Sod Culture with Vicia villosa Alters the Diversity of Fungal Communities in Walnut Orchards for Sustainability Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Soil Sampling and Experimental Design
2.3. Determinations of Root AMF Colonization Rate and Soil Mycelial Length
2.4. DNA Extraction, PCR Amplification, and Illumina Sequencing
2.5. Data Analysis
3. Results
3.1. Changes in Sequencing Data and OTUs
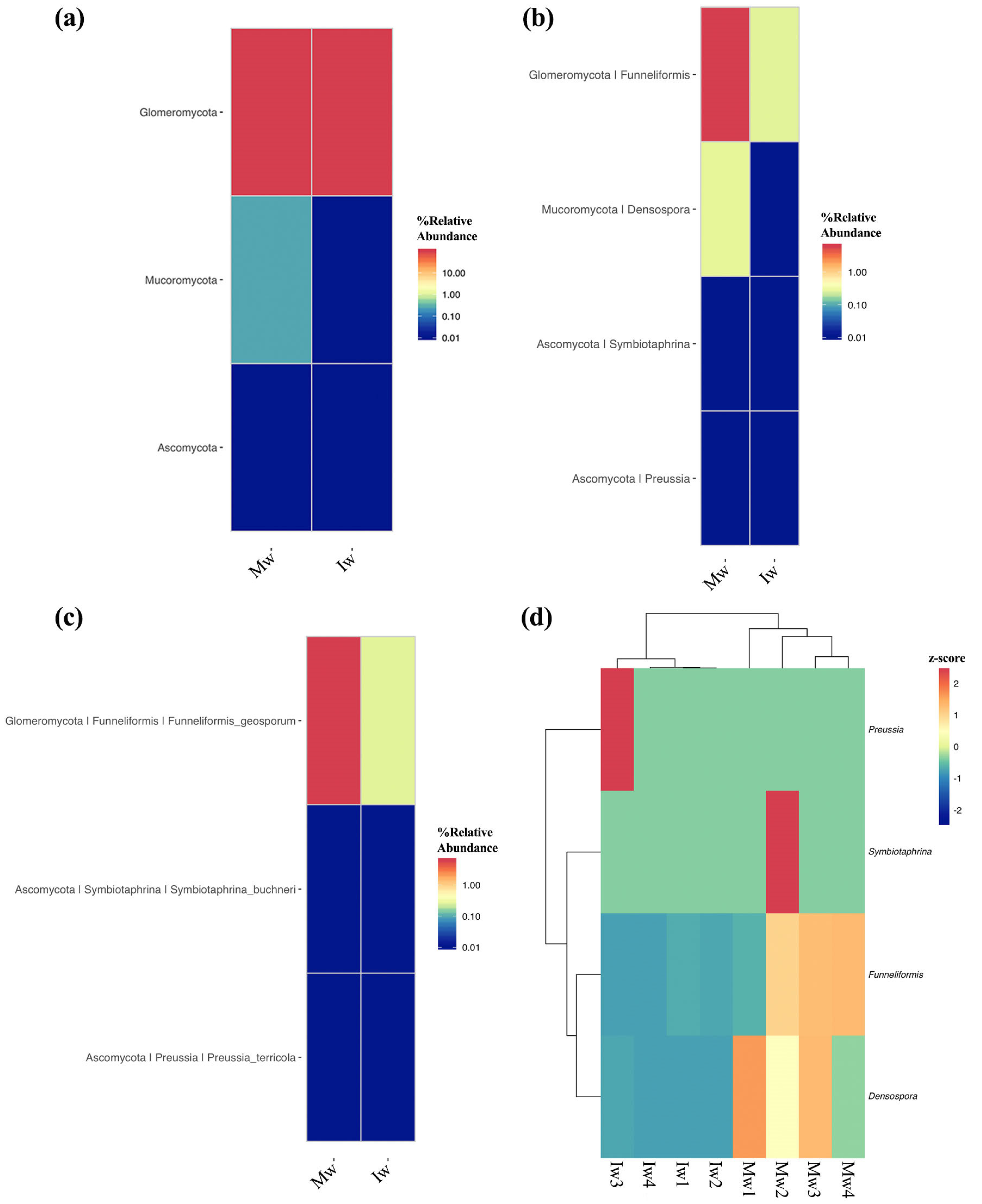
3.2. Changes in the Structural Composition of Soil Fungal Communities at the Phylum, Genus, and Species Level
3.3. Changes in the Diversity of Soil Fungal Communities
3.4. Changes in Mycorrhizal Fungal Growth in Soil and Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Li, C.; Cao, C.; Wang, L.; Li, X.; Che, J.; Yang, H.; Zhang, X.; Zhao, H.; He, G.; et al. Walnut fruit processing equipment: Academic insights and perspectives. Food Eng. Rev. 2021, 13, 822–857. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ali, S.; Ouyang, Q.; Li, H.; Wu, X.; Hashim, M.M.; Javaria, S.; Chen, Q. Application of benchtop NIR spectroscopy coupled with multivariate analysis for rapid prediction of antioxidant properties of walnut (Juglans regia). Food Chem. 2021, 359, 129928. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Paraskevopoulou, A.; Samanidou, V. Exploring the volatile metabolome of conventional and organic walnut oils by solid-phase microextraction and analysis by GC-MS combined with chemometrics. Food Chem. 2021, 363, 130331. [Google Scholar] [CrossRef]
- Liu, W.; Pu, X.; Sun, J.; Shi, X.; Cheng, W.; Wang, B. Effect of Lactobacillus plantarum on functional characteristics and flavor profile of fermented walnut milk. LWT—Food Sci. Technol. 2022, 160, 113254. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Brodribb, T.J.; Wang, J.; Yao, X.; Li, S. Long term effects of management practice intensification on soil microbial community structure and co-occurrence network in a non-timber plantation. For. Ecol. Manag. 2020, 459, 117805. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, B.X.; Xu, C.Y.; Raza, M.; Wang, Q.; Wang, Q.Z.; Fu, Y.N.; Hu, J.Y.; Imoulan, A.; Hussain, M.; et al. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Mwakilili, A.D.; Mwaikono, K.S.; Herrera, S.L.; Midega, C.A.; Magingo, F.; Alsanius, B.; Dekker, T.; Lyantagaye, S.L. Long-term maize-Desmodium intercropping shifts structure and composition of soil microbiome with stronger impact on fungal communities. Plant Soil 2021, 467, 437–450. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Du, H.; Zhu, W.; Zhou, Y.; Yin, Y. Effects of intercropping between Morus alba and nitrogen fixing species on soil microbial community structure and diversity. Forests 2022, 13, 1345. [Google Scholar] [CrossRef]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Liu, C.; Wang, X.; Tian, Q.; Jiao, W.; Hu, J.; Liu, L.; et al. Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef]
