Solar-Induced Photocatalytic Degradation of Reactive Red and Turquoise Dyes Using a Titanium Oxide/Xanthan Gum Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TiO2/XG
2.3. Characterization of the TiO2/XG Nanocomposite
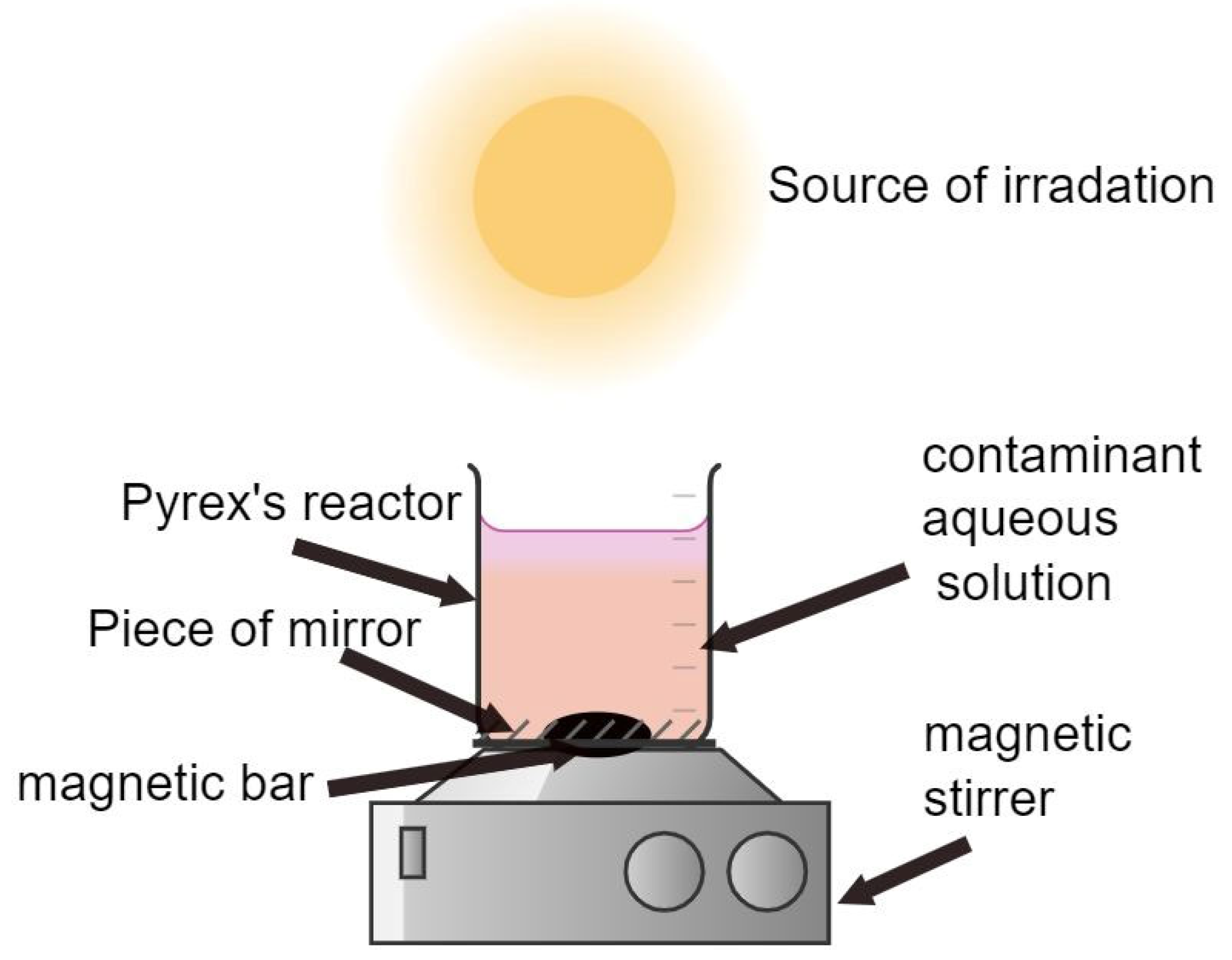
2.4. Experimental Procedure and Analysis
3. Results and Discussion
3.1. TiO2/XG Characteristics
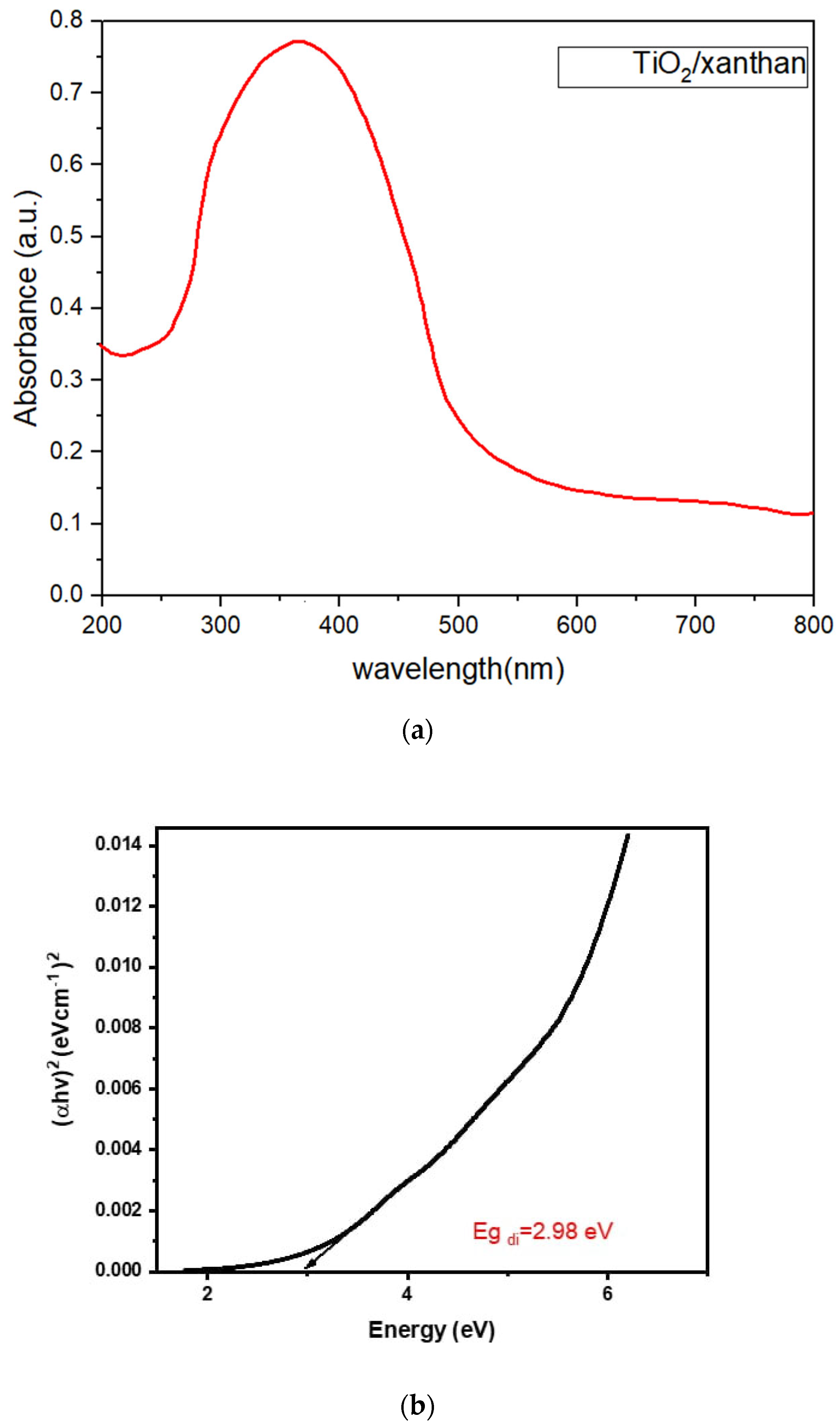
3.1.1. Optical Properties UV–Vis Spectroscopy
3.1.2. FTIR
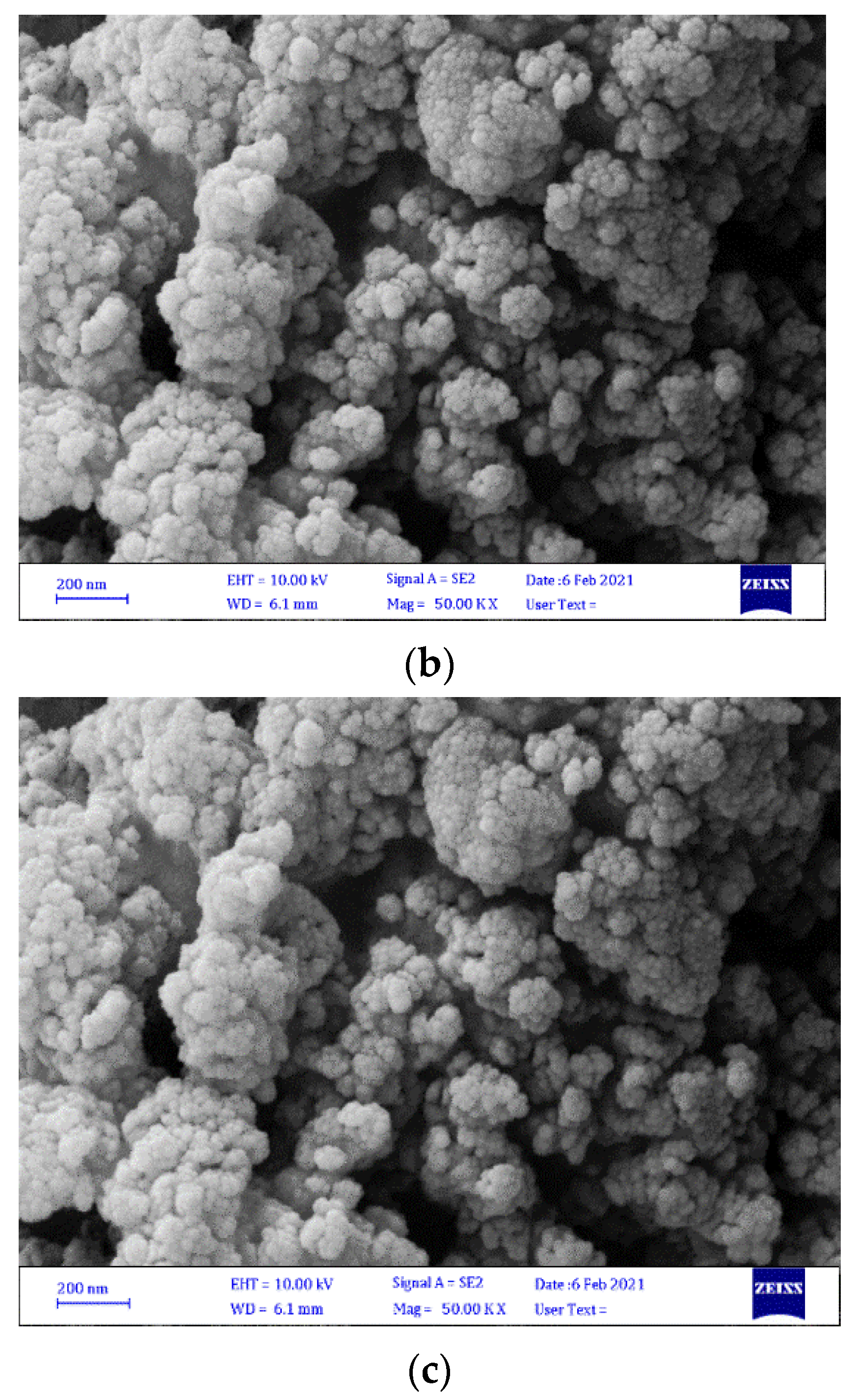
3.1.3. Scanning Electron Microscopy
3.1.4. X-ray Diffraction
3.2. Adsorption and Photocatalytic Activity of the TiO2/XG Composites
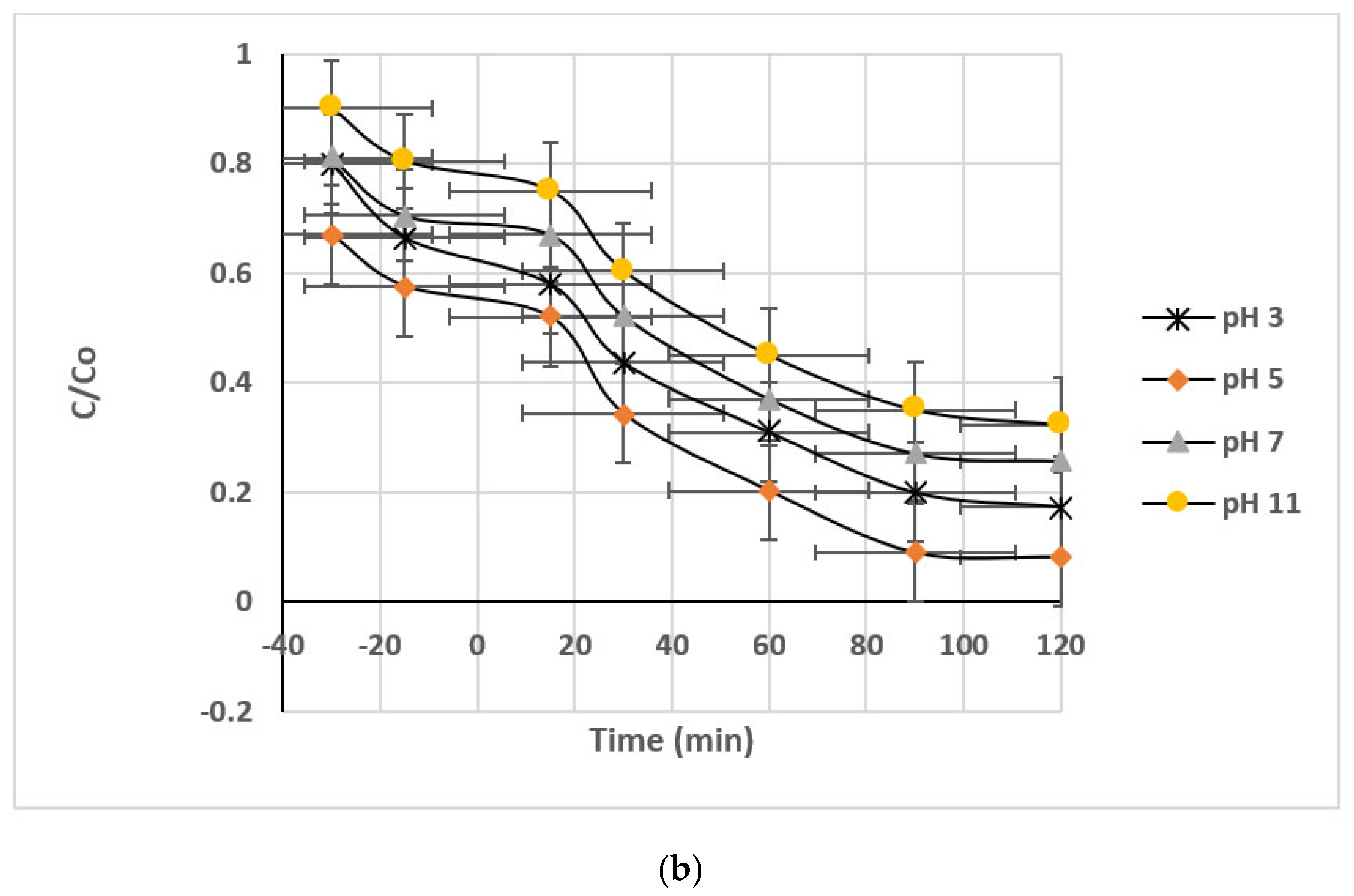
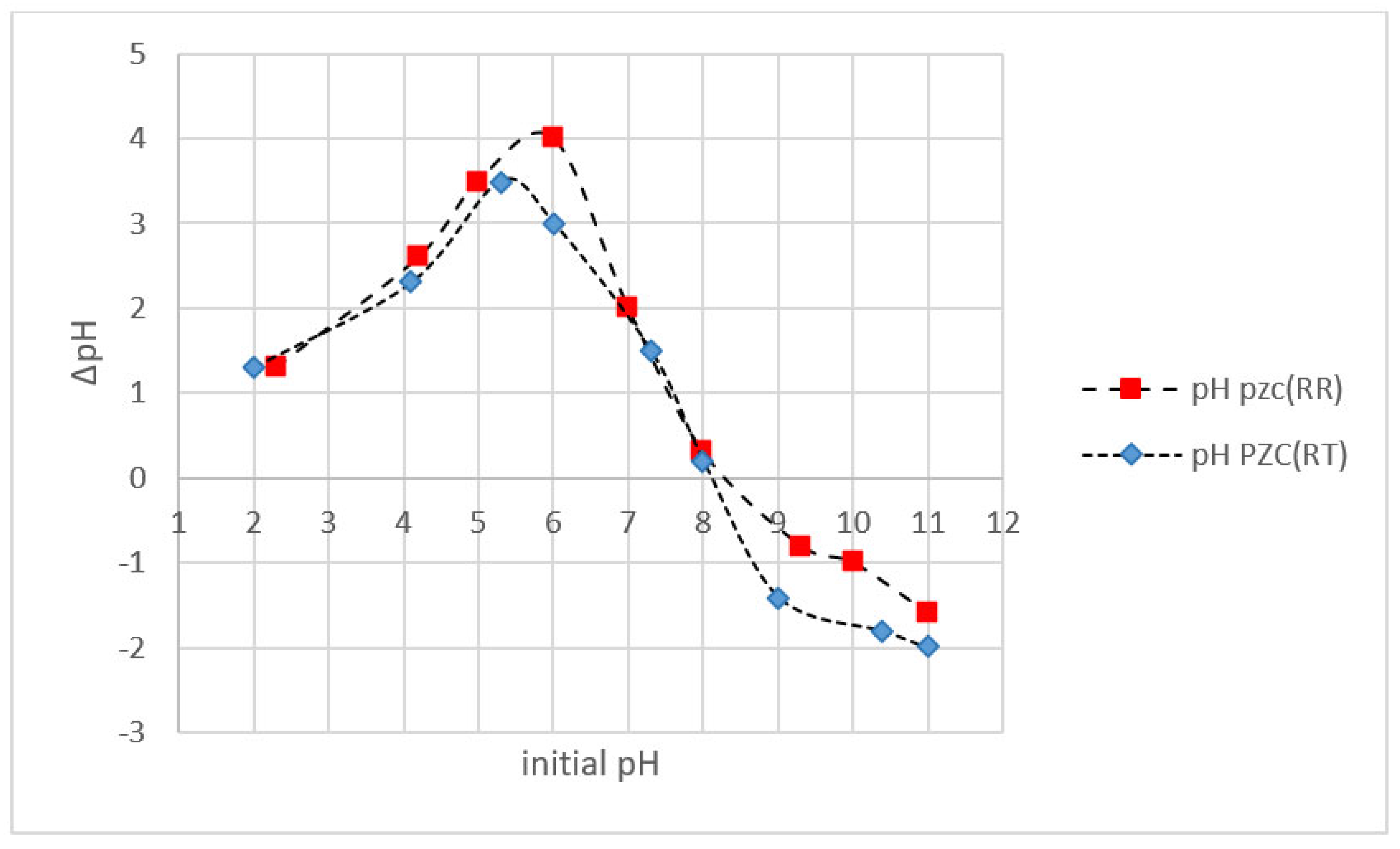
3.2.1. pH
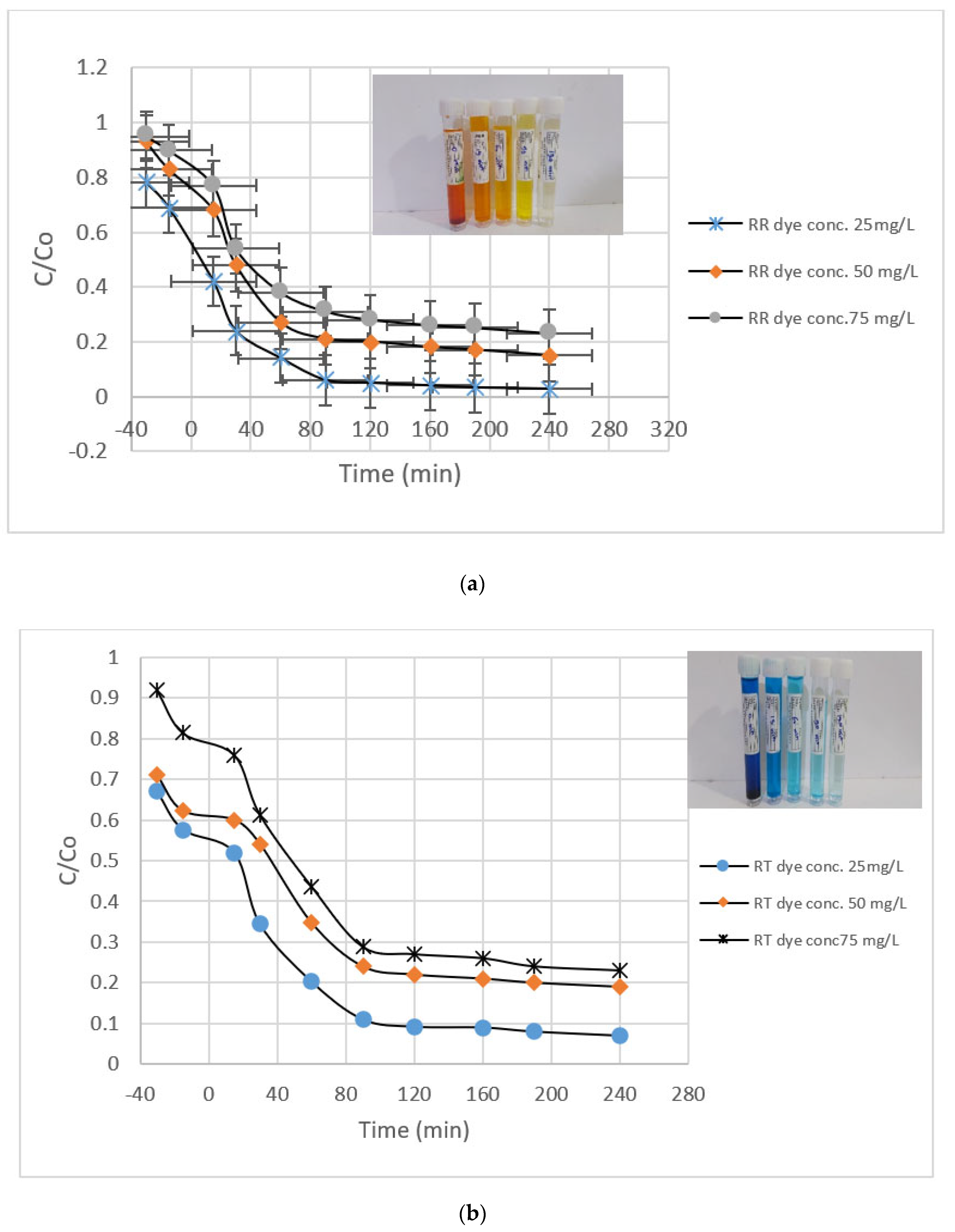
3.2.2. Dye Concentration and Time
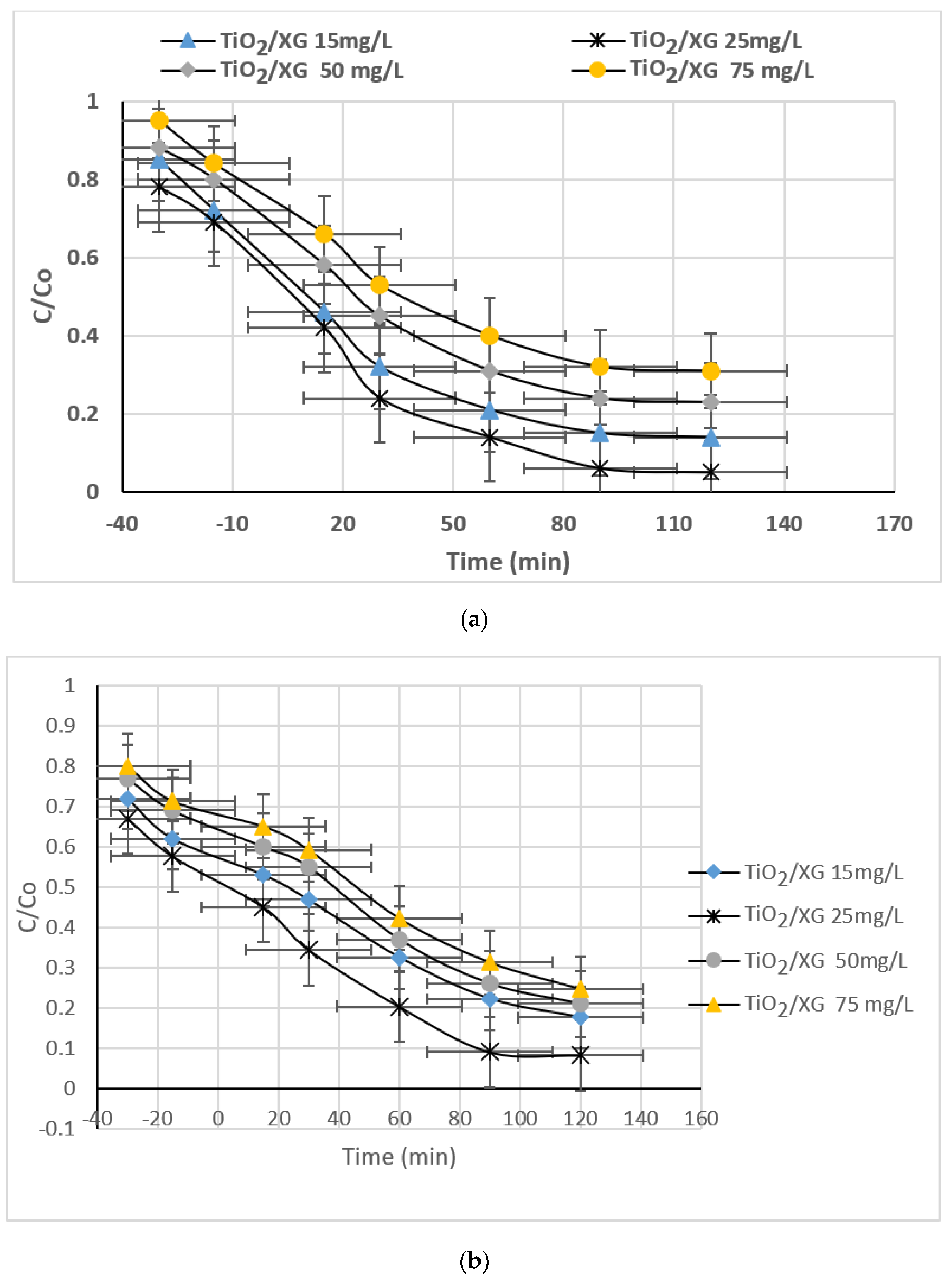
3.2.3. Effect of TiO2/XG
3.2.4. H2O2 Concentration
3.3. Photodegradation Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Alwared, A.I.; Balarak, D.; Sabaghi, R. Photocatalytic degradation of Ciprofloxacin by MMT/CuFe2O4 nanocomposite: Characteristics, response surface methodology, and toxicity analyses. Environ. Sci. Pollut. Res. 2023, 30, 70076–70093. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S. Solar- TiO2 immobilized photocatalytic reactors performance assessment in the degradation of methyl orange dye in aqueous solution Environ. Nanotechnol. Monit. Manag. 2021, 16, 100514. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of spherical-graphitic carbon nitride via hydrothermal method for enhanced photo-degradation ability towards antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Ali, H.Q.; Mohammed, A.A. Elimination of Congo red dyes from aqueous solution using Eichhornia Crassipes. Iraqi J. Chem. Pet. Eng. 2020, 21, 21–32. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang F and Mahmood, A. Fe-ZrO2 imbedded graphene like carbon nitride for acarbose (ACB) photo-degradation intermediate study. Adv. Powder Technol. 2018, 29, 3233–3240. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Mohammed, N.A.A.; Alwared, A.I.; Salman, M.S. Decolorization of reactive Yellow dye by advanced oxidation using continuous reactors. Iraqi J. Chem. Pet. Eng. 2020, 21, 1–6. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent achievements in dyes removal focused on advanced oxidation processes integrated with biological methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Hameed, F.M.; Mousa, K.M. Study on kinetic and optimization of continuous advanced oxidative decolorization of Brilliant reactive Red dye. Iraqi J. Chem. Pet. Eng. 2019, 20, 9–14. [Google Scholar] [CrossRef]
- Chaker, H.; Attar, A.E.; Djennas, M.; Fourmentin, S. A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: A central composite design in response surface methodology. Chem. Eng. Res. Des. 2021, 171, 198–212. [Google Scholar] [CrossRef]
