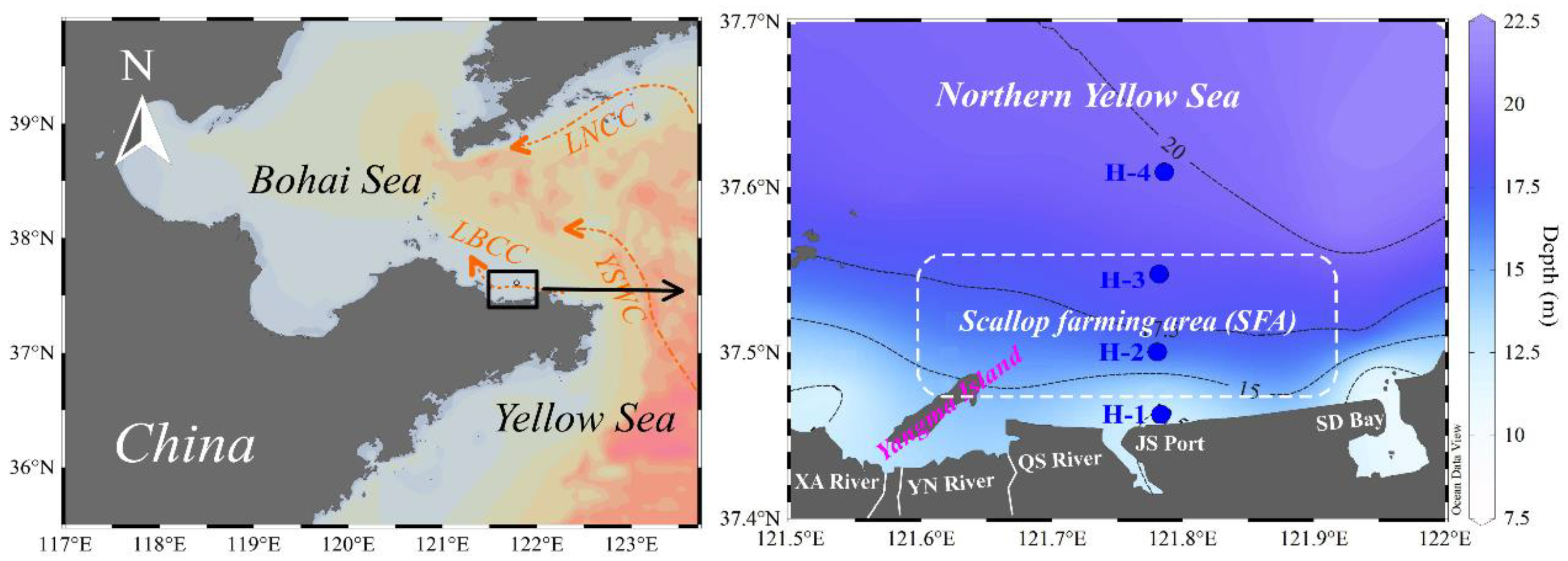
2.1. Field Sampling
2.2. Analytical Methods
2.3. Calculation of Source Proportions and Burial Fluxes
2.4. Data Analysis
3. Results
3.1. Bulk Sediment Properties
3.2. Biological Compositions of SOM
3.3. δ13C, δ15N, and the Sources of SOM
3.4. Burial Rates of SOM
3.5. Principal Component Analysis (PCA)
4. Discussion
4.1. General Characteristics of SOM
4.1.1. Composition and Distribution of SOM
4.1.2. Source and Storage of SOM
4.1.3. Burial of SOM
4.2. Effect Mechanism of Scallop Farming on SOM Dynamics
4.3. Response of SOM Burial to Mariculture Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Clay | Silt | Sand | TOC | TN | Terrestrial OM | Marine Autogenic OM | Scallop-Deposited OM | Protein | Carbohydrate | Lipid | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Silt | −0.021 | ||||||||||
| Sand | −0.417 c | −0.900 c | |||||||||
| TOC | 0.230 a | −0.440 c | 0.307 a | ||||||||
| TN | 0.241 a | −0.005 | −0.111 | 0.492 c | |||||||
| Terrestrial OM | 0.138 | −0.500 c | 0.415 b | 0.661 c | −0.057 | ||||||
| Marine autogenic OM | 0.318 a | −0.277 a | 0.131 | 0.620 c | 0.185 | 0.326 a | |||||
| Scallop-deposited OM | −0.084 | 0.103 | −0.062 | 0.289 a | 0.501 c | −0.199 | −0.356 b | ||||
| Protein | 0.462 c | −0.435 c | 0.195 | 0.241 a | 0.010 | 0.423 b | 0.306 a | −0.184 | |||
| Carbohydrate | −0.214 | 0.122 | −0.018 | 0.067 | −0.045 | −0.005 | −0.006 | 0.115 | −0.306 b | ||
| Lipid | 0.021 | 0.077 | −0.079 | −0.135 | 0.011 | −0.017 | −0.146 | 0.000 | 0.026 | 0.064 | |
| BPC | 0.171 | −0.103 | 0.019 | 0.017 | 0.002 | 0.195 | 0.028 | −0.059 | 0.390 c | 0.202 | 0.890 c |
| Core | SAR (cm yr−1) | Burial Flux (g m−2 yr−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| TOC | TN | BPC | Mar-OC | Ter-OC | Scal-OC | |||
| NSFA (H1) | Range | 22.19–34.73 | 2.71–4.21 | 18.83–29.29 | 7.33–16.56 | 8.28–15.52 | 0.05–11.83 | |
| Mean | 0.64 | 28.95 ± 3.88 | 3.48 ± 0.46 | 22.80 ± 2.60 | 11.97 ± 2.89 | 11.26 ± 2.32 | 5.49 ± 3.78 | |
| SFA (H2) | Range | 29.19–44.80 | 4.02–5.38 | 21.62–34.54 | 11.17–26.26 | 6.01–15.28 | 3.26–15.28 | |
| Mean | 0.81 | 35.91 ± 4.16 | 4.84 ± 0.34 | 26.54 ± 3.26 | 16.70 ± 3.64 | 9.94 ± 3.03 | 8.47 ± 3.50 | |
| SFA (H3) | Range | 33.35–44.28 | 4.65–5.73 | 26.73–37.85 | 11.40–18.64 | 10.17–15.85 | 4.83–15.42 | |
| Mean | 0.91 | 38.32 ± 3.55 | 5.04 ± 0.29 | 32.17 ± 3.64 | 15.47 ± 2.55 | 13.44 ± 1.66 | 9.23 ± 2.46 | |
| NSFA (H4) | Range | 17.84–32.03 | 3.34–4.20 | 21.15–30.94 | 6.88–15.94 | 7.57–11.64 | 3.17–8.37 | |
| Mean | 0.76 | 26.54 ± 4.21 | 3.73 ± 0.27 | 25.41 ± 2.89 | 10.83 ± 2.70 | 9.51 ± 1.23 | 5.44 ± 1.66 | |
| Location | TOC (%) | TN (‰) | TOC/TN | δ13C (‰) | δ15N (‰) | Reference | |
|---|---|---|---|---|---|---|---|
| Yellow Sea | Range | 0.08–1.07 | 0.20–1.03 | 5.4–14.7 | −23.14 to −21.26 | n.a. | Hu et al. [28] |
| Mean | 0.46 ± 0.25 | 0.60 ± 0.30 | 8.05 ± 1.54 | n.a. | n.a. | ||
| Bohai Bay | Range | 0.04–0.69 | 0.10–0.90 | 3.3–7.7 | −23.9 to −21.7 | n.a. | Hu et al. [30] |
| Mean | 0.38 ± 0.17 | n.a. | n.a. | n.a. | n.a. | ||
