Replacing Mineral Fertilizer with Nitrified Human Urine in Hydroponic Lettuce (Lactuca sativa L.) Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up, Plant Material, and Growing Conditions
2.2. Nutrient Solution Treatments
2.3. Nutrient Solution Analyses
2.4. Calculation of Fertilizer Savings and Carbon Footprints
2.5. Yield and Product Quality Parameters
2.6. Statistical Analysis
3. Results
3.1. Nutrient Solution Salinity and Fertilizer Savings
3.2. Carbon Footprint of Fertilizer Production and Wastewater Treatment
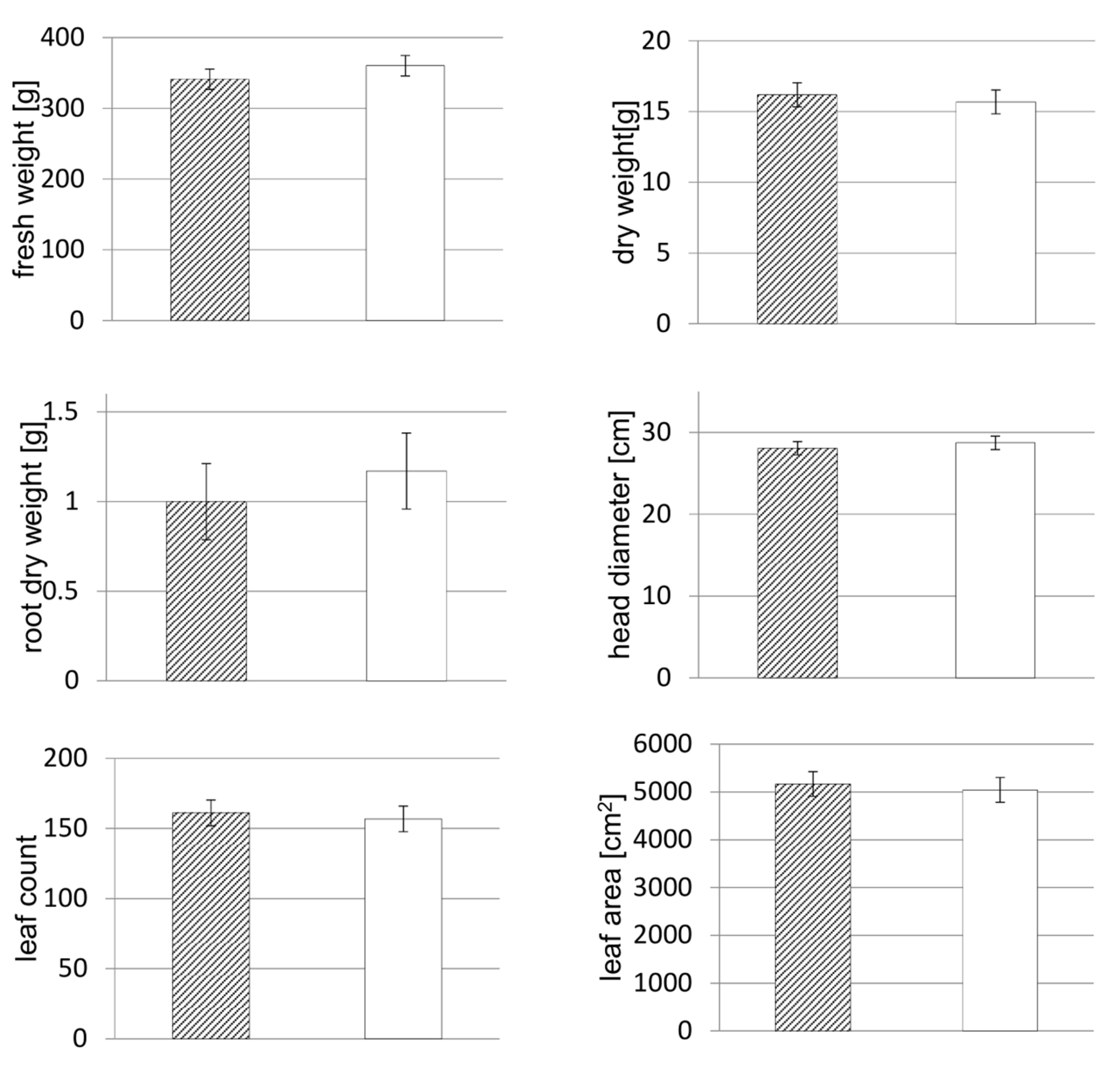
3.3. Yield and Morphological Parameters
3.4. Product Quality
4. Discussion
4.1. Yield, Salinity of the Nutrient Solution, and Cations in Plant Tissue
4.2. Fertilizer Savings and Carbon Footprint
4.3. Product Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spangberg, J.; Tidaker, P.; Jönsson, H. Environmental Impact of Recycling Nutrients in Human Excreta to Agriculture Compared with Enhanced Wastewater Treatment. Sci. Total Environ. 2014, 493, 209–219. [Google Scholar] [CrossRef]
- International Fertilizer Industry Association. Fertilizers, Climate Change and Enhancing Agricultural Productivity Sustainably; International Fertilizer Industry Association: Paris, France, 2009. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Nziguheba, G.; Smolders, E. Inputs of Trace Elements in Agricultural Soils via Phosphate Fertilizers in European Countries. Sci. Total Environ. 2008, 390, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.A.; Gujer, W. Separate Management of Anthropogenic Nutrient Solutions (Human Urine). Water Sci. Technol. 1996, 34, 87–94. [Google Scholar] [CrossRef]
- STOWA. Separate Urine Collection and Treatment: Options for Sustainable Wastewater Systems and Mineral Recovery. In STOWA Report 2001.39; Wilsenach, J., van Loosdrecht, M., Eds.; STOWA: Utrecht, The Netherlands, 2001. [Google Scholar]
- Jönsson, H.; Stenström, T.-A.; Svensson, J.; Sundin, A. Source Separated Urine-Nutrient and Heavy Metal Content, Water Saving and Faecal Contamination. Water Sci. Technol. 1997, 35, 145–152. [Google Scholar] [CrossRef]
- Fumasoli, A.; Etter, B.; Sterkele, B.; Morgenroth, E.; Udert, K.M. Operating a Pilot-Scale Nitrification/Distillation Plant for Complete Nutrient Recovery from Urine. Water Sci. Technol. 2016, 73, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Schwegler, P.; Larsen, T.A. Nutrients in Urine: Energetic Aspects of Removal and Recovery. Water Sci. Technol. 2003, 48, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Simha, P.; Ganesapillai, M. Ecological Sanitation and Nutrient Recovery from Human Urine: How Far Have We Come? A Review. Sustain. Environ. Res. 2017, 27, 107–116. [Google Scholar] [CrossRef]