- Yang, J.; Huang, R.; Cao, Y.; Wang, H.; Ivanets, A. Heterogeneous Fenton degradation of persistent organic pollutants using natural chalcopyrite: Effect of water matrix and catalytic mechanism. Environ. Sci. Pollut. Res. 2022, 29, 75651–75663. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Renteria, E.; Oliva, J.; Navarro-Garcia, N.E.; Rodriguez-Gonzalez, V. Novel sustainable composites made of car’s waste and sodium titanate for the efficient photocatalytic removal of the bromophenol blue dye: Study under solar and UV–Vis light. Environ. Sci. Pollut. Res. 2022, 29, 76752–76765. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liu, D. Rapid synthesis of recyclable and reusable magnetic TiO2@Fe3O4 for degradation of organic pollutant. Appl. Phys. A 2019, 125, 77. [Google Scholar] [CrossRef]
- Garcia, C.R.; Oliva, J.; Chavez, D.; Viesca-Villanueva, E.; Tejada-Velasquez, Y.; Mtz-Enriquez, A.I.; Diaz-Torres, L.A. Using a novel Sr2CeO4:Ni Photocatalyst for the degradation of the recalcitrant Congo Red dye under solar irradiation. Top. Catal. 2022, 65, 1102–1112. [Google Scholar] [CrossRef]
- Rana, A.G.; Minceva, M. Analysis of photocatalytic degradation of phenol with exfoliated graphitic carbon nitride and light-emitting diodes using response surface methodology. Catalysts 2021, 11, 898. [Google Scholar] [CrossRef]