| Sishili Bay | Range | 0.17–1.33 | 0.20–1.04 | 7.9–10.1 | −22.7 to −21.6 | 5.4–6.5 | Liu et al. [64] |
| Pearl River Estuary | Range | 0.48–1.60 | n.a. | 8.50–15.32 | n.a. | n.a. | |
| Sanggou Bay | Mean | 0.50 | 0.44 | 11.47 | n.a. | n.a. | Liu et al. [19] |
| Jiaozhou Bay | Range | 0.07–0.45 | 0.16–0.48 | n.a. | n.a. | n.a. | Dai et al. [29] |
| Mean | 0.38 | 0.32 | n.a. | n.a. | n.a. | ||
| East China Sea shelf | Range | 0.15–0.75 | 0.22–1.51 | 4.69–9.12 | −22.08 to −19.99 | 3.67–6.28 | Zhou et al. [31] |
| Mean | 0.54 | n.a. | n.a. | n.a. | n.a. | ||
| Coastal waters around Yangma Island | Range | 0.27–0.62 | 0.48–0.75 | 5.30–11.21 | −23.13 to −21.21 | 3.13–5.96 | This study |
| Mean | 0.47 ± 0.07 | 0.61 ± 0.06 | 7.68 ± 1.11 | −22.30 ± 0.38 | 4.54 ± 0.55 |
| Location | Protein | Carbohydrate | Lipid | BPC | Reference |
|---|---|---|---|---|---|
| Laizhou Bay | 0.65 ± 0.23 | 1.45 ± 0.74 | 0.28 ± 0.08 | 1.18 | Huang et al. [4] |
| Galician Coast | 1.04 | 0.24 | 0.44 | 0.94 | Cividanes et al. [41] |
| Northern Adriatic | 1.05 ± 0.68 | 0.25 ± 0.13 | 0.06 ± 0.05 | 0.65 | Bianchelli et al. [42] |
| Akrotiri Bay, Cyprus | 3.04 ± 1.59 | 3.79 ± 1.82 | 0.70 ± 0.30 | 3.53 | Pusceddu et al. [43] |
| Sounion Bay, Greece | 0.90 ± 0.56 | 4.78 ± 4.64 | 0.46 ± 0.14 | 2.70 | Pusceddu et al. [43] |
| Pachino Bay, Italy | 0.80 ± 0.60 | 4.58 ± 3.28 | 0.16 ± 0.10 | 2.34 | Bongiorni et al. [44] |
| Gulf of Alicante, Spain | 2.04 ± 0.21 | 8.25 ± 3.03 | 0.45 ± 0.17 | 4.64 | Pusceddu et al. [43] |
| Coastal waters of New Caledonia | 4.82 ± 1.85 | 4.45 ± 1.08 | 1.91 ± 0.64 | 5.57 | Pusceddu et al. [50] |
| Coastal waters around Yangma Island | 4.31 ± 0.59 | 2.46 ± 0.40 | 1.02 ± 0.49 | 3.86 | This study |






References
- Xu, J.; Han, L.; Yin, W. Research on the ecologicalization efficiency of mariculture industry in China and its influencing factors. Mar. Policy 2022, 137, 104935. [Google Scholar] [CrossRef]
- FAO. WaPOR Database Methodology: Version 2 Release, April 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Yang, B.; Gao, X.L.; Xing, Q.G. Geochemistry of organic carbon in surface sediments of a summer hypoxic region in the coastal waters of northern Shandong Peninsula. Cont. Shelf Res. 2018, 171, 113–125. [Google Scholar] [CrossRef]
- Huang, Q.; Olenin, S.; Jiang, T.; Sun, S.; Troch, M.D. Assessing environmental effects of the bay scallop Argopecten irradians culture in China: Using abiotic and biotic indicators. Aquaculture 2019, 499, 316–328. [Google Scholar] [CrossRef]
- Shi, R.; Han, T.; Xu, S.; Huang, H.; Qi, Z.; Zhu, Q. Bacterial community responses to the redox profile changes of mariculture sediment. Mar. Pollut. Bull. 2021, 166, 112250. [Google Scholar] [CrossRef]
- Souza, G.; Kuroshima, K.; Abreu, J.; Manzoni, G. Speciation and distribution of sedimentary phosphorus in an important mariculture area, Armação do Itapocoroy Bay, Southern Brazil. Reg. Stud. Mar. Sci. 2022, 49, 102137. [Google Scholar] [CrossRef]
- Tang, Q.S. Carbon sink fisheries and the development of modern fisheries. Jiangxi Fish. Sci. 2011, 2, 5–7. [Google Scholar]