- Alami, M.M.; Xue, J.; Ma, Y.; Zhu, D.; Abbas, A.; Gong, Z.; Wang, X. Structure, function, diversity, and composition of fungal communities in rhizospheric soil of Coptis chinensis Franch under a successive cropping system. Plants 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yan, W.; Wu, H.; Zhang, J.; Zhang, Z.; Zhang, Z.; Rensing, C.; Lin, W. Consecutive monoculture regimes differently affected the diversity of the rhizosphere soil viral community and accumulated soil-borne plant viruses. Agric. Ecosyst. Environ. 2022, 337, 108076. [Google Scholar] [CrossRef]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microb. Ecol. 2018, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, H.; Jiao, Y.; Zhang, Z.; Rensing, C.; Lin, W. The combination of biochar and PGPBs stimulates the differentiation in rhizosphere soil microbiome and metabolites to suppress soil-borne pathogens under consecutive monoculture regimes. GCB Bioenergy 2022, 14, 84–103. [Google Scholar] [CrossRef]
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Amjad, H.; Awais, S.; Lin, X.; Lin, W.; Lin, S. Long-term monoculture negatively regulates fungal community composition and abundance of tea orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pan, F.; Wang, Q.; Luo, J.; Zhang, Q.; Pan, Y.; Wu, C.; Liu, W. The effect of different remediation treatments on soil fungal communities in rare earth tailings soil. Forests 2022, 13, 1987. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.; Lin, Y.; Luo, J.; Di, H.J.; Lindsey, S.; Liu, D.; Fan, J.; Ding, W. Responses of soil fungal diversity and community composition to long-term fertilization: Field experiment in an acidic Ultisol and literature synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.N.; Shu, B.; Wu, Q.S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Carron, A.I.; Garibaldi, L.A.; Marquez, S.; Fontenla, S. The soil fungal community of native woodland in Andean Patagonian forest: A case study considering experimental forest management and seasonal effects. For. Ecol. Manag. 2020, 461, 117955. [Google Scholar] [CrossRef]
- Odelade, K.A.; Babalola, O.O. Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int. J. Environ. Res. Public Health 2019, 16, 3873. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Yang, J.; Su, D.; Li, Z.; Xiao, W.; Wang, Y.; Cui, X. Comparative analysis of fungal diversity in rhizospheric soil from wild and reintroduced Magnolia sinica estimated via high-throughput sequencing. Plants 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Yuan, P.; Ullah, S.; Iqbal, A.; Zhao, Q.; Liang, H.; Khan, A.; Imran; Zhang, H.; Wu, X.; et al. Biochar amendment and nitrogen fertilizer contribute to the changes in soil properties and microbial communities in a Paddy field. Front. Microbiol. 2022, 13, 834751. [Google Scholar] [CrossRef]