- Ong, S.; Lee, C.; Zainal, Z.; Keng, P.; Ha, S. Photocatalytic degradation of basic and reactive dyes in both single and binary systems using immobilized TiO2. J. Fundam. Sci. 2009, 5, 2, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Senthilnathan, J.; Philip, L. Photocatalytic degradation of lindane under UV and visible light using N-doped TiO2. Chem. Eng. J. 2010, 161, 83–92. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior Fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Jouali, A.; Salhi, A.; Aguedach, A.; Lhadi, E.K.; El Krati, M.; Tahiri, S. Photo-catalytic degradation of polyphenolic tannins in continuous-flow reactor using titanium dioxide immobilized on a cellulosic material. Water Sci. Technol. 2020, 82, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Dalto, F.; Kuźniarska-Biernacka, I.; Pereira, C.; Mesquita, E.; Soares, O.S.G.P.; Pereira, M.F.R.; Rosa, M.J.; Mestre, A.S.; Carvalho, A.P.; Freire, C. Solar light-induced methylene blue removal over TiO2/AC composites and photocatalytic regeneration. Nanomaterials 2021, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin. Xanthan gum/titanium dioxide nanocomposite for photocatalytic degradation of methyl orange dye. Int. J. Biol. Macromol. 2019, 12, 1046–1053. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.K.; Imam, E.; Ramadan, A.R. Sand supported TiO2 photocatalyst in a tray photo-reactor for the removal of emerging contaminants in wastewater. Catal. Today 2018, 313, 55–62. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Singh, J.; Rawat, M.; Kumar, S. Expanding horizon: Green synthesis of TiO2 nanoparticles using Carica papaya leaves for photocatalysis application. Mater. Res. Express. 2019, 6, 095034. [Google Scholar] [CrossRef]
