Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bio-Sorbent
2.2. Characterization of the Adsorbent
2.2.1. Total Ash
2.2.2. Moisture
2.2.3. Total Solids
2.2.4. Density
Density
Specific Gravity (Relative Density)
Porosity
2.2.5. Isoelectric Point
2.3. Preparing the Methylene Blue Stock Solution
2.4. Quantifying the Methylene Blue Concentration
2.5. Effect of the Solution Initial PH (Batch Experiment)
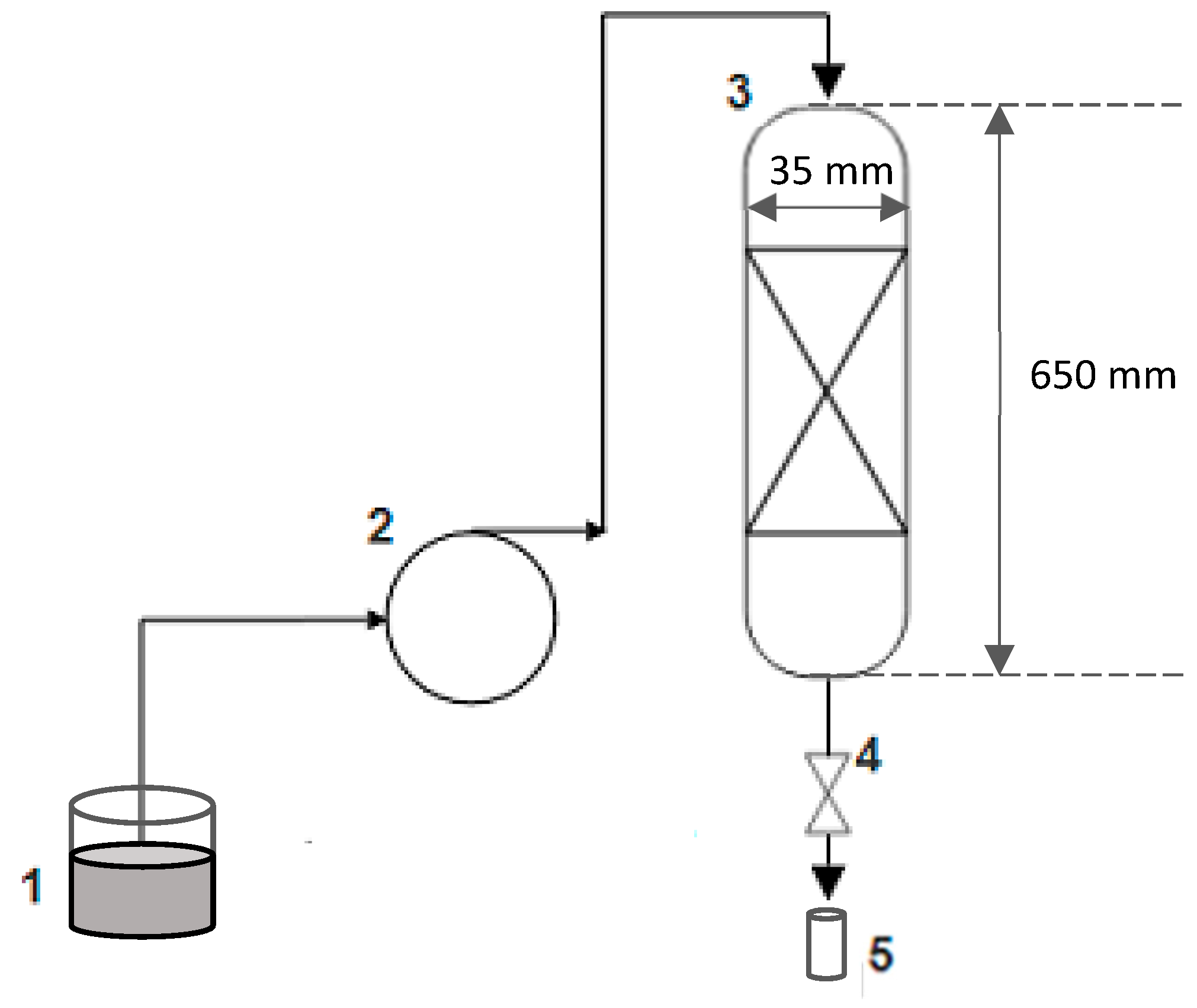
2.6. Experimental Setup
2.7. Determining the Removal Percentage of Methylene Blue
- Determining the Breakthrough Curve
3. Results
3.1. Characterization of the Adsorbent
3.1.1. Physical Characterization of the Adsorbent
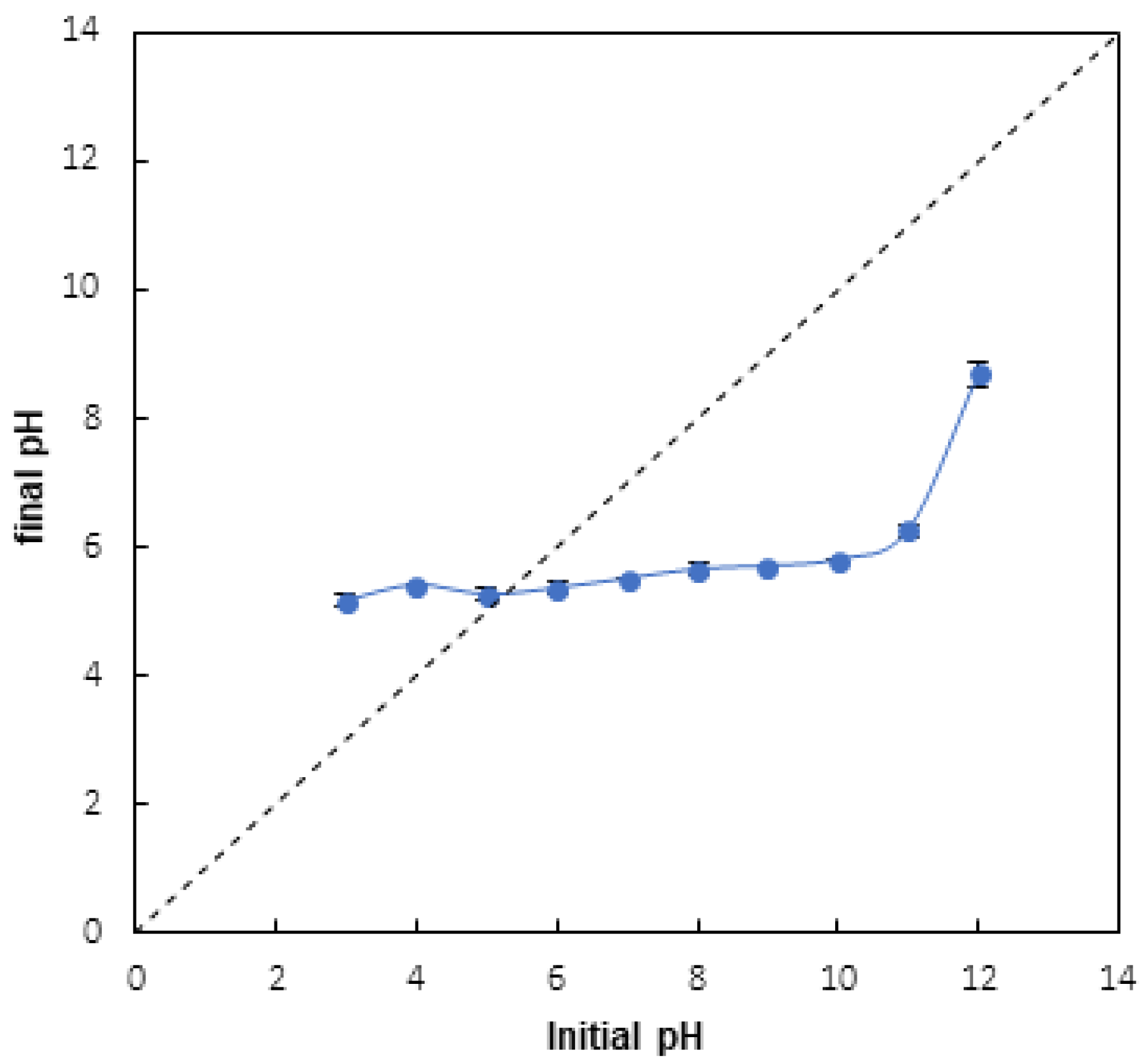
3.1.2. Adsorbent Zero-Point Charge
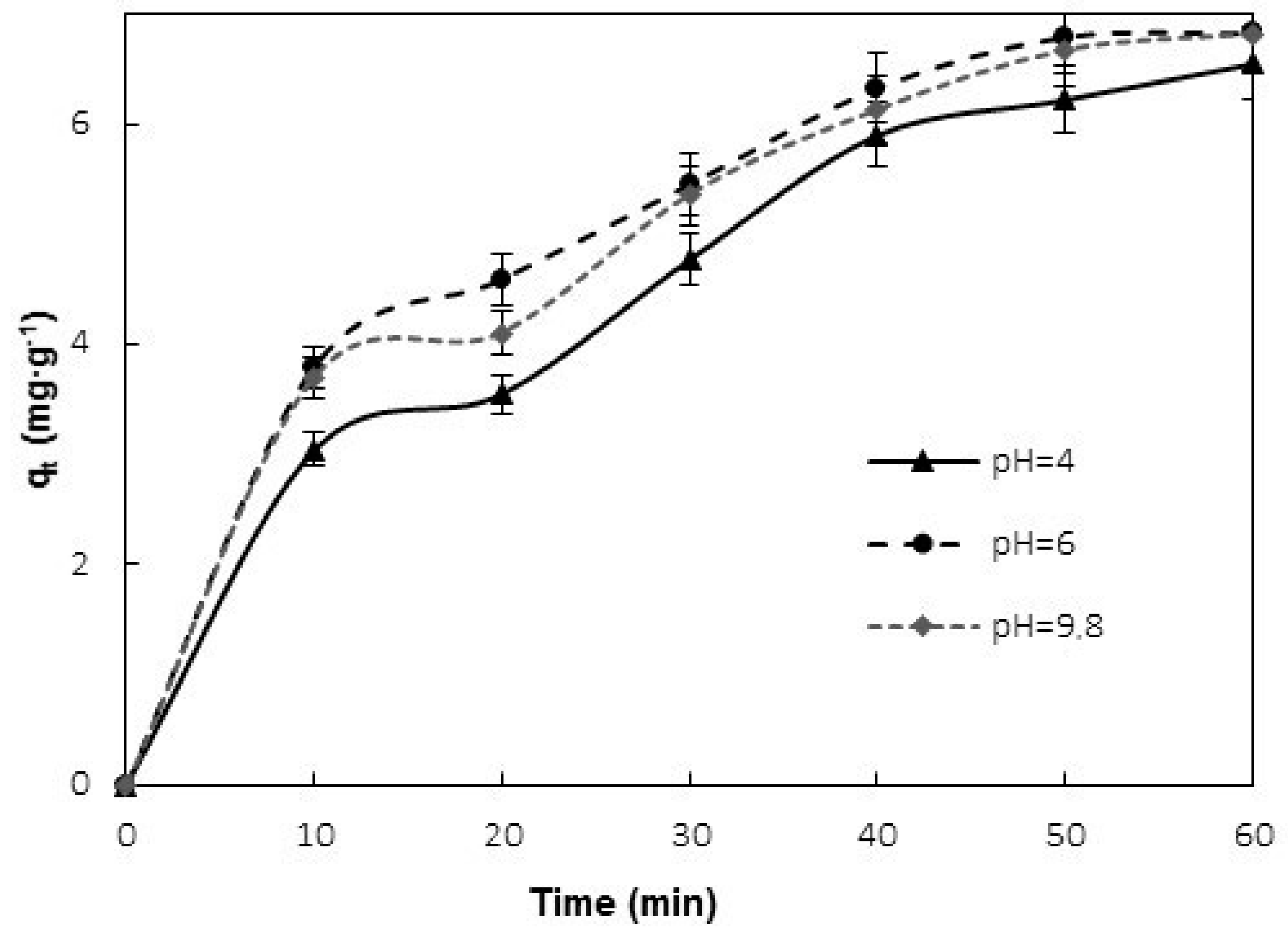
3.2. Effect of PH
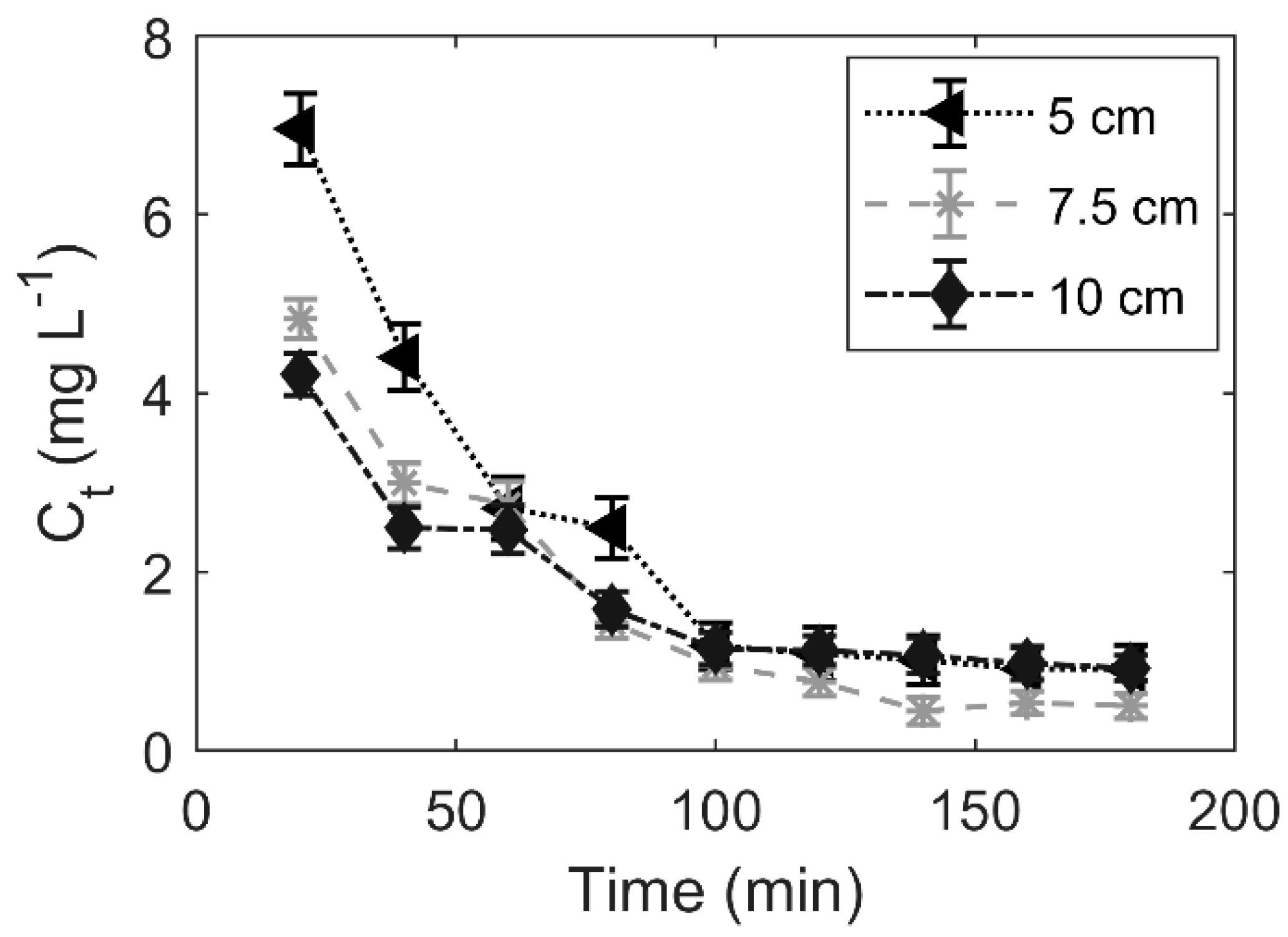
3.3. Effect of the Fixed-Bed Height
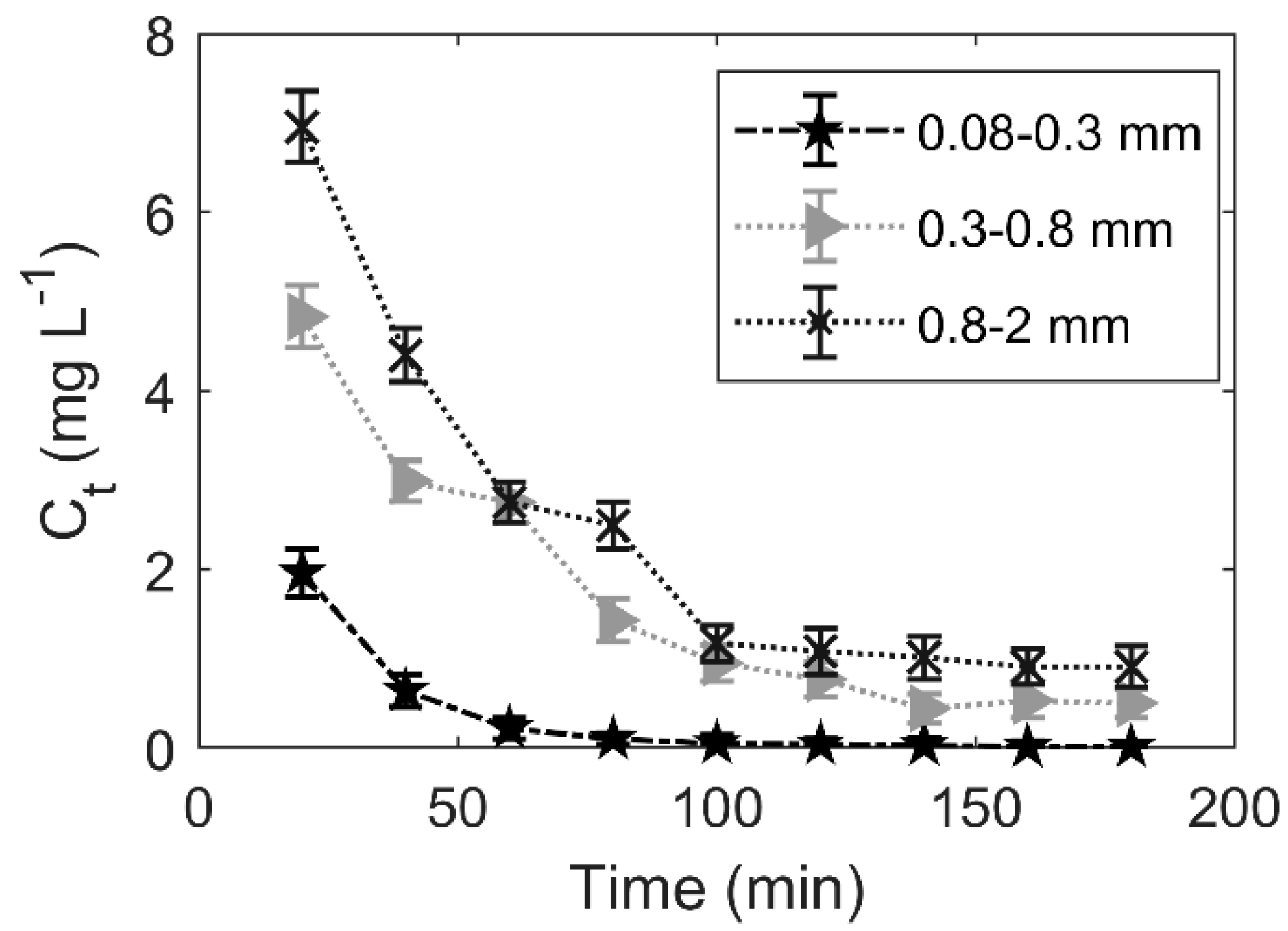
3.4. Effect of Particle Size
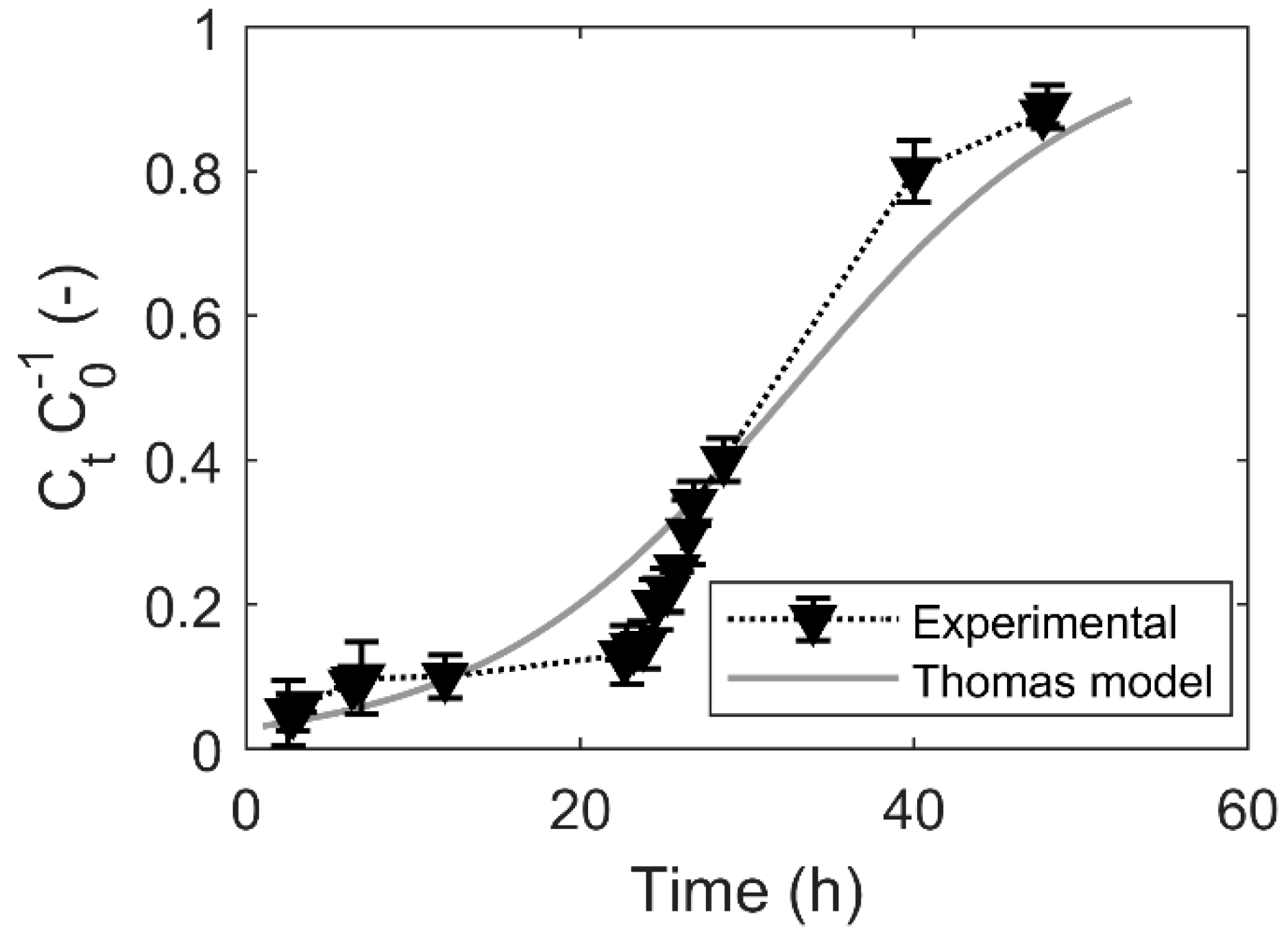
3.5. Breakthrough Curve
4. Discussion
4.1. Effect of PH
4.2. Effect of the Fixed-Bed Height
4.3. Effect of Particle Size
4.4. Breakthrough Curve
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandhru, M.; Rani, S.K.; Vasimalai, N. Reductive degradation of toxic six dyes in industrial wastewater using diaminobenzoic acid capped silver nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104225. [Google Scholar] [CrossRef]
- Ayele, A.; Getachew, D.; Kamaraj, M.; Suresh, A. Phycoremediation of synthetic dyes: An effective and eco-friendly algal technology for the dye abatement. J. Chem. 2021, 2021, 9923643. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid Treatment Systems for Dye Wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Amel, K.; Meniai, A.; Kerroum, D. Isotherm and Kinetics Study of Biosorption of Cationic Dye onto Banana Peel. Energy Procedia 2012, 19, 286–295. [Google Scholar] [CrossRef]
