Physical-Mechanical and Electrical Resistivity Properties of Cementitious Mortars Containing Fe3O4-MWCNTs Nanocomposite
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.1.1. Ordinary Portland Cement
2.1.2. Aggregate
2.2. Synthesis of MWCNTs and Fe3O4-MWCNTs
2.3. Mixture Proportions and Testing
3. Results and Discussion
3.1. Characterization of Synthesised MWCNTs and Fe3O4-MWCNTs
3.1.1. X-ray Diffractometer (XRD)
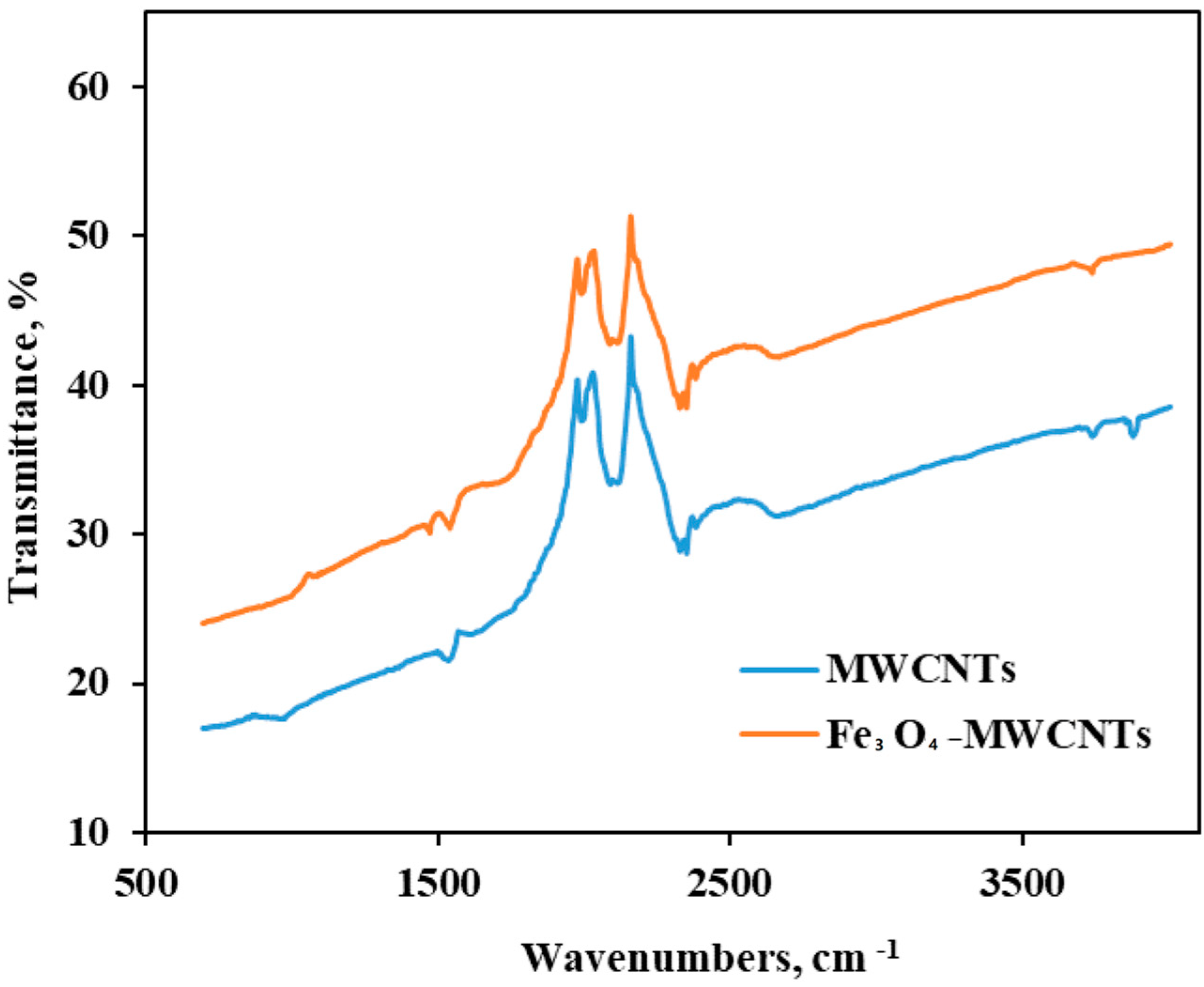
3.1.2. FTIR Spectroscopy
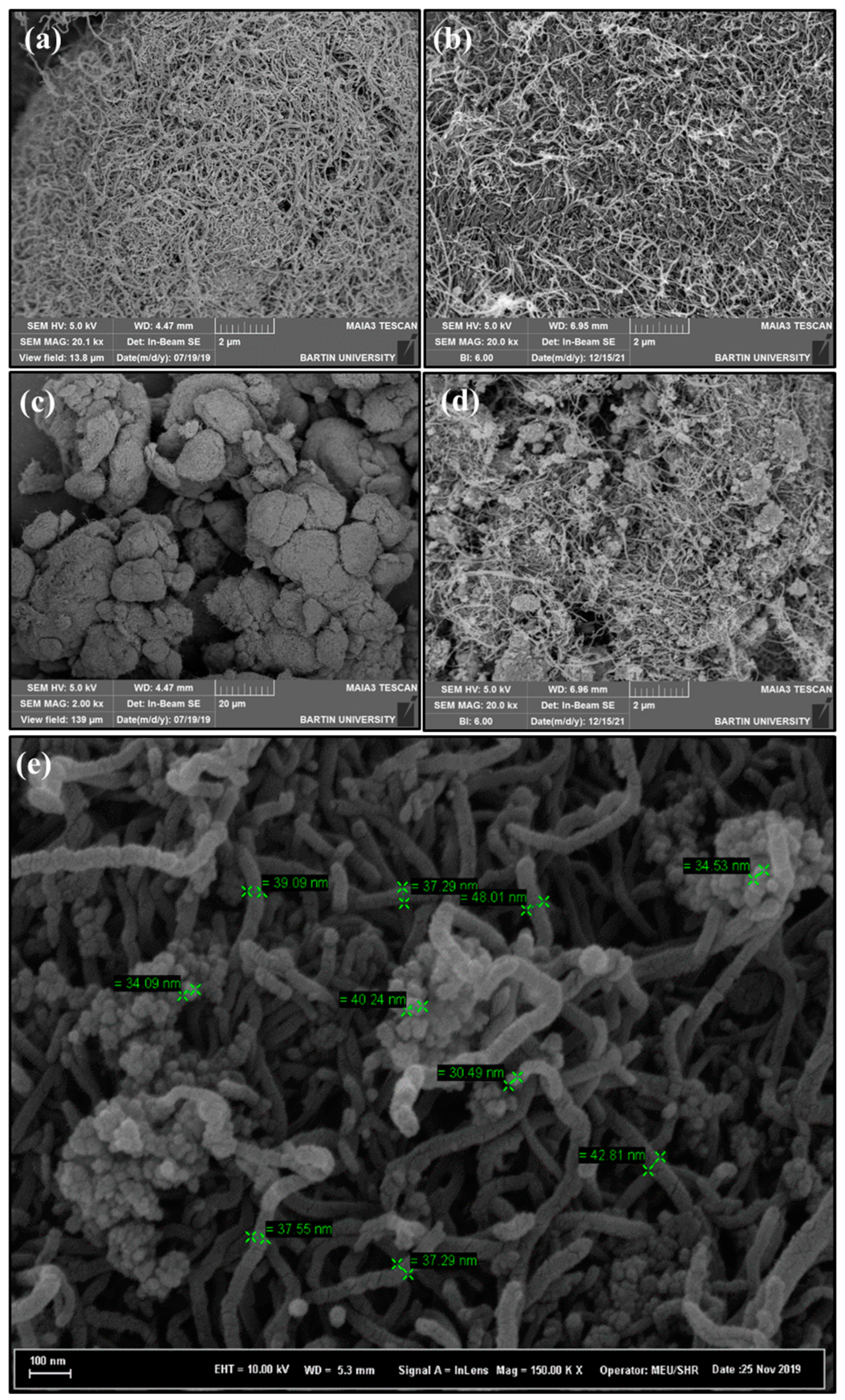
3.1.3. TEM