- Zaier, M.; Vidal, L.; Garreau, S.H.; Balan, L. Generating highly reflective and conductive metal layers through a light-assisted synthesis and assembling of silver nanoparticles. Sci. Rep. 2017, 7, 12410. [Google Scholar] [CrossRef] [Green Version]
- Giwa, A.; Nkeonye, P.O.; Bello, K.A.; Kolawole, E.G.; Campos, A.M.F.O. Solar photocatalytic degradation of reactive Yellow 81 and reactive Violet 1 in aqueous solution containing semiconductor oxides. Int. J. Appl. Sci. Technol. 2012, 2, 90–105. [Google Scholar]
- Alobaidi, T.; Alwared, A.I. Biosynthetic of titanium dioxide nanoparticles using Zizyphus Spina-Christi leaves extract: Properties. J. Ecol. Eng. 2022, 23, 315–324. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green synthesis of TiO2 nanoparticles using Acorus calamus leaf extract and evaluating its photocatalytic and in Vitro Antimicrobial Activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Taghdiri, M.; Doolabi, S.D. Shift the photocatalytic activity of P25 TiO2 nanoparticles toward the visible region upon surface modification with organic hybrid of phosphotungstate. Int. J. Photoenergy 2022, 2020, 8870194. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and optimization of terbinafine HCl loaded chitosan/xanthan gum nanoparticles containing gel: Ex-vivo permeation and in-vivo antifungal studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Mecozzi, M.; Sturchio, E. Computer assisted