Influence of Low Air Pressure on the Partial Denitrification-Anammox (PD/A) Process
Abstract
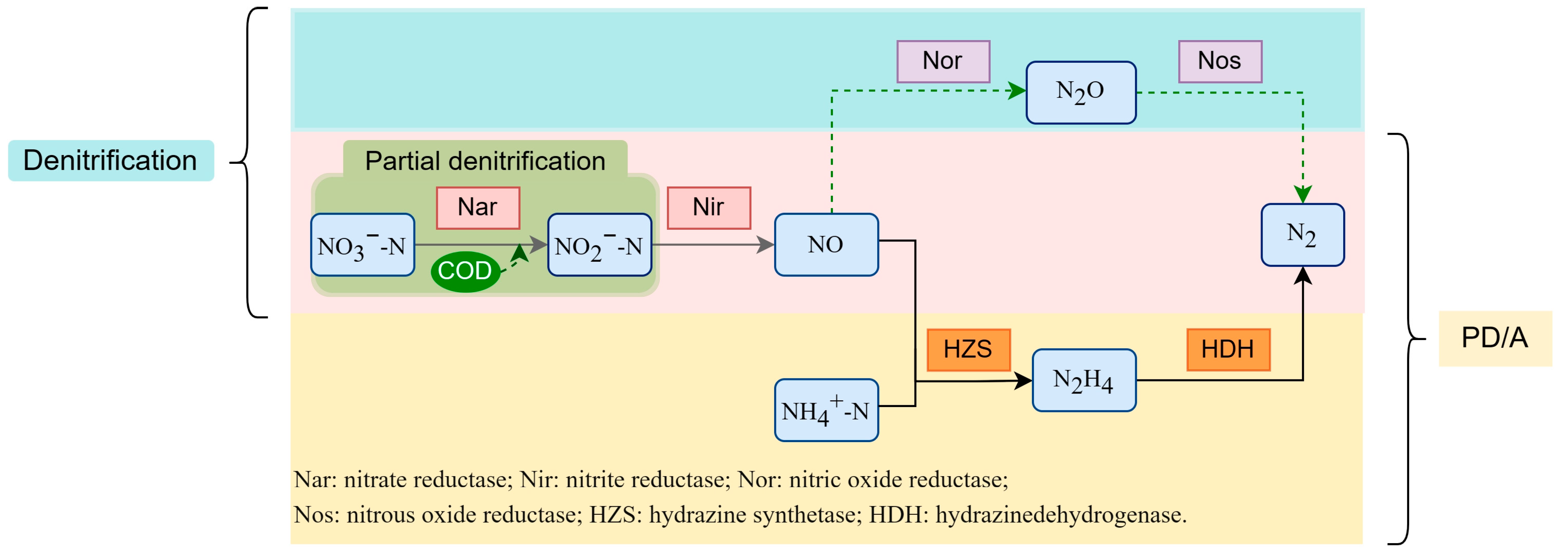
:1. Introduction
2. Materials and Methods
2.1. Reactor Setup
2.2. Seeding Sludge and Synthetic Wastewater
2.3. Operating Conditions
2.4. Batch Tests
2.5. Analytical Methods
2.6. Calculations
2.7. Microbial Community Structure Analysis
3. Results
3.1. PD Process Performance
3.2. Anammox Process Performance
3.3. Nitrogen Removal Performance in the Combined PD/A System
3.4. Microbial Community Succession in the PD/A System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Zheng, J.; Zhao, L.; Liu, W.; Chen, L.; Cai, T.; Ji, X.-M. Succession of microbial communities reveals the inevitability of anammox core in the development of anammox processes. Bioresour. Technol. 2023, 371, 128645. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Peng, Y.; Du, R.; Wang, S. Feasibility of enhancing the DEnitrifying AMmonium OXidation (DEAMOX) process for nitrogen removal by seeding partial denitrification sludge. Chemosphere 2016, 148, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Rosenthal, A.; Ramalingam, K.; Fillos, J.; Chandran, K. Linking Community Profiles, Gene Expression and N-Removal in Anammox Bioreactors Treating Municipal Anaerobic Digestion Reject Water. Environ. Sci. Technol. 2010, 44, 6110–6116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, Y.; Wang, Q.; Zhang, Z.; Wu, C.; Gao, M.; Liu, F. The Summary of Nitritation Process in Mainstream Wastewater Treatment. Sustainability 2022, 14, 16453. [Google Scholar] [CrossRef]
- Cao, S.B.; Wang, S.Y.; Peng, Y.Z.; Wu, C.C.; Du, R.; Gong, L.X.; Ma, B. Achieving partial denitrification with sludge fermentation liquid as carbon source: The effect of seeding sludge. Bioresour. Technol. 2013, 149, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, H.E.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Hassan, G.K.; Lu, X.; Piechota, G.; Mannina, G.; Bott, C.B.; Mąkinia, J. An advanced synergy of partial denitrification-anammox for optimizing nitrogen removal from wastewater: A review. Bioresour. Technol. 2023, 381, 129168. [Google Scholar] [CrossRef]
- Lu, W.K.; Zhang, Y.L.; Wang, Q.Q.; Wei, Y.; Bu, Y.N.; Ma, B. Achieving advanced nitrogen removal in a novel partial denitrification/anammox-nitrifying (PDA-N) biofilter process treating low C/N ratio municipal wastewater. Bioresour. Technol. 2021, 340, 125661. [Google Scholar] [CrossRef]
- Gao, R.T.; Peng, Y.Z.; Li, J.W.; Liu, Y.; Deng, L.Y.; Li, W.Y.; Kao, C.K. Mainstream partial denitrification-anammox (PD/A) for municipal sewage treatment from moderate to low temperature: Reactor performance and bacterial structure. Sci. Total Environ. 2022, 806, 150267. [Google Scholar] [CrossRef]
- Liu, Y. Calculation of air supply of surface aerator in high altitude municipal sewage treatment plant. Nonferrous Met. Eng. Res. 2015, 36, 3. (In Chinese) [Google Scholar]
