Pilot-Scale Anaerobic Co-Digestion of Food Waste and Polylactic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Substrate and Inoculum
2.2. Biochemical Methane Potential Experiments
2.3. Experimental Procedure and Pilot-Scale Basic Anaerobic Digester
2.4. Analytical Methods
3. Results and Discussion
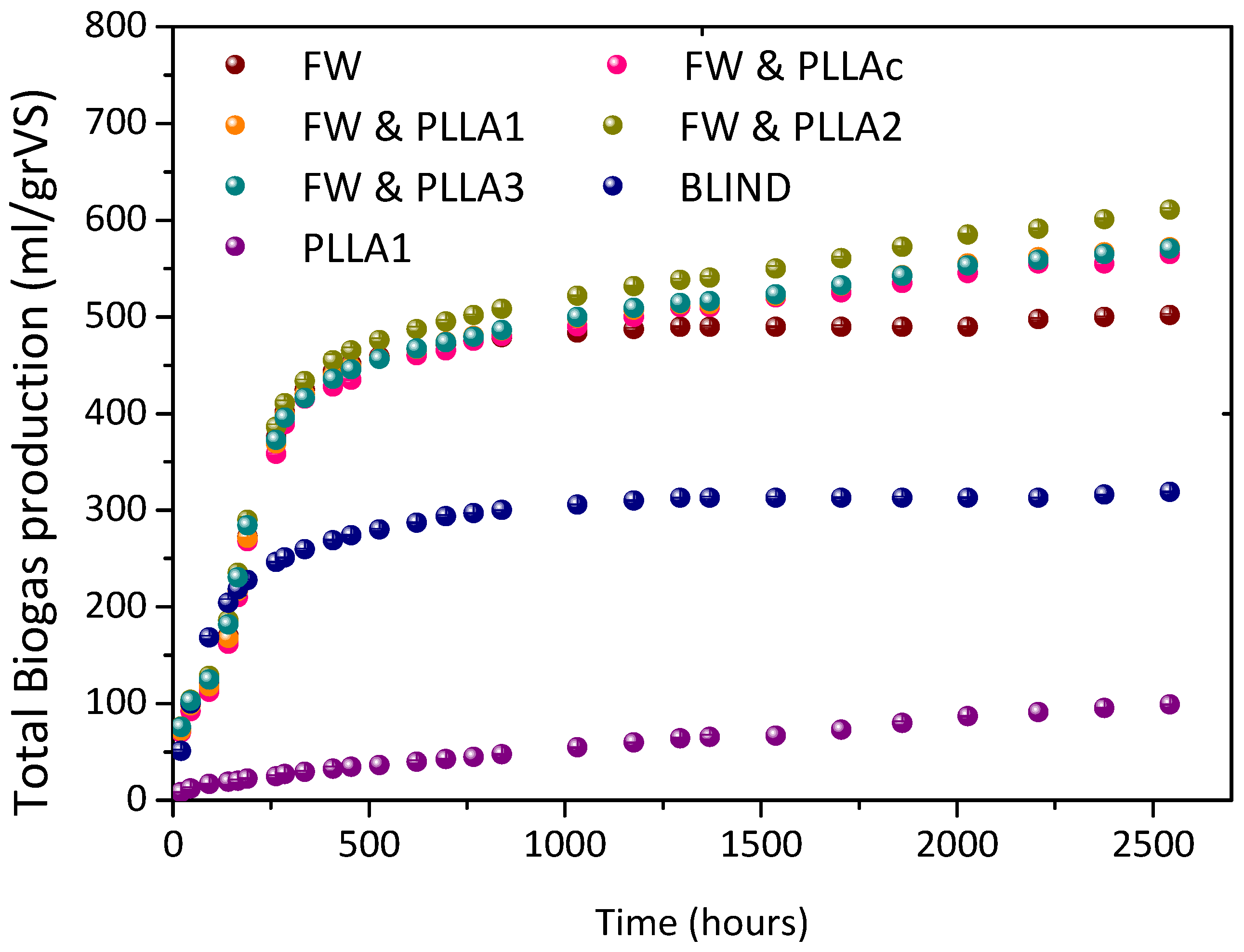
3.1. BMP Experiment
3.2. Pilot Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- European Parliament, 2019. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019L0904 (accessed on 24 January 2023).