- Xia, B.; Han, Q.; Chen, B.J.; Sui, Q.; Jiang, T.; Sun, X.M.; Zhu, L.; Chai, C.; Qu, K.M. Influence of shellfish biodeposition on coastal sedimentary organic matter: A case study from Sanggou Bay, China. Cont. Shelf Res. 2019, 172, 12–21. [Google Scholar] [CrossRef]
- Zhang, J.; Hansen, P.K.; Wu, W. Sediment-focused environmental impact of long-term large- scale marine bivalve and seaweed farming in Sungo Bay, China. Aquaculture 2020, 528, 735561. [Google Scholar] [CrossRef]
- Gao, X.L.; Yang, Y.W.; Wang, C.Y. Geochemistry of organic carbon and nitrogen in surface sediments of coastal Bohai Bay inferred from their ratios and stable isotopic signatures. Mar. Pollut. Bull. 2012, 64, 1148–1155. [Google Scholar] [CrossRef]
- Li, R.; Liu, S.; Zhang, J.; Jiang, Z.; Fang, J. Sources and export of nutrients associated with integrated multi-trophic aquaculture in Sanggou Bay, China. Aquacult. Env. Interac. 2016, 8, 285–309. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Gao, X.L.; Zhao, J.M.; Liu, Y.L.; Gao, T.C.; Lui, H.K.; Huang, T.H.; Chen, C.T.A.; Xing, Q.G. The influence of summer hypoxia on sedimentary phosphorus biogeochemistry in a coastal scallop farming area, North Yellow Sea. Sci. Total Environ. 2021, 11, 143486. [Google Scholar] [CrossRef] [PubMed]
- Larowe, D.E.; Arndt, S.; Bradley, J.A. The fate of organic carbon in marine sediments—New insights from recent data and analysis. Earth-Sci. Rev. 2020, 204, 103146. [Google Scholar] [CrossRef]
- Yang, B.; Gao, X.L.; Zhao, J.M.; Lu, Y.X.; Gao, T.C. Biogeochemistry of dissolved inorganic nutrients in an oligotrophic coastal mariculture region of the northern Shandong Peninsula, north Yellow Sea. Mar. Pollut. Bull. 2020, 150, 110693. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, X.; Zhao, J.; Liu, Y.; Xie, L.; Lv, X.; Xing, Q. Summer deoxygenation in a bay scallop (Argopecten irradians) farming area: The decisive role of water temperature, stratification and beyond. Mar. Pollut. Bull. 2021, 173, 113092. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Zeng, L.; Zhang, H.; Tu, C.; Zhou, Q.; Luo, Y. Stocks and losses of soil organic carbon from Chinese vegetated coastal habitats. Global Chang. Biol. 2020, 27, 202–214. [Google Scholar] [CrossRef]
- Danovaro, R.; Fabiano, M. Seasonal changes in quality and quantity of food available for benthic suspension-feeders in the Golfo Marconi (North-western Mediterranean). Estuar. Coast. Shelf S. 1997, 44, 723–736. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R.; Fraschetti, S. A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the Ligurian Sea (northwestern Mediterranean). Cont. Shelf Res. 1995, 15, 1453–1469. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.S.; Yang, Q.; Yang, S.; Yang, G.P.; Sun, Y. Burial fluxes and source apportionment of carbon in culture areas of Sanggou Bay over the past 200 years. Acta Oceanol. Sin. 2015, 34, 23–30. [Google Scholar] [CrossRef]