- Chen, Y.; Li, S.; Lu, Y.; Zhu, G.; Cheng, H. Simultaneous nitrification, denitrification and phosphorus removal (SNDPR) at low atmosphere pressure. Biochem. Eng. J. 2020, 160, 107629. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Han, J.; Zhang, W.-J.; Sun, J.; Li, X.; Yang, Z.-l.; Yang, J.-L.; Li, S.-P.; Zhu, G.-C. Influence of low air pressure on combined nitritation and anaerobic ammonium oxidation process. Sci. Total Environ. 2022, 838, 156556. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Rice, E.W.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Berry, E.A.; Trumpower, B.L. Simultaneous determination of Hemes-A, Hemes-B, and Hemes-C from pyridine hemochrome spectra. Anal. Biochem. 1987, 161, 1–15. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, J.G.; Yan, X.Q.; Wang, Z.Q. Discussion on the Detection method of hydrazine concentration in Water. Meas. Tech. 2014, 5, 23–26. (In Chinese) [Google Scholar]
- Hu, T.; Peng, Y.; Yuan, C.; Zhang, Q. Enhanced nutrient removal and facilitating granulation via intermittent aeration in simultaneous partial nitrification endogenous denitrification and phosphorus removal (SPNEDpr) process. Chemosphere 2021, 285, 131443. [Google Scholar] [CrossRef]
- Cao, S.B.; Peng, Y.Z.; Du, R.; Zhang, H.Y. Characterization of partial-denitrification (PD) granular sludge producing nitrite: Effect of loading rates and particle size. Sci. Total Environ. 2019, 671, 510–518. [Google Scholar] [CrossRef]
- Kartal, B.; Maalcke, W.J.; de Almeida, N.M.; Cirpus, I.; Gloerich, J.; Geerts, W.; Op den Camp, H.J.M.; Harhangi, H.R.; Janssen-Megens, E.M.; Francoijs, K.-J.; et al. Molecular mechanism of anaerobic ammonium oxidation. Nature 2011, 479, 127–130. [Google Scholar] [CrossRef]
- Peng, L.; Shi, R.; Tao, Y.; Huang, Q.; Yang, M.; He, Y.; Xu, W. Starting up anammox system with high efficiency nitrogen removal at low temperatures: Performance optimization, sludge characterization and microbial community analysis. J. Environ. Manag. 2023, 325, 116542. [Google Scholar] [CrossRef]
- Jiang, H.; Peng, Y.; Li, X.; Zhang, F.; Wang, Z.; Ren, S. Advanced nitrogen removal from mature landfill leachate via partial nitrification-Anammox biofilm reactor (PNABR) driven by high dissolved oxygen (DO): Protection mechanism of aerobic biofilm. Bioresour. Technol. 2020, 306, 123119. [Google Scholar] [CrossRef]
- Ma, J.Y.; Yao, H.; Yu, H.Q.; Zuo, L.S.; Li, H.Y.; Ma, J.G.; Xu, Y.R.; Pei, J.; Li, X.Y. Hydrazine addition enhances the nitrogen removal capacity in an anaerobic ammonium oxidation system through accelerating ammonium and nitrite degradation and reducing nitrate production. Chem. Eng. J. 2018, 335, 401–408. [Google Scholar] [CrossRef]