- Seow, T.W.; Lim, C.K.; Nor, M.H.M.; Mubarak, M.F.M.; Lam, C.Y.; Yahya, A.; Ibrahim, Z. Review on wastewater treatment technologies. Int. J. Appl. Environ. Sci. 2016, 11, 111–126. [Google Scholar]
- Fontana, I.B.; Peterson, M.; Cechinel, M.A.P. Application of brewing waste as biosorbent for the removal of metallic ions present in groundwater and surface waters from coal regions. J. Environ. Chem. Eng. 2018, 6, 660–670. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B. Removal of methyl violet 2B from aqueous solution using Casuarina equisetifolia needle. Int. Sch. Res. Not. 2013, 2013, 619819. [Google Scholar]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B. Removal of the methyl violet 2B dye from aqueous solution using sustainable adsorbent Artocarpus odoratissimus stem axis. Appl. Water Sci. 2017, 7, 3573–3581. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Mahesh, R.; Gadekar, M. Mansoor Ahammed. Coagulation/flocculation process for dye removal using water treatment residuals: Modelling through artificial neural networks. Des. Water Treat. 2016, 57, 26392–26400. [Google Scholar] [CrossRef]
- Rodrigues, C.; Madeira, L.; Boaventura, R. Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ. Technol. 2012, 34, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.Y.; Budiman, P.M.; Shak, P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Bakheet, B.; Yuan, S.; Li, Z.; Wang, H.; Zuo, J.; Komarneni, S.; Wang, Y. Electro-peroxone treatment of Orange II dye wastewater. Water Res. 2013, 47, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Benincá, C.; Peralta-Zamora, P.; Tavares, C.; Igarashi-Mafra, L. Degradation of an Azo Dye Ponceau 4R and Treatment of Wastewater from a Food Industry by Ozonation.Ozone. Sci. Eng. 2013, 354, 295–301. [Google Scholar] [CrossRef]