examination of Infrared and near Infrared spectra to assess structural and molecular changes in biological samples exposed to pollutants: A case of study. J. Imaging 2017, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Dhanya, A.; Aparna, K. Synthesis and evaluation of TiO2/Chitosan based hydrogel for the adsorptional photocatalytic degradation of Azo and Anthraquinone dye under UV Light Irradiation. Procedia Technol. 2016, 24, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Fardood, S.T.; Ramazani, A.; Golfar, Z.; Joo, S.W. Green synthesis of α-Fe2O3 (hematite) nanoparticles using Tragacanth Gel. J. Appl. Chem. Res. 2017, 3, 19–27. [Google Scholar]
- Maleki, A.; Moradi, F.; Shahmoradi, B.; Rezaee, R.; Lee, S.M. The photocatalytic removal of diazinon from aqueous solutions using tungsten oxide doped zinc oxide nanoparticles immobilized on glass substrate. J. Mol. Liq. 2019, 297, 111918. [Google Scholar] [CrossRef]
- Mansouri, L.; Sabelfeld, M.; Geissen, S.; Bousselmi, L. Catalysed Ozonation for removal of an endocrine-disrupting compound using the O3/Fenton reagents system. Environ. Tech. 2015, 36, 1721–1730. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Niaei, A.; Rasoulifard, M.H.; Khataee, A.R. Immobilization of TiO2 nano powder on glass beads for the photocatalytic decolorization of an azo dye C.I. Direct Red 23. J. Environ. Sci Health A Toxic Hazard. Subst. Environ. Eng. 2005, 40, 1605–1617. [Google Scholar] [CrossRef]