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Cucina, M.; de Nisi, P.; Tambone, F.; Adani, F. The role of waste management in reducing bioplastics’ leakage into the environment: A review. Bioresour. Technol. 2021, 337, 125459. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Park, H.; Choi, O.; Sang, B.I. Anaerobic co-digestion of bioplastics as a sustainable mode of waste management with improved energy production—A review. Bioresour. Technol. 2021, 322, 124537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Heaven, S.; Banks, C.J. Degradation of some EN13432 compliant plastics in simulated mesophilic anaerobic digestion of food waste. Polym. Degrad. Stab. 2018, 147, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Papa, G.; Cucina, M.; Echchouki, K.; De Nisi, P.; Adani, F. Anaerobic digestion of organic waste allows recovering energy and enhancing the subsequent bioplastic degradation in soil. Resour. Conserv. Recycl. 2023, 188, 106694. [Google Scholar] [CrossRef]
- Nsair, A.; Onen Cinar, S.; Alassali, A.; Abu Qdais, H.; Kuchta, K. Operational parameters of biogas plants: A review and evaluation study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Faisal, S.; Thakur, N.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Saif, I.; Ali, G.; Zheng, Y.; Salama, E.S. Facilitated lignocellulosic biomass digestibility in anaerobic digestion for biomethane production: Microbial communities’ structure and interactions. J. Chem. Technol. Biotechnol. 2021, 96, 1798–1817. [Google Scholar] [CrossRef]
- Ekielski, A.; Zelazinski, T.; Mishra, P.K.; Skudlarski, J. Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics. Materials 2021, 14, 6092. [Google Scholar] [CrossRef]
- Cazaudehore, G.; Guyoneaud, R.; Evon, P.; Martin-Closas, L.; Pelacho, A.; Raynaud, C.; Monlau, F. Can anaerobic digestion be a suitable end-of-life scenario for biodegradable plastics? A critical review of the current situation, hurdles, and challenges. Biotechnol. Adv. 2022, 56, 107916. [Google Scholar] [CrossRef]