- Masoumbeigi, H.; Rezaee, A. Removal of Methylene Blue (MB) dye from synthetic wastewater using UV/H2O2 advanced oxidation process. Health Policy Sustain. Health 2015, 2, 160–166. [Google Scholar]
- Wawrzkiewicz, M.; Hubicki, Z.; Kilislioglu, A. Anion Exchange Resins as Effective Sorbents for Removal of Acid, Reactive, and Direct Dyes from Textile Wastewaters. Ion. Exchange. 2015, 2, 37–72. [Google Scholar] [CrossRef]
- Suteu, D.; Bilba, D.; Coseri, S. Macroporous polymeric ion exchangers as adsorbents for the removal of cationic dye basic blue 9 from aqueous solutions. J. Appl. Polym. Sci. 2014, 131, 39620. [Google Scholar] [CrossRef]
- Mondal, S.; Ouni, H.; Dhahbi, M.; De, S. Kinetic modeling for dye removal using polyelectrolyte enhanced ultrafiltration. J. Hazard. Mater. 2012, 229–230, 381–389. [Google Scholar] [CrossRef]
- Buscio, V.; García-Jiménez, M.; Vilaseca, M.; López-Grimau, V.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials 2016, 9, 490. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Sie Yon, J. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S.; Yue, Q.; Li, Q. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Biores. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.E.; El-Sayed, M.M. Assessment of food processing and pharmaceutical industrial wastes as potential biosorbents: A review. Biomed. Res. Int. 2014, 2014, 146769. [Google Scholar] [CrossRef] [PubMed]
- Hayder, A.A.; Mohammed, A.K.; Alaa, H.A. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Suppl. Res. Technol. Aqua. 2020, 69, 99–112. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid. Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Proc. Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Ahmadabadi, M.; Mahvi, A. Reactive red-120 removal by activated carbon obtained from cumin herb wastes. Fresenius Environ. Bulletin. 2013, 22, 584–590. [Google Scholar]