- Talebi, S.; Chaibakhsh, N.; Moradi-Shoeili, Z. Application of nanoscale ZnS/TiO2 composite for optimized photocatalytic decolorization of a textile dye. J. Appl. Res. Technol. 2017, 15, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Durán, A.; Monteagudo, J.M. Solar photocatalytic degradation of reactive blue 4 using a Fresnel lens. Water Res. 2007, 41, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Muruganandham, M.; Swaminathan, M. Solar driven decolorization of reactive Yellow 14 by advanced oxidation processes in heterogeneous and homogeneous media. Dye. Pigment. 2007, 72, 137–143. [Google Scholar] [CrossRef]
- Salarian, A.A.; Hami, Z.; Mirzaie, N.; Mohseni, S.M.; Asadi, A.; Bahrami, H.; Vosoughi, M.; Alinejad, A.; Zare, M. N-doped TiO2 nanosheets for photocatalytic degradation and mineralization of diazinon under simulated solar irradiation: Optimization and modeling using a response surface methodology. J. Mol. Liq. 2016, 220, 183–191. [Google Scholar] [CrossRef]
- Rastkari, N.; Eslami, A.; Nasseri, S.; Piroti, E.; Asadi, A. Optimizing parameters on nano photocatalytic degradation of Ibuprofen using UVC/ZnO processes by response surface methodology. Pol. J. Environ. Stud. 2017, 26, 785–794. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
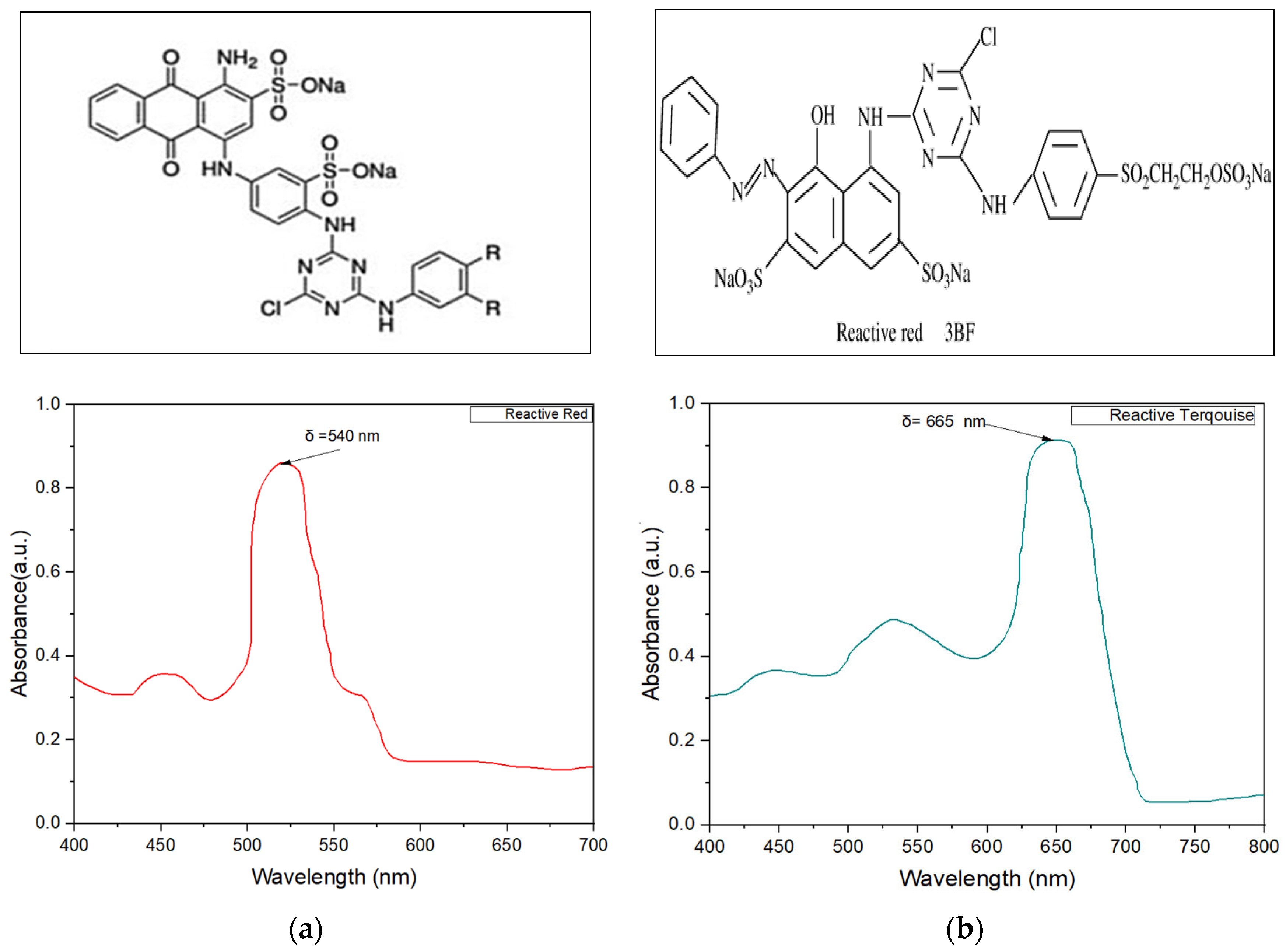









| Item | Reactive Turquoise | Reactive Red |
|---|---|---|
| Trade name | Blue 77 | Red 3B |
| Origin | China | China |
| Phase | Solid/Powder Package 5 kg | Solid/Powder Package 5 kg |