- Bornemann, G.; Waßer, K.; Hauslage, J. The Influence of Nitrogen Concentration and Precipitation on Fertilizer Production from Urine Using a Trickling Filter. Life Sci. Space Res. 2018, 18, 12–20. [Google Scholar] [CrossRef]
- Udert, K.M.; Wächter, M. Complete Nutrient Recovery from Source-Separated Urine by Nitrification and Distillation. Water Res. 2012, 46, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Özel Duygan, B.D.; Udert, K.M.; Remmele, A.; McArdell, C.S. Removal of Pharmaceuticals from Human Urine during Storage, Aerobic Biological Treatment, and Activated Carbon Adsorption to Produce a Safe Fertilizer. Resour. Conserv. Recycl. 2021, 166, 105341. [Google Scholar] [CrossRef]
- Muskolus, A. Anthropogenic Plant Nutrients as Fertiliser. Ph.D. Thesis, Humboldt-University of Berlin, Berlin, Germany, 2008. [Google Scholar]
- Pradhan, S.K.; Holopainen, J.K.; Heinonen-Tanski, H. Stored Human Urine Supplemented with Wood Ash as Fertilizer in Tomato (Solanum lycopersicum) Cultivation and Its Impacts on Fruit Yield and Quality. J. Agric. Food Chem. 2009, 57, 7612–7617. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Nerg, A.M.; Sjöblom, A.; Holopainen, J.K.; Heinonen-Tanski, H. Use of Human Urine Fertilizer in Cultivation of Cabbage (Brassica oleracea)—Impacts on Chemical, Microbial, and Flavor Quality. J. Agric. Food Chem. 2007, 55, 8657–8663. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, C.; Etter, B.; Udert, K.M.; Frossard, E.; Nanzer, S.; Tamburini, F.; Oberson, A. Plant Uptake of Phosphorus and Nitrogen Recycled from Synthetic Source-Separated Urine. Ambio 2015, 44, 217–227. [Google Scholar] [CrossRef]
- Zabel, P.; Bornemann, G.; Tajmar, M.; Schubert, D. Yield of Dwarf Tomatoes Grown with a Nutrient Solution Based on Recycled Synthetic Urine. Life Sci. Space Res. 2019, 20, 62–71. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Geelen, D.; De Paepe, J.; Clauwaert, P.; De Pascale, S.; Rouphael, Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability 2021, 13, 4218. [Google Scholar] [CrossRef]
- Mauerer, M.; Rocksch, T.; Dannehl, D.; Schuch, I.; Mewis, I.; Förster, N.; Ulrichs, C.; Schmidt, U. Impact of Different Concentrations of Nitrified Urine in a Recirculating Nutrient Solution on Growth, Yield and Quality of Lettuce. DGG-Proceedings 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Halbert-Howard, A.; Häfner, F.; Karlowsky, S.; Schwarz, D.; Krause, A. Evaluating Recycling Fertilizers for Tomato Cultivation in Hydroponics, and Their Impact on Greenhouse Gas Emissions. Environ. Sci. Pollut. Res. 2021, 28, 59284–59303. [Google Scholar] [CrossRef]