- Benadjemia, M.; Millière, L.; Reinert, L.; Benderdouche, N.; Declaux, L. Preparation, characterization and Methylene Blue adsorption of phosphoric acid activated carbons from globe artichoke leaves. Fuel Proc. Technol. 2011, 92, 1203–1212. [Google Scholar] [CrossRef]
- Din, A.; Hameed, B.; Ahmad, A. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Haz. Mat. 2009, 161, 1522–1529. [Google Scholar] [CrossRef]
- Anisuzzaman, S.; Bono, A.; Krishnaiah, D.; Tan, Y. A study on dynamic simulation of phenol adsorption in activated carbon packed bed column. J. King Saud. Univ. Eng. Sci. 2016, 28, 47–55. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.; Fouad, Y. Liquid–liquid extraction of methylene blue dye from aqueous solutions using sodium dodecylbenzenesulfonate as an extractant. Alex. Eng. J. 2015, 54, 77–81. [Google Scholar] [CrossRef]
- Lee, D.; Hong, W.; Hwang, K. Removal of an Organic Dye from Water Using a Predispersed Solvent Extraction. Sep. Sci. Technol. 2000, 35, 1951–1962. [Google Scholar] [CrossRef]
- Fox, M.; Dulay, M. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R. Photocatalytic degradation of methylene blue with Fe doped ZnS nanoparticles. Spectrochim. Acta Part. A Mol. Biomol. Spectroscop. 2013, 113, 250–256. [Google Scholar] [CrossRef]
- Keweloh, H.; Heipieper, H.-J.; Rehm, H.-J. Protection of bacteria against toxicity of phenol by immobilization in calcium alginate. Appl. Micro. Biotechnol. 1989, 31, 383–389. [Google Scholar] [CrossRef]
- Bülbül, G.; Aksu, Z. Investigation of the combined effects of external mass transfer and biodegradation rates on phenol removal using immobilized P. putida in a packed-bed column reactor. Enz. Micro. Technol. 1998, 22, 397–403. [Google Scholar] [CrossRef]