- Dan, S.F.; Liu, S.M.; Yang, B.; Udoh, E.C.; Umoh, U.; Ewa-Oboho, I. Geochemical discrimination of bulk organic matter in surface sediments of the Cross River estuary system and adjacent shelf, South East Nigeria (West Africa). Sci. Total Environ. 2019, 678, 351–368. [Google Scholar] [CrossRef]
- Brandes, J.A.; Devol, A.H. A global marine-fixed nitrogen isotopic budget: Implications for Holocene nitrogen cycling. Glob. Biogeochem. Cycles 2002, 16, 67-1–67-14. [Google Scholar] [CrossRef]
- Guo, Z.; Juyuan, L.I.; Feng, J.; Ming, F.; Yang, Z. Compound-specific carbon isotope compositions of individual long-chain n-alkanes in severe Asian dust episodes in the North China coast in 2002. Sci. Bull. 2006, 51, 2133–2140. [Google Scholar] [CrossRef]
- Xu, C.; Yang, B.; Dan, S.F.; Zhang, D.; Liao, R.; Lu, D.; Peng, S. Spatiotemporal variations of biogenic elements and sources of sedimentary organic matter in the largest oyster mariculture bay (Maowei Sea), Southwest China. Sci. Total Environ. 2020, 730, 139056. [Google Scholar] [CrossRef] [PubMed]
- Ingall, E.; Jahnke, R. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 1994, 58, 2571–2575. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sanchez-Cabeza, J.A.; Ruiz-Fernández, A.C. 210Pb sediment radiochronology: An integrated formulation and classification of dating models. Geochim. Cosmochim. Acta 2012, 82, 183–200. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Serrano, O.; Masqué, P. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 2018, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Shi, X.; Guo, Z.; Wang, H.; Yang, Z. Sources, dispersal and preservation of sedimentary organic matter in the Yellow Sea: The importance of depositional hydrodynamic forcing. Mar. Geol. 2013, 335, 52–63. [Google Scholar] [CrossRef]
- Dai, J.; Song, J.; Li, X.; Yuan, H.; Li, N.; Zheng, G. Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China. Environ. Pollut. 2007, 145, 656–667. [Google Scholar] [CrossRef]
- Hu, L.; Guo, Z.; Feng, J.; Yang, Z.; Fang, M. Distributions and sources of bulk organic matter and aliphatic hydrocarbons in surface sediments of the Bohai Sea, China. Mar. Chem. 2009, 113, 197–211. [Google Scholar] [CrossRef]
- Zhou, F.X.; Gao, X.L.; Yuan, M.H.; Song, J.M.; Chen, F.J. The distribution and seasonal variations of sedimentary organic matter in the East China Sea shelf. Mar. Pollut. Bull. 2018, 129, 163–171. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, J.; Mao, Y.; Zhang, J.; Fang, J. Contribution of culturing scallop to carbon cycle in Sanggou Bay. Fish. Mod. 2011, 38, 13–16. [Google Scholar]
- Keil, R. Anthropogenic forcing of carbonate and organic carbon preservation in marine sediments. Annu. Rev. Mar. Sci. 2017, 9, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Gao, X.L.; Zhang, Y.; Xing, Q.G.; Tosi, L.; Qin, S. Geochemical characteristics of phosphorus in surface sediments of two major Chinese mariculture areas: The Laizhou Bay and the coastal waters of the Zhangzi Island. Mar. Pollut. Bull. 2014, 83, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xu, S.; Qi, Z.; Zhu, Q.; Huang, H.; Weber, F. Influence of suspended mariculture on vertical distribution profiles of bacteria in sediment from Daya Bay, Southern China. Mar. Pollut. Bull. 2019, 146, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gao, Q.F.; Dong, S.L.; Wang, F.; Li, H.D.; Zhao, K. Effects of abalone (Haliotis discus hannai Ino) and kelp (Saccharina japonica) mariculture on sources, distribution, and preservation of sedimentary organic carbon in Ailian Bay, China: Identified by coupling stable isotopes (δ13C and δ15N) with C/N ratio analyses. Mar. Pollut. Bull. 2019, 141, 387–397. [Google Scholar]
- Tian, Y.; Chen, G.; Lu, H.; Zhu, H.; Ye, Y. Effects of shrimp pond effluents on stocks of organic carbon, nitrogen and phosphorus in soils of Kandelia obovata forests along Jiulong River Estuary. Mar. Pollut. Bull. 2019, 149, 110657. [Google Scholar] [CrossRef]
- Pusceddu, A.; Dell’Anno, A.; Fabiano, M.; Danovaro, R. Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar. Ecol. Prog. Ser. 2009, 375, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Dalu, T.; Cuthbert, R.N.; Chavalala, T.L.; Froneman, P.W.; Wasserman, R.J. Assessing sediment particle-size effects on benthic algal colonisation and total carbohydrate production. Sci. Total Environ. 2020, 710, 136348. [Google Scholar] [CrossRef]
- Cividanes, S.; Incera, M.; Lopez, J. Temporal variability in the biochemical composition of sedimentary organic matter in an intertidal flat of the Galician coast (NW Spain). Oceanol. Acta 2002, 25, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bianchelli, S.; Pusceddu, A.; Buschi, E.; Danovaro, R. Trophic status and meiofauna biodiversity in the Northern Adriatic Sea: Insights for the assessment of good environmental status. Mar. Environ. Res. 2016, 113, 18–30. [Google Scholar] [CrossRef]
- Pusceddu, A.; Fraschetti, S.; Mirto, S.; Holmer, M.; Danovaro, R. Effects of intensive mariculture on sediment biochemistry. Ecol. Appl. 2007, 17, 1366–1378. [Google Scholar] [CrossRef]
- Bongiorni, L.; Mirto, S.; Pusceddu, A.; Danovaro, R. Response of benthic protozoa and thraustochytrid protists to fish farm impact in seagrass (Posidonia oceanica) and soft-bottom sediments. Microb. Ecol. 2005, 50, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, A.; Dell’Anno, A.; Fabiano, M. Organic matter composition in coastal sediments at Terra Nova Bay (Ross Sea) during summer 1995. Polar. Biol. 2000, 23, 288–293. [Google Scholar] [CrossRef]
- Kautsky, N.; Evans, S. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in a Baltic coastal ecosystem. Mar. Ecol. Prog. Ser. 1987, 38, 201–212. [Google Scholar] [CrossRef]
- Miller, D.C.; Norkko, A.; Pilditch, C.A. Influence of diet on dispersal of horse mussel Atrina zelandica biodeposits. Mar. Ecol. Prog. Ser. 2002, 242, 153–167. [Google Scholar] [CrossRef]
- Danovaro, R.; Corinaldesi, C.; La Rosa, T.; Luna, G.; Mazzola, A.; Mirto, S.; Vezzulli, L.; Fabiano, M. Aquaculture impact on benthic microbes and organic matter cycling in coastal Mediterranean sediments: A synthesis. Chem. Ecol. 2003, 19, 59–65. [Google Scholar] [CrossRef]