- Wdowikowska, A.; Reda, M.; Kabała, K.; Chohura, P.; Jurga, A.; Janiak, K.; Janicka, M. Water and Nutrient Recovery for Cucumber Hydroponic Cultivation in Simultaneous Biological Treatment of Urine and Grey Water. Plants 2023, 12, 1286. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of Vegetable Crops to Salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J.; Ephrath, J.E. A Model for Nutrient and Water Flow and Their Uptake by Plants Grown in a Soilless Culture. Plant Soil 2005, 271, 309–319. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffmann, G.J. Crop Salt Tolerance—Current Assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Tas, G.; Papadandonakis, N.; Savvas, D. Responses of Lettuce (Lactuca sativa L. var. longifolia) Grown in a Closed Hydroponic System to NaCl- or CaCl2-Salinity. J. Appl. Bot. Food Qual. 2005, 79, 136–140. [Google Scholar]
- Göhler, F.; Molitor, H.-D. Kopfsalat. In Erdelose Kulturverfahren im Gartenbau; Göhler, F., Molitor, H.-D., Eds.; Eugen Ulmer: Stuttgart, Germany, 2002; pp. 227–231. ISBN 3-8001-5053-0. [Google Scholar]
- Andriolo, J.L.; da Luz, G.L.; Witter, M.H.; Godoi, R.d.S.; Barros, G.T.; Bortolotto, O.C. Growth and Yield of Lettuce Plants under Salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef]
- Collier, G.F.; Tibbitts, T.W. Tipburn of Lettuce. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1982; Volume 4, pp. 49–65. [Google Scholar]
- Köpping, I.; McArdell, C.S.; Borowska, E.; Böhler, M.A.; Udert, K.M. Removal of Pharmaceuticals from Nitrified Urine by Adsorption on Granular Activated Carbon. Water Res. X 2020, 9, 100057. [Google Scholar] [CrossRef]
- Dannehl, D.; Huyskens-Keil, S.; Wendorf, D.; Ulrichs, C.; Schmidt, U. Influence of Intermittent-Direct-Electric-Current (IDC) on Phytochemical Compounds in Garden Cress during Growth. Food Chem. 2012, 131, 239–246. [Google Scholar] [CrossRef]
- VDLUFA. Bestimmung von Mineralischem Stickstoff (Nitrat Und Ammonium) in Bodenprofilen A 6.1.4.1. In Band I: Die Untersuchung von Böden, 3. Teillfg; VDLUFA-Verlag: Darmstadt, Germany, 2002; ISBN 978-3-941273-13-9. [Google Scholar]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced Aquaponics: Evaluation of Intensive Tomato Production in Aquaponics vs. Conventional Hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Raaphorst, M.G.M.; Benninga, J. Kwantitatieve Informatie Voor de Glastuinbou w 2019: Kengetallen Voor Groenten-, Snijbloemen-, Pot-En Perkplanten Teelten; Wageningen UR: Wageningen, The Netherlands, 2019. [Google Scholar]
- Blom, T.; Jenkins, A.; Pulselli, R.M.; van den Dobbelsteen, A.A.J.F. The Embodied Carbon Emissions of Lettuce Production in Vertical Farming, Greenhouse Horticulture, and Open-Field Farming in the Netherlands. J. Clean. Prod. 2022, 377, 134443. [Google Scholar] [CrossRef]