- Gemeiner, P.; Štefuca, V.; Báleš, V. Biochemical engineering of biocatalysts immobilized on cellulosic materials. Enz. Micro. Technol. 1993, 7, 551–566. [Google Scholar] [CrossRef]
- Giannakoudakis, D.; Kyzas, G.; Avranas, A.; Lazaridis, N. Multi-parametric adsorption effects of the reactive dye removal with commercial activated carbons. J. Molec. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Giannakoudakis, D.A.; Sarafidis, C.S.; Lazaridis, N.K.; Deliyanni, E.A. Effective impregnation for the preparation of magnetic mesoporous carbon: Application to dye adsorption. J. Chem. Technol. Biotechnol. 2017, 92, 1899–1911. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 11, 235–253. [Google Scholar] [CrossRef]
- Akkaya, G.; Güzel, F. Application of some domestic wastes as new low-cost biosorbents for removal of methylene blue: Kinetic and equilibrium studies. Chem. Eng. Commun. 2014, 201, 557–578. [Google Scholar] [CrossRef]
- de Oliveira Brito, S.M.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2013, 174, 84–92. [Google Scholar] [CrossRef] [PubMed]
- López-Cabeza, R.; Gámiz, B.; Cornejo, J.; Celis, R. Behavior of the enantiomers of the herbicide 982 imazaquin in agricultural soils under different application regimes. Geoderma 2017, 293, 64–72. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar]
- Tang, C.F.; Shu, Y.; Zhang, R.Q.; Li, X.; Song, J.F.; Li, B.; Zhang, Y.T.; Ou, D.L. Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv. 2017, 26, 16092–16103. [Google Scholar] [CrossRef]
- Tonucci, M.C.; Gurgel, L.V.A.; Aquino, S.F.D. Activated carbons from agricultural byproducts (pine tree and coconut shell), coal, and carbon nanotubes as adsorbents for removal of sulfamethoxazole from spiked aqueous solutions: Kinetic and thermodynamic studies. Ind. Crop. Prod. 2015, 74, 111–121. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.; Berry, R.; Tam, K. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Oguz, E. Fixed-bed column studies on the removal of Fe3+ and neural network modelling. Arab. J. Chemist. 2018, 10, 313–320. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.; Muñoz, A.; Arboleda-Faini, X.; Streitwieseer, D. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Env. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Sarici-Özdemir, Ç. Removal of Methylene Blue by Activated Carbon Prepared from Waste in a Fixed-Bed Column. Partic. Sci. Technol. 2014, 32, 311–318. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A Rev. Env. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, B92, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Achack, M.; Hafdini, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 1661, 117–125. [Google Scholar] [CrossRef]
- Uddin, T.; Rukanuzzaman, M.; Khan, M.; Islam, A. Adsorption of methylene blue from aqueous solution by jackfruit (Artocarpus heteropyllus) leaf powder: A fixed-bed column study. J. Env. Mang. 2009, 90, 3443–3450. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Zhao, X.; Wang, Y.; Xie, F.; Cheng, J.; Tang, M. Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: Experiments and prediction of breakthrough curves. Desalination 2009, 245, 284–297. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.; Ismail, K. Adsorptive removal of methylene blue by chemically treated cellulosic waste banana (Musa sapientum) peels. J. Taibah Univ. Sci. 2018, 6–12, 809–819. [Google Scholar] [CrossRef]
- Ponnusami, V.; Vikram, S.; Srivastava, S.N. Guava Psidium guajava leaf powder: Novel adsorbent for removal of methylene blue from aqueous solutions. J. Hazard. Mater. 2008, 1521, 276–286. [Google Scholar] [CrossRef]
- INN. NCh842 Alimentos—Determinación de Ceniza. 2018. Available online: http://www.inn.cl/inn-informa-nuevas-normas-chilenas (accessed on 12 April 2022).