- Tselepides, A.; Polychronaki, T.; Marrale, D.; Akoumianaki, I.; Dell’Anno, A.; Pusceddu, A.; Danovaro, R. Organic matter composition of the continental shelf and bathyal sediments of the Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 311–344. [Google Scholar] [CrossRef]
- Pusceddu, A.; Fraschetti, S.; Scopa, M.; Rizzo, L.; Danovaro, R. Meiofauna communities, nematode diversity and C degradation rates in seagrass (Posidonia oceanica L.) and unvegetated sediments invaded by the algae Caulerpa cylindracea (Sonder). Mar. Environ. Res. 2016, 119, 88–99. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Y.; Ma, F.; Ren, G.; Xiong, H.; Zhao, P.; Wang, J. Research progress of algae protein. Bot. Res. 2019, 8, 79–87. [Google Scholar]
- Rizzo, L.; Pusceddu, A.; Bianchelli, S.; Fraschetti, S. Potentially combined effect of the invasive seaweed Caulerpa cylindracea (sonder) and sediment deposition rates on organic matter and meiofaunal assemblages. Mar. Environ. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Redfield, A.C. The influence of organisms on the composition of sea-water. Sea 1963, 40, 640–644. [Google Scholar]
- Lu, F.Y.; Liu, Z.Q.; Ji, H.B. Carbon and nitrogen isotopes analysis and sources of organic matter in the upper reaches of the Chaobai River near Beijing, China. Sci. China Earth Sci. 2012, 42, 1912–1922. [Google Scholar] [CrossRef]
- Xia, B.; Cui, Y.; Chen, B.; Cui, Z.; Qu, K.; Ma, F. Carbon and nitrogen isotopes analysis and sources of organic matter in surface sediments from the Sanggou Bay and its adjacent areas, China. Acta Oceanol. Sin. 2014, 33, 48–57. [Google Scholar] [CrossRef]
- Kuwae, M.; Yamaguchi, H.; Tsugeki, N.K.; Miyasaka, H.; Fukumori, K.; Ikehara, M.; Genkai-Kato, M.; Omori, K.; Sugimoto, T.; Ishida, S.; et al. Spatial distribution of organic and sulfur geochemical parameters of oxic to anoxic surface sediments in Beppu Bay in southwest Japan. Estuar. Coast. Shelf Sci. 2007, 72, 348–358. [Google Scholar] [CrossRef]
- Pan, Z.; Tan, Y.M.; Gao, Q.F.; Dong, S.L.; Fang, X.D.; Yan, J.L. A 120-year record of burial fluxes and source apportionment of sedimentary organic carbon in Alian Bay, China: Implication for the influence of mariculture activities, and regional environment changes. Aquaculture 2021, 535, 736421. [Google Scholar] [CrossRef]
- Bianchi, T.S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1984; pp. 196–229. [Google Scholar]
- Torres, I.; Inglett, P.; Brenner, M.; Kenney, W.; Ramesh Reddy, K. Stable isotope (δ13C and δ15N) values of sediment organic matter in subtropical lakes of different trophic status. J. Paleolimnol. 2012, 47, 693–706. [Google Scholar] [CrossRef]
- Pancost, R.D.; Boot, C.S. The palaeoclimatic utility of terrestrial biomarkers in marine sediments. Mar. Chem. 2004, 92, 239–261. [Google Scholar] [CrossRef]