- Brentrup, F.; Lammel, J.; Stephani, T.; Christensen, B. Updated Carbon Footprint Values for Mineral Fertilizer from Different World Regions. In Proceedings of the 11th International Conference on Life Cycle Assessment of Food 2018 (LCA Food) in Conjunction with the 6th LCA AgriFood Asia and 7th International Conference on Green and Sustainable Innovation (ICGSI) on “Global Food Challenges towards Sustainable Consum”, Bangkok, Thailand, 17–19 October 2018. [Google Scholar]
- Umweltbundesamt. Prozessorientierte Basisdaten Für Umweltmanagement-Instrumente (ProBas). Available online: https://www.probas.umweltbundesamt.de/ (accessed on 6 March 2023).
- Faust, V.; Gruber, W.; Ganigué, R.; Vlaeminck, S.E.; Udert, K.M. Nitrous Oxide Emissions and Carbon Footprint of Decentralized Urine Fertilizer Production by Nitrification and Distillation. ACS EST Eng. 2022, 2, 1745–1755. [Google Scholar] [CrossRef]
- ISO 19747:2009-07; Investigation of Solids—Pre-Treatment, Preparation and Processing of Samples for Chemical, Biological and Physical Investigations. DIN: Berlin, Germany, 2009.
- ISO 15936:2012; Sludge, Treated Biowaste, Soil and Waste—Determination of Total Organic Carbon (TOC) by Dry Combustion. DIN: Berlin, Germany, 2012.
- ISO 16168:2012-11; Sludge, Treated Biowaste and Soil—Determination of Total Nitrogen Using Dry Combustion Method. DIN: Berlin, Germany, 2012.
- Taylor, K.L.; Brackenridge, A.E.; Vivier, M.A.; Oberholster, A. High-Performance Liquid Chromatography Profiling of the Major Carotenoids in Arabidopsis Thaliana Leaf Tissue. J. Chromatogr. A 2006, 1121, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mewis, I.; Smetanska, I.M.; Müller, C.T.; Ulrichs, C. Specific Poly-Phenolic Compounds in Cell Culture of Vitis Vinifera L. Cv. Gamay Fréaux. Appl. Biochem. Biotechnol. 2011, 164, 148–161. [Google Scholar] [CrossRef]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype Variability in Growth and Secondary Metabolite Profile in Moringa Oleifera: Impact of Sulfur and Water Availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef]
- De Pascale, S.; Barbieri, G. Effects of Soil Salinity from Long-Term Irrigation with Saline-Sodic Water on Yield and Quality of Winter Vegetable Crops. Sci. Hortic. 1995, 64, 145–157. [Google Scholar] [CrossRef]
- Sonneveld, C.; van den Bos, A.L.; Voogt, W. Modeling Osmotic Salinity Effects on Yield Characteristics of Substrate-Grown Greenhouse Crops. J. Plant Nutr. 2005, 27, 1931–1951. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Influence of NaCl and Calcium Nitrate on Lettuce and Endive Growth Using Nutrient Film Technique. Int. J. Veg. Sci. 2008, 15, 44–56. [Google Scholar] [CrossRef]
- Marschner’s Mineral Nutrition of Plants; Rengel, Z., Cakmak, I., White, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780323853521. [Google Scholar]