- Eurofins. Laboratorio de AlimentosIDA. 2016. Available online: https://www.eurofins.cl/media/166258/le340-qu%C3%ADmica-alimentos.pdf (accessed on 12 April 2022).
- IDAL. Determinación Humedad—Método en Estufa de Aire. 2011. Available online: http://www.idal.cl/sgcidal/images/stories/Procedimientos/Laboratorio/Determinacion%20humedad%20metodo%20estufa%20de%20aire.pdf (accessed on 12 April 2022).
- ASTM International. ASTM E1756-08(2015), Standard Test Method for Determination of Total Solids in Biomass. 2015. Available online: http://www.astm.org/cgi-bin/resolver.cgi?E1756-08(2015) (accessed on 12 April 2022).
- ASTM International. ASTM D6683-14, Standard Test Method for Measuring Bulk Density Values of Powders and Other Bulk Solids as Function of Compressive Stress. 2014. Available online: http://www.astm.org/cgi-bin/resolver.cgi?D6683-14 (accessed on 15 April 2022).
- ASTM International. ASTM D854-14, Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. 2014. Available online: http://www.astm.org/cgi-bin/resolver.cgi?D854-14 (accessed on 15 April 2022).
- Manger, E. Porosity and Bulk. Density of Sedimentary Rocks; United States Departament of the Interior: Washington, DC, USA, 1963. Available online: https://pubs.usgs.gov/bul/1144e/report.pdf (accessed on 3 May 2022).
- Amaringo Villa, F.; Hormaza Anaguano, A. Determinación del punto de carga cero y punto isoeléctrico de dos residuos agrícolas y su aplicación en la remoción de colorantes. Rev. De. Investig. Agrar. Y Ambiental. 2013, 4, 27–36. Available online: https://dialnet.unirioja.es/descarga/articulo/5344979.pdf (accessed on 15 May 2022). [CrossRef]
- Archibald, J. Nutrient Composition of Banana Skins. J. Dairy. Sci. 1949, 32, 969–971. [Google Scholar] [CrossRef]
- Oberoi, H.; Sandhu, S.; Vadlani, P. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioprod. Proc. 2012, 90, 257–265. [Google Scholar] [CrossRef]
- Abubakar, U.; Yusuf, K.; Safiyanu, I.; Abdullahi, S.; Saidu, S.; Abdu, G.; Indee, A. Proximate and mineral composition of corn cob, banana and plantain peels. Int. J. Food Sci. Nut. 2016, 1, 25–27. [Google Scholar]
- Mandavgane, S.; Pathak, P.; Kulkarni, B. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Mondal, N.K. Natural Banana (Musa acuminate) Peel: An Unconventional Adsorbent for Removal of Fluoride from Aqueous Solution through Batch Study. Water Conserv. Sci. Eng. 2017, 1, 223–232. [Google Scholar] [CrossRef]
- Bhaumik, R.; Mondal, N. Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl. Water Sci. 2016, 6, 115–135. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.-U.; Ogada, T. Combustion of agricultural residues. Prog. Energy Comb. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Gao, X.; Driver, L.; Kasin, I.; Masiello, C.; Pyle, L.; Dugan, B.; Ohlson, M. Effect of environmental exposure on charcoal density and porosity in a boreal forest. Sci. Tot. Env. 2017, 592, 316–325. [Google Scholar] [CrossRef]
- Fogler, H.S. Catalysis and catalitic reactors. In Elements of Chemical Reaction Engineering; Amundson, N.R., Ed.; Prentice Hall Professional Technical Reference: Hoboken, NJ, USA, 2005; pp. 645–680. [Google Scholar]
- Castellar Ortega, G.; Cardozo Arrieta, B.; Suarez Guerrero, J.; Vega Taboada, J. Adsorción por lote y en una columna de lecho fijo del colorante B39 sobre carbón activado granular. Prospect. 2013, 11, 66–75. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Env. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef]
- Dahiru, M.; Zango, Z.U. Maje Alhaji Haruna, Cationic Dyes Removal Using Low-Cost Banana Peel Biosorbent. Am. J. Mat. Sci. 2018, 8, 32–38. [Google Scholar] [CrossRef]
- Salleh, A.; Mahmoud, D.; Ghani, W.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Stavrinou, A.; Aggelopoulos, C.A.; Tsakiroglou, C.D. A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels. Processes 2020, 8, 1467. [Google Scholar] [CrossRef]