- Ma, W.Y.; Wu, Q.S.; Xu, Y.J.; Kuča, K. Exploring mycorrhizal fungi in walnut with a focus on physiological roles. Not. Bot. Horti Agrobo. 2021, 49, 12363. [Google Scholar] [CrossRef]
- Huang, G.M.; Zou, Y.N.; Wu, Q.S.; Xu, Y.J.; Kuča, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Zou, Y.N.; Xu, Y.J.; Liu, R.C.; Huang, G.M.; Kuča, K.; Srivastava, A.K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Two different strategies of Diversispora spurca-inoculated walnut seedlings to improve leaf P acquisition at low and moderate P levels. Front. Plant Sci. 2023, 14, 1140467. [Google Scholar] [CrossRef] [PubMed]
- Mortier, E.; Lamotte, O.; Martin-Laurent, F.; Recorbet, G. Forty years of study on interactions between walnut tree and arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2020, 40, 43. [Google Scholar] [CrossRef]
- Achatz, M.; Rillig, M.C. Arbuscular mycorrhizal fungal hyphae enhance transport of the allelochmical juglone in the field. Soil Biol. Biochem. 2014, 78, 76–82. [Google Scholar] [CrossRef]
- Webber, S.M.; Bailey, A.P.; Huxley, T.; Potts, S.G.; Lukac, M. Traditional and cover crop-derived mulches enhance soil ecosystem services in apple orchards. Appl. Soil Ecol. 2022, 178, 104569. [Google Scholar] [CrossRef]
- Wang, R.; Cao, B.; Sun, Q.; Song, L. Response of grass interplanting on bacterial and fungal communities in a jujube orchard in Ningxia, northwest China. Heliyon 2020, 6, e03489. [Google Scholar] [CrossRef]
- Xiao, L.; Lai, S.; Chen, M.; Long, X.; Fu, X.; Yang, H. Effects of grass cultivation on soil arbuscular mycorrhizal fungi community in a tangerine orchard. Rhizosphere 2022, 24, 100583. [Google Scholar] [CrossRef]
- Cloutier, M.L.; Murrell, E.; Barbercheck, M.; Kaye, J.; Finney, D.; García-González, I.; Bruns, M.A. Fungal community shifts in soils with varied cover crop treatments and edaphic properties. Sci. Rep. 2020, 10, 6198. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Yan, Z.; Zhang, W.; Duan, T. Green manure crops affected soil chemical properties and fungal diversity and community of apple orchard in the Loess Plateau of China. J. Soil Sci. Plant Nut. 2021, 21, 1089–1102. [Google Scholar] [CrossRef]
- Qian, Y.L.; Wang, X.Z.; Lai, X.F.; Li, J.C.; Shen, Y.Y. Effects of perennial forage on characteristics of the soil fungal community in an apple orchard. Acta Pratac. Sin. 2019, 28, 124–132. [Google Scholar]
- Grant, J.; Anderson, K.K.; Prichard, T.; Bugg, R.L.; Thomas, F.; Johnson, T. Cover Crops for Walnut Orchards, 1st ed.; UCANR Publications: Davis, CA, USA, 2006; pp. 1–19. [Google Scholar]