- Resh, H.M. 3 Nutrient Solution. In Hydroponic Food Production. A Definitive Guidebook for the Advanced Home Gardener; Woodbridge Press: New York, NY, USA, 2013; pp. 31–88. [Google Scholar]
- Commission of the European Communities. Commission Regulation (EC) No 118/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Gunes, A.; Post, W.N.K.; Kirkby, E.A.; Aktas, M. Influence of Partial Replacement of Nitrate by Amino Acid Nitrogen or Urea in the Nutrient Medium on Nitrate Accumulation in NFT Grown Winter Lettuce. J. Plant Nutr. 1994, 17, 1929–1938. [Google Scholar] [CrossRef]
- Caldwell, C.R.; Britz, S.J. Effect of Supplemental Ultraviolet Radiation on the Carotenoid and Chlorophyll Composition of Green House-Grown Leaf Lettuce (Lactuca sativa L.) Cultivars. J. Food Compos. Anal. 2006, 19, 637–644. [Google Scholar] [CrossRef]
- Winter, M.; Herrmann, K. Esters and Glucosides of Hydroxycinnamic Acids in Vegetables. J. Agric. Food Chem. 1986, 34, 616–620. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Dae, Y.K. Salt in Irrigation Water Affects the Nutritional and Visual Properties of Romaine Lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Huang, J.; Gruber, M.Y.; Kaddour, R.; Lachaâl, M.; Ouerghi, Z.; Hannoufa, A. The Impact of Genotype and Salinity on Physiological Function, Secondary Metabolite Accumulation, and Antioxidative Responses in Lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef] [PubMed]

| Element | Unit | Concentration |
|---|---|---|
| NH4-N | 26.3 | |
| NO3-N | 31.9 | |
| Total N | 58.2 | |
| P | 4.4 | |
| K | 24.1 | |
| Na | [g/L] | 28.1 |
| Cl | 36.0 | |
| Ca | 1.3 | |
| Mg | 0.2 | |
| S | 5.7 | |
| Fe | 3.8 | |
| B | 42.6 | |
| Cu | 9.4 | |
| Mn | [mg/L] | 0.6 |
| Mo | 0.5 | |
| Zn | 23.2 |
| Fertilizer | Unit | Control | Aurin + mf | Product |
|---|---|---|---|---|
| Nitrified urine fertilizer | [L] | 0 | 0.432 | Aurin 1 |
| CaNO3 | [g] | 61.171 | 78.741 | YaraLiva Calcinit 2 |
| KH2PO4 | [g] | 62.252 | 57.285 | YaraTera KRISTA MKP 2 |
| KNO3 | [g] | 138.752 | 113.038 | YaraTera KRISTA K PLUS 2 |
| KAS (76% NH4NO3 + 24% CaCO3) | [g] | 90.614 | 0 | Triferto KAS 27% 3 |
| Yara Krista MgS | [g] | 28.412 | 28.963 | Yara Krista MgS 2 |
| Fe-EDTA 13% | [g] | 3.759 | 4.017 | COMPO Fetrilon 13 4 |
| Microelement stock | [L] | 0.037 | 0.032 | Premixed stock solution 5 |
| KOH 20% | [L] | 0.054 | 0.059 | Potash lye 6 |
| H3PO4 2.5% | [L] | 0.237 | 0.205 | Phosphoric acid 6 |
| Control | Aurin + mf | ||||
|---|---|---|---|---|---|
| Unit | Mean | SD | Mean | SD | |
| Y 1 | [g] | 8188 | 387 | 8647 | 195 |
| FN 2 | [g] | 30.8 | 3.0 | 33.5 | 0.9 |
| NUEN 3 | - | 268 | 31 | 258 | 9 |
| FK 2 | [g] | 34.7 | 0.9 | 34.5 | 3.3 |