- Delgado, J.M.P.Q. A critical review of dispersion in packed beds. Heat. Mass. Transfer. 2006, 42, 279–310. [Google Scholar] [CrossRef]
- Luzi, C.D.; Martinez, O.M.; Barreto, G.F. Effect of radial velocity profiles on axial dispersion in packed beds: Asymptotic behaviour. Braz. J. Chem. Eng. 2021, 38, 865–885. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, L.; Yan, H.; Li, H.; Jiang, Z.; Kan, X.; Cheng, R. Removal of methylene blue from aqueous solutions by straw based adsorbent in a fixed-bed column. Chem. Eng. J. 2011, 173, 429–436. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef]
- Song, J.; Zou, W.; Bian, Y.; Su, F.; Han, R. Adsorption characteristics of methylene blue by peanut husk in batch and. Desalination 2011, 265, 119–125. [Google Scholar] [CrossRef]
- Singh, B.; Thakur, V.; Bhatia, G.; Verma, D.; Singh, K. Eco-friendly and Cost-effective Use of Rice Straw in the Form of Fixed Bed Column to Remove Water Pollutants. J. Bioremediat Biodegrad. 2016, 7, 374. [Google Scholar] [CrossRef]
- Abbas, S.; Kamar, F.; Hossien, Y. Adsorption of methyl violet 2B dye from aqueous solutions onto waste of Banana peel using fixed-bed column. Int. J. Civ. Eng. Technol. 2018, 9, 2094–2109. [Google Scholar]
| Parameter | Value | Unit |
|---|---|---|
| Ash | 12.88 ± 0.1 | % |
| Moisture | 84.51 ± 0.24 | % |
| Total Solids | 15.49 ± 0.24 | % |
| Moisture | Operating Conditions | Reference |
|---|---|---|
| 2.98 ± 0.02 | Particle size: 0.2 mm | [72] |
| Drying temperature: 60 °C, 24 h | ||
| 6.1 | Particle size: 0.25 mm Drying temperature: 50 °C, 24 h | [73] |
| 9.8 | Particle size: 0.106–0.9 mm | [71] |
| Drying temperature: 70 °C |
| Particle Size Range (mm) | Density (g/cm3) | Specific Gravity (g/cm3) | Porosity (%) |
|---|---|---|---|
| 0.08–0.3 | 0.248 | 0.38 | 54.74 |
| 0.3–0.8 | 1.005 | 0.39 | 47.46 |
| 0.8–2 | 1.025 | 0.4 | 47.46 |
| Result | Value |
|---|---|
| Experimental | |
| Removal, % | 62.5 |
| Total adsorption capacity, mg g−1 | 22.11 |
| Thomas model | |
| , mg g−1 | 25.40 |
| , mL mg−1 min−1 | 4.5 × 10−2 |
| , h | 53.0 |
| 0.914 |
| Bioadsorbent | Dye | Flow | Particle | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Jackfruit Leaf Powder | Methylene blue | 7.5 | 300 | 40 | 0.075–0.3 | 350 | 264 | [56] |
| Straw | Methylene blue | 7.8 | 2000 | 1.28 | 0.063–0.15 | 150 | 227.8 | [84] |
| Phoenix Tree Leaf Powder | Methylene blue | 10 | 50 | 8 | 0.25–0.425 | 1200 | 131 | [57] |
| Hazelnut shell | Methylene blue | 8.3 | 100 | 19.1 | 0.125 | 300 | 50 | [85] |
| Peanut shell | Methylene blue | 8.5 | 40 | 7.5 | 0.425–0.85 | 400 | 42.2 | [86] |
| Rice straw | Methylene blue | 2.5 | 100 | 10 | 0.3–0.5 | 110 | 27.5 | [87] |
| Banana Peel | Methyl violet 2B | 10 | 50 | 8 | 0.15–0.3 | 60 | 28.33 | [88] |
| 32 | 0.15–0.3 | 60 | 16.64 | |||||
| Banana Peel | Methylene blue | 5 | 40 | 10 | 0.8–2 | 180 | 138 | This study |
| 7.5 | 40 | 10 | 0.8–2 | 180 | 140.3 | |||
| 10 | 40 | 10 | 0.8–2 | 180 | 140 | |||
| 5 | 40 | 10 | 0.08–0.3 | 180 | 145.1 | |||
| 5 | 40 | 10 | 0.3–0.8 | 180 | 100.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yhon, J.; Mendoza, J.; Osorio, E.; Domínguez, M.P. Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment. Sustainability 2023, 15, 9870. https://doi.org/10.3390/su15139870
Yhon J, Mendoza J, Osorio E, Domínguez MP. Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment. Sustainability. 2023; 15(13):9870. https://doi.org/10.3390/su15139870
Chicago/Turabian StyleYhon, Jennifer, Jeamilette Mendoza, Efren Osorio, and María Paz Domínguez. 2023. "Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment" Sustainability 15, no. 13: 9870. https://doi.org/10.3390/su15139870
APA StyleYhon, J., Mendoza, J., Osorio, E., & Domínguez, M. P. (2023). Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment. Sustainability, 15(13), 9870. https://doi.org/10.3390/su15139870