| Wavelength (nm) | 665 | 540 |
| Solubility g/L | 100–300 | 100 |
| Purity | 99% | 99% |
| CAS | 12236-86-1 | 70210-20-7 |
| pH(1% aqueous solution) | 7 ± 0.5 | 7.2 ± 0.3 |
| Molecular formula | C19H10Cl2N6Na2O7S2 | C41H25ClCuN14Na4O14S5 |
| Molecular weight | 615.3 | 1289.0 |
| Dye Concentration | First-Order for RR Dye | First-Order For RT Dye | ||||
|---|---|---|---|---|---|---|
| Removal % at 120 min | R2 | Kobs (min−1) | Removal % at 120 min | R2 | Kobs (min−1) | |
| 25 | 92.5 | 0.9424 | 0.0183 | 90.8 | 0.9428 | 0.0151 |
| 50 | 77 | 0.9205 | 0.011 | 74 | 0.9486 | 0.0094 |
| 75 | 66 | 0.9066 | 0.0083 | 70 | 0.9447 | 0.0085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwared, A.I.; Mohammed, N.A.; Al-Musawi, T.J.; Mohammed, A.A. Solar-Induced Photocatalytic Degradation of Reactive Red and Turquoise Dyes Using a Titanium Oxide/Xanthan Gum Composite. Sustainability 2023, 15, 10815. https://doi.org/10.3390/su151410815
Alwared AI, Mohammed NA, Al-Musawi TJ, Mohammed AA. Solar-Induced Photocatalytic Degradation of Reactive Red and Turquoise Dyes Using a Titanium Oxide/Xanthan Gum Composite. Sustainability. 2023; 15(14):10815. https://doi.org/10.3390/su151410815
Chicago/Turabian StyleAlwared, Abeer I., Noor A. Mohammed, Tariq J. Al-Musawi, and Ahmed A. Mohammed. 2023. "Solar-Induced Photocatalytic Degradation of Reactive Red and Turquoise Dyes Using a Titanium Oxide/Xanthan Gum Composite" Sustainability 15, no. 14: 10815. https://doi.org/10.3390/su151410815