- Liu, D.Y.; Shen, X.H.; Wang, Y.J.; Chen, Y.J.; Li, L. Tracking the sources of organic matter in the surface sediments of Sishili Bay, Northern Yellow Sea and the environmental implication. Acta Oceanol. Sin. 2012, 34, 205–212. [Google Scholar]
- Dan, S.F.; Cui, D.; Yang, B.; Wang, X.; Ning, Z.; Lu, D.; Kang, Z.; Huang, H.; Zhou, J.; Cui, D.; et al. Sources, burial flux and mass inventory of black carbon in surface sediments of the Daya Bay, a typical mariculture bay of China. Mar. Pollut. Bull. 2022, 179, 113708. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Fang, J.; Tang, Q.; Zhang, M.; Du, M. Impact of shellfish biodeposits and rotten seaweed on the sediments of Ailian Bay, China. Aquac. Int. 2013, 22, 811–819. [Google Scholar] [CrossRef]
- Bhushan, R.; Dutta, K.; Somayajulu, B.L.K. Concentrations and burial fluxes of organic and inorganic carbon on the eastern margins of the Arabian Sea. Mar. Geol. 2001, 178, 95–113. [Google Scholar] [CrossRef]
- Gordon, E.S.; Goñi, M.A.; Roberts, Q.N.; Kineke, G.C.; Allison, M.A. Organic matter distribution and accumulation on the inner Louisiana shelf west of the Atchafalaya River. Cont. Shelf Res. 2001, 21, 1691–1721. [Google Scholar] [CrossRef]
- Winogradow, A.; Pempkowiak, J. Organic carbon burial rates in the Baltic Sea sediments. Estuar. Coast. Shelf Sci. 2014, 138, 27–36. [Google Scholar] [CrossRef]
- Berner, R.A. Burial of organic carbon and pyrite sulfur in the modern ocean: Its geochemical and environmental significance. Am. J. Sci. 1982, 282, 451–473. [Google Scholar] [CrossRef]
- Plew, D.R.; Stevens, C.L.; Spigel, R.H.; Hartstein, N.D. Hydrodynamic implications of large offshore mussel farms. IEEE J. Ocean. Eng. 2005, 30, 95–108. [Google Scholar] [CrossRef]
- Shi, J.; Wei, H. Simulation of hydrodynamic structures in a semi-enclosed bay with dense raft-culture. J. Ocean U. China 2009, 39, 1181–1187. [Google Scholar]
- Gao, S. Shallow marine sedimentat ion and physical environment evolution as a part of global change: An example from the Bohai, yellow and East China Sea regions. Earth Sci. Front. 2002, 9, 329–335. [Google Scholar]
- Chen, Y.; Zhao, Y.Y.; Liu, J.Y.; Qiu, J.G. Distribution characteristics of 226Ra in sediments of the East China Sea and determination of sedimentation rate in near-shore region. Oceanol. Limnol. Sin. 1982, 13, 381–387. [Google Scholar]
- Dong, T. Modern sedimentation models in the Bohai Sea. Mar. Geol. Quat. Geol. 1996, 16, 45–53. [Google Scholar]
- Li, F.Y.; Song, J.M.; Li, X.G.; Wang, Y.P.; Qi, J. Modern sedimentation rate and flux in the Jiaozhou Bay. Mar. Geol. Quat. Geol. 2003, 23, 29–33. [Google Scholar]
- Liu, G.S.; Li, D.M.; Yi, Y.; Liu, S.M.; Bai, J.; Zhang, J. Radionuclide distribution in sediments and sedimentary rates in the Jiaozhou Bay. Acta Geosci. Sin. 2008, 29, 769–777. [Google Scholar]
- Hu, B.Q.; Li, G.; Li, J.; Yang, M.; Wang, L.B.; Bu, R.Y. Spatial variability of the 210Pb sedimentation rates in the Bohai and Huanghai Seas and its influencing factors. Acta Oceanol. Sin. 2012, 33, 127–132. [Google Scholar]