- Russo, M.A.; O’Sullivan, C.; Rounsefell, B.; Halley, P.J.; Truss, R.; Clarke, W.P. The anaerobic degradability of thermoplastic starch: Polyvinyl alcohol blends: Potential biodegradable food packaging materials. Bioresour. Technol. 2009, 100, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Taniguchi, M.; Miura, S.; Ohara, H.; Matsumoto, T.; Shirai, Y. Making Plastics from Garbage: A Novel Process for Poly-L-Lactate Production from Municipal Food Waste. J. Ind. Ecol. 2003, 7, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Angelidaki, I.; Sanders, W. Assessment of anaerobic biodegradability of macropollutants. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–144. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Cazaudehore, G.; Monlau, F.; Gassie, C.; Lallement, A.; Guyoneaud, R. Methane production and active microbial communities during anaerobic digestion of three commercial biodegradable coffee capsules under mesophilic and thermophilic conditions. Sci. Total Environ. 2021, 784, 146972. [Google Scholar] [CrossRef]
- Wang, S.; Lydon, K.A.; White, E.M.; Grubbs, J.B.; Lipp, E.K.; Locklin, J.; Jambeck, J.R. Biodegradation of Poly(3-hydroxybutyrate- co -3-hydroxyhexanoate) plastic under anaerobic sludge and aerobic seawater conditions: Gas evolution and microbial diversity. Environ. Sci. Technol. 2018, 52, 5700–5709. [Google Scholar] [CrossRef]
- Nachod, B.; Keller, E.; Hassanein, A.; Lansing, S. Assessment of petroleum-based plastic and bioplastics degradation using anaerobic digestion. Sustainability 2021, 13, 13295. [Google Scholar] [CrossRef]
- Shrestha, A.; van-Eerten Jansen, M.C.A.A.; Acharya, B. Biodegradation of Bioplastic Using Anaerobic Digestion at Retention Time as per Industrial Biogas Plant and International Norms. Sustainability 2020, 12, 4231. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation assessment of poly (lactic acid) filled with functionalized titania nanoparticles (PLA/TiO2) under compost conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.-H.; Kang, S.-W.; Kim, W.-J.; Kim, D.-H.; Im, S.-W. Anaerobic Co-Digestion of Bioplastics and Food Waste under Mesophilic and Thermophilic Conditions: Synergistic Effect and Biodegradation. Fermentation 2022, 8, 638. [Google Scholar] [CrossRef]
- Benn, N.; Zitomer, D. Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Naran, E.; Toor, U.A.; Kim, D. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Cucina, M.; de Nisi, P.; Trombino, L.; Tambone, F.; Adani, F. Degradation of bioplastics in organic waste by mesophilic anaerobic digestion, composting and soil incubation. Waste Manag. 2021, 134, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; Carlet, L.; De Nisi, P.; Somensi, A.; Giordano, A.; Adani, F. Degradation of biodegradable bioplastics under thermophilic anaerobic digestion: A full-scale approach. J. Clean. Prod. 2022, 368, 133232. [Google Scholar] [CrossRef]