- Xu, Y.J.; Fu, Y.N.; Chen, Y.X.; Xu, C.Y.; Liao, S.; Wang, Q.Z.; Xu, C.Y. Decomposition dynamics and nutrient release of Vicia villosa in walnut forest. J. Inner Mongolia Agric. Univ. (Nat. Sci. Ed.) 2022, 43, 24–29. [Google Scholar]
- Jiang, L.L.; Sun, R.H.; Zhang, G.Y.; Gong, Q.T.; Wu, H.B.; Du, X.K. Effects of Vicia villosa Roth cultivation on soil microbial community structure in apple orchards. Acta Agric. Boreali Sin. 2022, 37, 291–299. [Google Scholar]
- Guo, Y.; Nie, C.J.; Xiang, Y.Z.; Xu, H.; Liu, X.; Zeng, H.; Li, C. Effect of different agroferestry patterns on soil fertility in Juglans region Orchards. Chin. J. Soil Sci. 2016, 47, 391–397. [Google Scholar]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Xiang, X.; Xu, H.; Han, G. Analysis of microbial diversity and succession during Xiaoqu Baijiu fermentation using high-throughput sequencing technology. Eng. Life Sci. 2022, 22, 495–504. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Liu, S.; Xu, S.; Wang, F.; Chen, H.; Liu, Y. Use of high-throughput sequencing to identify fungal communities on the surface of citri reticulatae pericarpium during the 3-year aging process. Curr. Microbiol. 2021, 78, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.J.; Yu, T.T.; Wang, J.M.; Gao, Y.B.; Wang, S.Z.; Li, Z.; Yun, X.F. Soil fungal ITS diversity in cucumber-celery intercropping. Chin. J. Eco-Agric. 2019, 27, 529–536. [Google Scholar]
- Wang, Y.; Liu, L.; Yang, J.; Duan, Y.; Luo, Y.; Taherzadeh, M.J.; Li, Y.; Li, H.; Awasthi, M.K.; Zhao, Z. The diversity of microbial community and function varied in response to different agricultural residues composting. Sci. Total Environ. 2020, 715, 136983. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Campbell, B.C. Regulation of aflatoxin production by naphthoquinones of walnut (Juglans regia). J. Agric. Food Chem. 2000, 48, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Mao, Y.F.; Hu, Y.L.; Zhang, L.L.; Yin, Y.J.; Pang, H.l.; Su, X.F.; Yang, L.; Shen, X. Effects of grass planting in apple orchard on soil microbial diversity, enzyme activities and carbon components. J. Plant Nutr. Fertil. 2021, 27, 1792–1805. [Google Scholar]
- Ma, W.; Yang, Z.; Hou, S.; Ma, Q.; Liang, L.; Wang, G.; Liang, C.; Zhao, T. Effects of living cover on the soil microbial communities and ecosystem functions of hazelnut orchards. Front. Plant Sci. 2021, 12, 652493. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Liang, C.F.; Jin, J.; Wang, X.X.; Ye, Z.H.; Wu, J.S. Effects of long-term sod cultivation on chinese hickory plantation soil fungal community and enzyme activities. Environ. Sci. 2023, 44, 2945–2954. [Google Scholar]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Mao, J.H.; Li, R.B.; Jing, Y.B.; Ning, D.L.; Li, Y.P.; Chen, H.Y. Arbuscular mycorrhizal fungi associated with walnut trees and their effect on seedling growth. J. Forest Environ. 2022, 42, 71–80. [Google Scholar]