| NUEK 3 | - | 236.0 | 16.2 | 252.3 | 26.6 |
| FP 2 | [g] | 10.6 | 1.3 | 10.9 | 0.7 |
| NUEP 3 | - | 784.9 | 136.4 | 795.5 | 55.4 |
| Unit | Control | Aurin + mf | |
|---|---|---|---|
| Mineral fertilizers used | - | CaNO3, KAS, KH2PO4, KNO3 | CaNO3, KH2PO4, KNO3 |
| Mineral fertilizer amounts | [kg ha−1] | 2115.49, 3133.72, 2152.88, 4798.52 | 2723.11, 1981.11, 3909.24 |
| CF mineral fertilizers | [CO2-eq ha−1] | 12,801.80 | 8930.08 |
| N from Aurin® fertilizer | [kg ha−1] | - | 869.90 |
| CF urine fertilizer | [CO2-eq ha−1] | - | 8003.1 |
| CF wastewater treatment | [CO2-eq ha−1] | 8503.24 | - |
| CF Total | [CO2-eq ha−1] | 21,305.05 | 16,933.13 |
| Control | Aurin + mf | |
|---|---|---|
| [µg/g Dry Weight] 1 | ||
| Chlorophyll a | 4287.68 (±197.50) | 4206.54 (±626.96) |
| Chlorophyll b | 2332.45 (±181.96) | 2334.73 (±424.07) |
| Cis-β-carotene | 16.53 (±2.21) | 16.26 (±3.14) |
| Lutein | 391.48 (±36.67) | 341.20 (±43.98) |
| Neoxanthin | 160.80 (±15.70) | 154.63 (±30.31) |
| Trans-β-carotene | 105.95 (±14.57) | 102.90 (±20.25) |
| Control | Aurin + mf | |
|---|---|---|
| [nmol/g Dry Weight] 1 | ||
| Caffeoylmalic acid | 522.33 (±179.87) | 535.44 (±233.12) |
| Caffeoylquinic acid | 665.81 (±207.43) | 750.09 (±315.18) |
| Caffoyltartaric acid | 414.27 (±105.56) | 494.97 (±177.46) |
| Dicaffeoylquinic acid | 67.74 (±7.77) | 102.70 (±59.98) |
| Dicaffeoyltartaric acid | 2071.25 (±583.11) | 2441.38 (±971.56) |
| Quercetin-3-O (6-malonyl) glucoside | 15.38 (±5.57) | 16.36 (±6.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauerer, M.; Rocksch, T.; Dannehl, D.; Schuch, I.; Mewis, I.; Förster, N.; Ulrichs, C.; Schmidt, U. Replacing Mineral Fertilizer with Nitrified Human Urine in Hydroponic Lettuce (Lactuca sativa L.) Production. Sustainability 2023, 15, 10684. https://doi.org/10.3390/su151310684
Mauerer M, Rocksch T, Dannehl D, Schuch I, Mewis I, Förster N, Ulrichs C, Schmidt U. Replacing Mineral Fertilizer with Nitrified Human Urine in Hydroponic Lettuce (Lactuca sativa L.) Production. Sustainability. 2023; 15(13):10684. https://doi.org/10.3390/su151310684
Chicago/Turabian StyleMauerer, Mareike, Thorsten Rocksch, Dennis Dannehl, Ingo Schuch, Inga Mewis, Nadja Förster, Christian Ulrichs, and Uwe Schmidt. 2023. "Replacing Mineral Fertilizer with Nitrified Human Urine in Hydroponic Lettuce (Lactuca sativa L.) Production" Sustainability 15, no. 13: 10684. https://doi.org/10.3390/su151310684
APA StyleMauerer, M., Rocksch, T., Dannehl, D., Schuch, I., Mewis, I., Förster, N., Ulrichs, C., & Schmidt, U. (2023). Replacing Mineral Fertilizer with Nitrified Human Urine in Hydroponic Lettuce (Lactuca sativa L.) Production. Sustainability, 15(13), 10684. https://doi.org/10.3390/su151310684