- Kang, D.; Li, Y.; Xu, D.; Li, W.; Li, W.; Ding, A.; Wang, R.; Zheng, P. Deciphering correlation between chromaticity and activity of anammox sludge. Water Res. 2020, 185, 116184. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, Y.; Xue, Y.; Zhang, Y.; Li, Y.-Y. Relationship of heme c, nitrogen loading capacity and temperature in anammox reactor. Sci. Total Environ. 2019, 659, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Peng, Y. Mechanisms and microbial structure of partial denitrification with high nitrite accumulation. Appl. Microbiol. Biotechnol. 2016, 100, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, Z.; Yin, T.; Liu, T.; Hu, Z. Feasibility of Achieving Efficient Nitrite Accumulation in Moving Bed Biofilm Reactor: The Influencing Factors, Microbial Structures, and Biofilm Characteristics. Water 2023, 15, 998. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Li, B.; Wang, S.; Ren, N.; Peng, Y. Nitrite production from partial-denitrification process fed with low carbon/nitrogen (C/N) domestic wastewater: Performance, kinetics and microbial community. Chem. Eng. J. 2017, 326, 1186–1196. [Google Scholar] [CrossRef]
- Wang, J.; Chi, Q.; Zhang, R.; Wu, X.; Jiang, X.; Mu, Y.; Tu, Y.; Shen, J. Evaluation of N-methylpyrrolidone bio-mineralization mechanism and bacterial community evolution under denitrification environment. J. Clean. Prod. 2022, 343, 130945. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, L.; Liu, J.; Wang, X.; Gao, R.; Pang, L.; Zhou, Y. Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res. 2019, 160, 178–187. [Google Scholar] [CrossRef]
- Wen, X.; Gong, B.; Zhou, J.; He, Q.; Qing, X. Efficient simultaneous partial nitrification, anammox and denitrification (SNAD) system equipped with a real-time dissolved oxygen (DO) intelligent control system and microbial community shifts of different substrate concentrations. Water Res. 2017, 119, 201–211. [Google Scholar] [CrossRef]
- Sengar, A.; Aziz, A.; Farooqi, I.H.; Basheer, F. Development of denitrifying phosphate accumulating and anammox micro-organisms in anaerobic hybrid reactor for removal of nutrients from low strength domestic sewage. Bioresour. Technol. 2018, 267, 149–157. [Google Scholar] [CrossRef]









| Group | SAA12h (mg·N·g−1· VSS−1·d−1) | NH4+-N Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | ||
| 96 kPa | 38.64 | 30.17 | 23.36 | 18.21 | 13.77 | 9.97 | 6.55 | 3.52 |
| 72 kPa | 46.56 | 29.90 | 22.62 | 16.97 | 12.08 | 7.90 | 4.33 | 1.24 |
| 65 kPa | 52.08 | 30.01 | 22.72 | 16.81 | 11.67 | 7.30 | 3.52 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Han, Z.; Lu, Y.; Li, S.; Yan, G.; Zhu, G. Influence of Low Air Pressure on the Partial Denitrification-Anammox (PD/A) Process. Sustainability 2023, 15, 9907. https://doi.org/10.3390/su15139907
Dai W, Han Z, Lu Y, Li S, Yan G, Zhu G. Influence of Low Air Pressure on the Partial Denitrification-Anammox (PD/A) Process. Sustainability. 2023; 15(13):9907. https://doi.org/10.3390/su15139907
Chicago/Turabian StyleDai, Wen, Zhenpeng Han, Yongze Lu, Shuping Li, Gangyin Yan, and Guangcan Zhu. 2023. "Influence of Low Air Pressure on the Partial Denitrification-Anammox (PD/A) Process" Sustainability 15, no. 13: 9907. https://doi.org/10.3390/su15139907