- Kolstad, J.; Vink, Ε.; De Wilde, Β.; Debeer, L. Assessment of anaerobic degradation of Ingeo polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]



| Parameter | LPM | FW |
|---|---|---|
| pH | 7.7 ± 0.1 | 4.5 ± 0.0 |
| TS (g/kg) | 9.4 ± 9.2 | 256.3 ± 2.0 |
| VS (g/kg) | 5.7 ± 5.3 | 242.7 ± 5.0 |
| TCOD (g/L) | 11.8 ± 5.2 | 151.3 ± 10.0 |
| TN (g/L) | 0.2 ± 0.1 | 2.5 ± 0.0 |
| Parameter | Commercial PLLAc | PLLA1 | PLLA2 | PLLA3 |
|---|---|---|---|---|
| Weight Average Molecular Weight (Mw) | 153,373 | 149,452 | 27,987 | 55,663 |
| Number Average Molecular Weight (Mn) | 76,640 | 81,146 | 13,552 | 26,332 |
| Polydispersity Đ | 2.00 | 1.84 | 1.84 | 1.55 |
| Parameter | Unit | A | B | ||
|---|---|---|---|---|---|
| Operational parameters | |||||
| Operating volume | L | 180 | 180 | ||
| Retention time | days | 50 | 50 | ||
| Production Parameters | |||||
| Total Biogas | L | 1.710 | 1.889 | ||
| Total Biogas | L/kg VS | 185.5 | 197.6 | ||
| CH4 | % | 64 ± 4.7 | 65 ± 3.4 | ||
| Total Biomethane | L | 1.094 | 1.228 | ||
| Total Biomethane | L/kg VS | 118.7 | 128.5 | ||
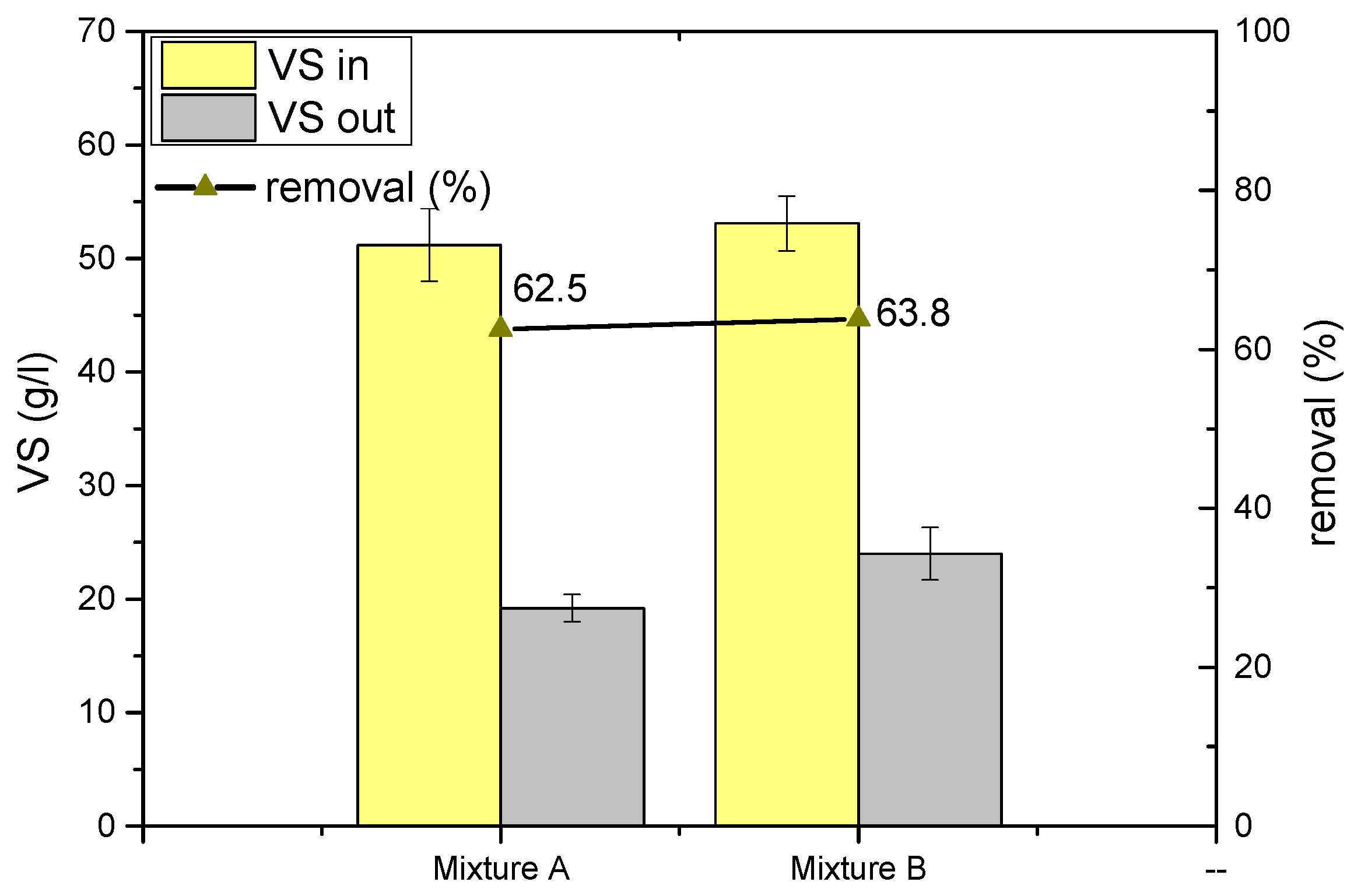
| Characteristics of Mixture and digestate | Mixture | Digestate | Mixture | Digestate | |
| VS | g/L | 51.2 ± 3.2 | 19.2 ± 1.2 | 53.1 ± 2.4 | 24.0 ± 2.3 |
| TCOD | g/L | 38.7 ± 3.1 | 23.7 ± 1.5 | 30.1 ± 2.7 | 19.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragkaki, A.; Tsompanidis, C.; Velonia, K.; Manios, T. Pilot-Scale Anaerobic Co-Digestion of Food Waste and Polylactic Acid. Sustainability 2023, 15, 10944. https://doi.org/10.3390/su151410944
Maragkaki A, Tsompanidis C, Velonia K, Manios T. Pilot-Scale Anaerobic Co-Digestion of Food Waste and Polylactic Acid. Sustainability. 2023; 15(14):10944. https://doi.org/10.3390/su151410944
Chicago/Turabian StyleMaragkaki, Angeliki, Christos Tsompanidis, Kelly Velonia, and Thrassyvoulos Manios. 2023. "Pilot-Scale Anaerobic Co-Digestion of Food Waste and Polylactic Acid" Sustainability 15, no. 14: 10944. https://doi.org/10.3390/su151410944