- Luo, H.; Liang, B.; Bao, C.G.; Li, M.; Yu, C.Y.; Ma, M.H. Optimization design for marine sediment monitoring frequency based on sedimentation rate. Mar. Sci. 2015, 39, 52–59. [Google Scholar]
- Molinaroli, E.; Guerzoni, S.; Sarretta, A.; Masiol, M.; Pistolato, M. Thirty-year changes (1970 to 2000) in bathymetry and sediment texture recorded in the lagoon of Venice sub-basins, Italy. Mar. Geol. 2009, 258, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Hedges, J.I.; Keil, R.G. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar. Chem. 1995, 49, 123–126. [Google Scholar] [CrossRef]
- McLaren, P.; Bowles, D. The effects of sediment transport on grain-size distributions. J. Sediment. Res. 1985, 55, 457–470. [Google Scholar]
- Biggs, R.B.; Howell, B.A. The estuary as a sediment trap: Alternate approaches to estimating its filtering efficiency. In The Estuary as a Filter; Kennedy, V.S., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 107–129. [Google Scholar]
- Franzo, A.; Cibic, T.; Del, N.P.; Solidoro, C. Microphytobenthic response to mussel farm biodeposition in coastal sediments of the northern Adriatic Sea. Mar. Pollut. Bull. 2014, 79, 379–388. [Google Scholar] [CrossRef]
- Burdige, D.J. Burial of terrestrial organic matter in marine sediments: A reassessment. Global Biogeochem. Cycles 2005, 19, 922–932. [Google Scholar] [CrossRef] [Green Version]
- Hartnett, H.E.; Keil, R.G.; Al, E. Influence of oxygen exposure time on organic carbon preservation in continental marine regions. Nature 1998, 391, 572–575. [Google Scholar] [CrossRef]
- Wiyoto, W.; Sukenda, S.; Harris, E.; Nirmala, K.; Djokosetiyanto, D.; Ekasari, J. The effects of sediment redox potential and stocking density on Pacific white shrimp Litopenaeus vannamei production performance and white spot syndrome virus resistance. Aquac. Res. 2017, 48, 2741–2751. [Google Scholar] [CrossRef]
- Zhou, P.; Li, D.M.; Zhao, L.; Li, H.T.; Ni, Z.X.; Zhao, F.; Yu, H.S.; Li, X.M. 120-year sedimentary record and its environmental implications, in a dated marine sediment core from Daya bay in the northeastern South China Sea. Mar. Pollut. Bull. 2019, 145, 248–253. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Gao, X.; Liu, J.; Xie, L.; Zhao, J.; Xing, Q.; Donnici, S.; Tosi, L.; Tang, C. Biogeochemical Characteristics of Sedimentary Organic Matter in Coastal Waters of a Mariculture Area: The Big Impact of Bay Scallop Farming. Sustainability 2023, 15, 10595. https://doi.org/10.3390/su151310595
Yang B, Gao X, Liu J, Xie L, Zhao J, Xing Q, Donnici S, Tosi L, Tang C. Biogeochemical Characteristics of Sedimentary Organic Matter in Coastal Waters of a Mariculture Area: The Big Impact of Bay Scallop Farming. Sustainability. 2023; 15(13):10595. https://doi.org/10.3390/su151310595
Chicago/Turabian StyleYang, Bo, Xuelu Gao, Jin Liu, Lei Xie, Jianmin Zhao, Qianguo Xing, Sandra Donnici, Luigi Tosi, and Cheng Tang. 2023. "Biogeochemical Characteristics of Sedimentary Organic Matter in Coastal Waters of a Mariculture Area: The Big Impact of Bay Scallop Farming" Sustainability 15, no. 13: 10595. https://doi.org/10.3390/su151310595