- Xiang, D.; Veresoglou, S.D.; Rillig, M.C.; Xu, T.; Li, H.; Hao, Z.; Chen, B. Relative importance of individual climatic drivers shaping arbuscular mycorrhizal fungal communities. Microb. Ecol. 2016, 72, 418–427. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zhang, Y.C.; Zhang, Z.Z.; Srivastava, A.K. Underground communication of root hormones by common mycorrhizal network between trifoliate orange and white clover. Arch. Agron. Soil Sci. 2017, 63, 1187–1197. [Google Scholar] [CrossRef]
- He, W.X.; Wu, Q.S.; Hashem, A.; Abd_Allah, E.F.; Muthuramalingam, P.; Al-Arjani, A.-B.F.; Zou, Y.N. Effects of symbiotic fungi on sugars and soil fertility and structure-mediated changes in plant growth of Vicia villosa. Agriculture 2022, 12, 1523. [Google Scholar] [CrossRef]
- Draghi, W.O.; Alvarez, F.; Russo, D.M.; Lagares, A.; Wall, L.G.; Zorreguieta, A. Root-associated Burkholderia spp. on the hairy vetch (Vicia villosa Roth.) cover crop vary depending on soil history of use. Rhizosphere 2021, 17, 100297. [Google Scholar] [CrossRef]
- Pott, L.P.; Amado, T.J.C.; Schwalbert, R.A.; Gebert, F.H.; Reimche, G.B.; Pes, L.Z.; Ciampitti, I.A. Effect of hairy vetch cover crop on maize nitrogen supply and productivity at varying yield environments in Southern Brazil. Sci. Total Environ. 2021, 759, 144313. [Google Scholar] [CrossRef] [PubMed]
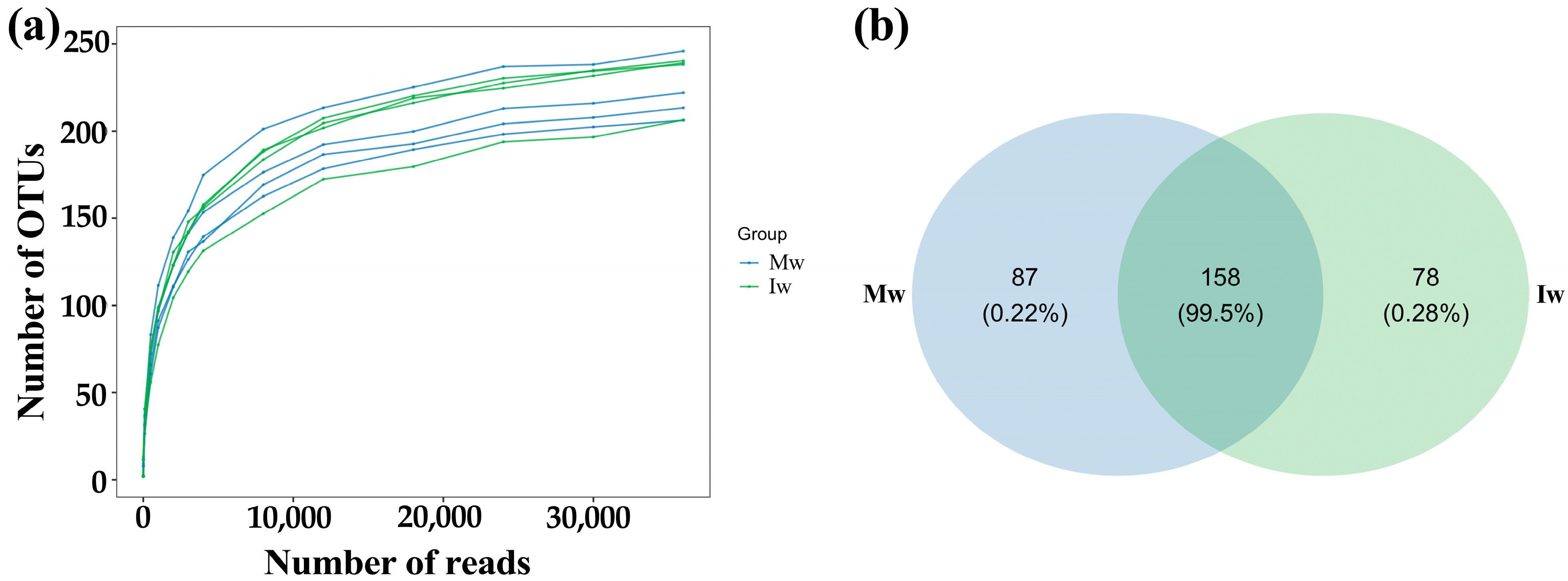


| Treatments | Raw Tags | Effective Tags | Effective Rates (%) | Average Length (bp) |
|---|---|---|---|---|
| Mw | 35,184 | 33,511 | 92.01 | 258 |
| Iw | 36,315 | 34,620 | 90.28 | 258 |
| Treatments | Kingdom | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|
| Mw | 34 | 36 | 244 | 245 | 245 | 245 | 245 |
| Iw | 18 | 19 | 234 | 236 | 236 | 236 | 236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.-X.; Sun, Q.-F.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S.; Xu, Y.-J. Sod Culture with Vicia villosa Alters the Diversity of Fungal Communities in Walnut Orchards for Sustainability Development. Sustainability 2023, 15, 10731. https://doi.org/10.3390/su151310731
He W-X, Sun Q-F, Hashem A, Abd_Allah EF, Wu Q-S, Xu Y-J. Sod Culture with Vicia villosa Alters the Diversity of Fungal Communities in Walnut Orchards for Sustainability Development. Sustainability. 2023; 15(13):10731. https://doi.org/10.3390/su151310731
Chicago/Turabian StyleHe, Wan-Xia, Qiao-Feng Sun, Abeer Hashem, Elsayed Fathi Abd_Allah, Qiang-Sheng Wu, and Yong-Jie Xu. 2023. "Sod Culture with Vicia villosa Alters the Diversity of Fungal Communities in Walnut Orchards for Sustainability Development" Sustainability 15, no. 13: 10731. https://doi.org/10.3390/su151310731