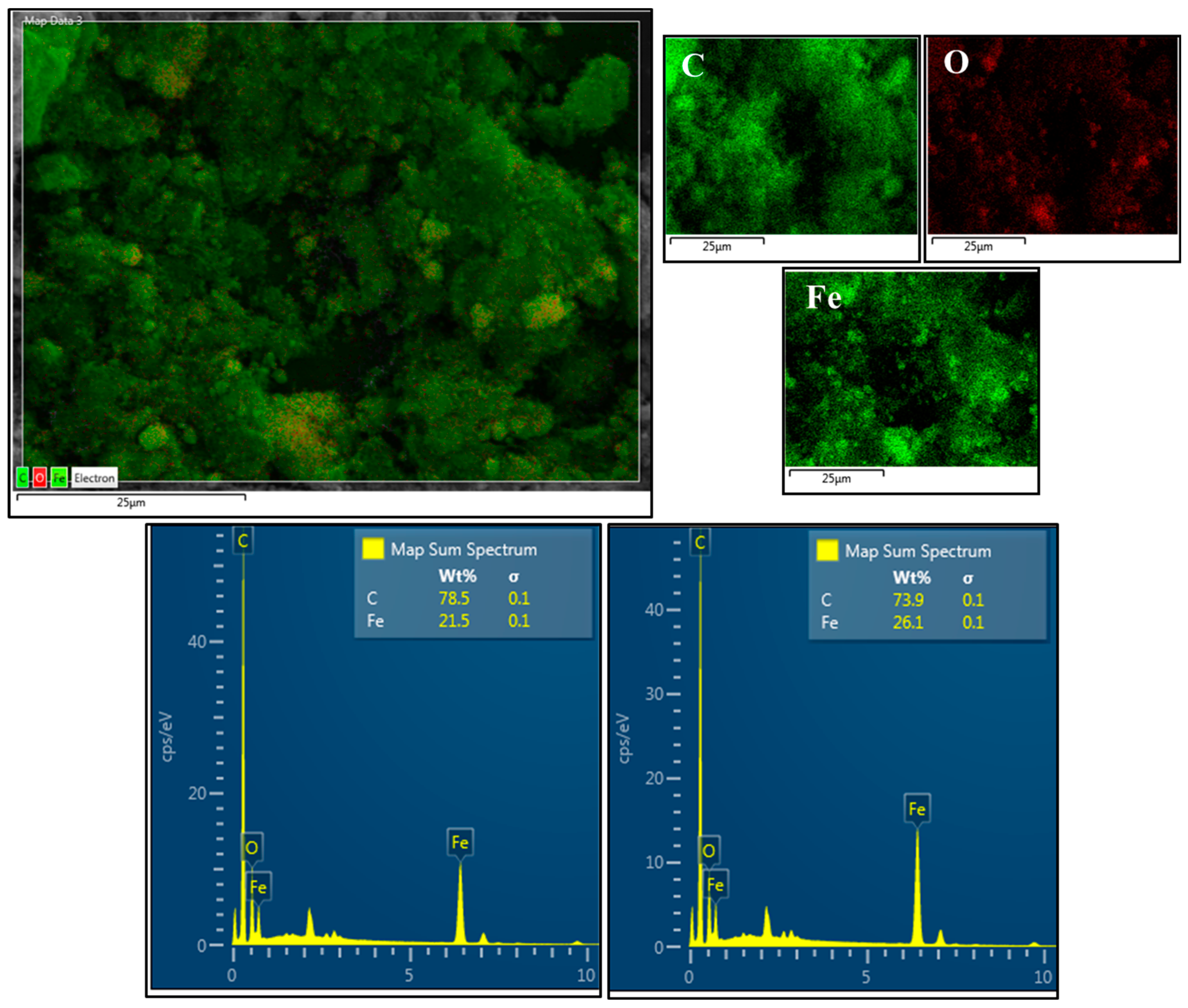
3.1.4. SEM/EDS
3.2. Characterization of Mortars
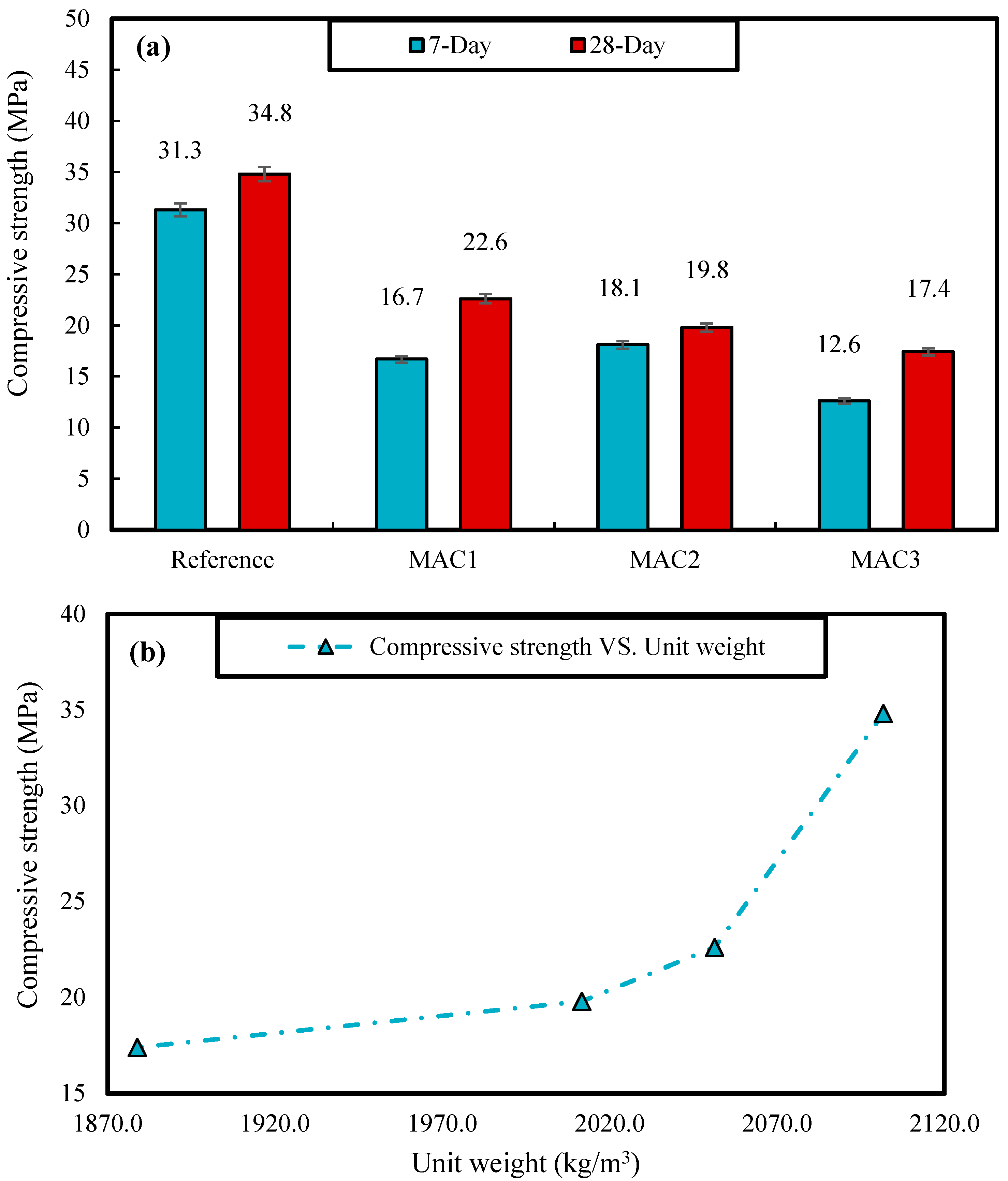
3.2.1. Unit Weight
3.2.2. Compressive Strength
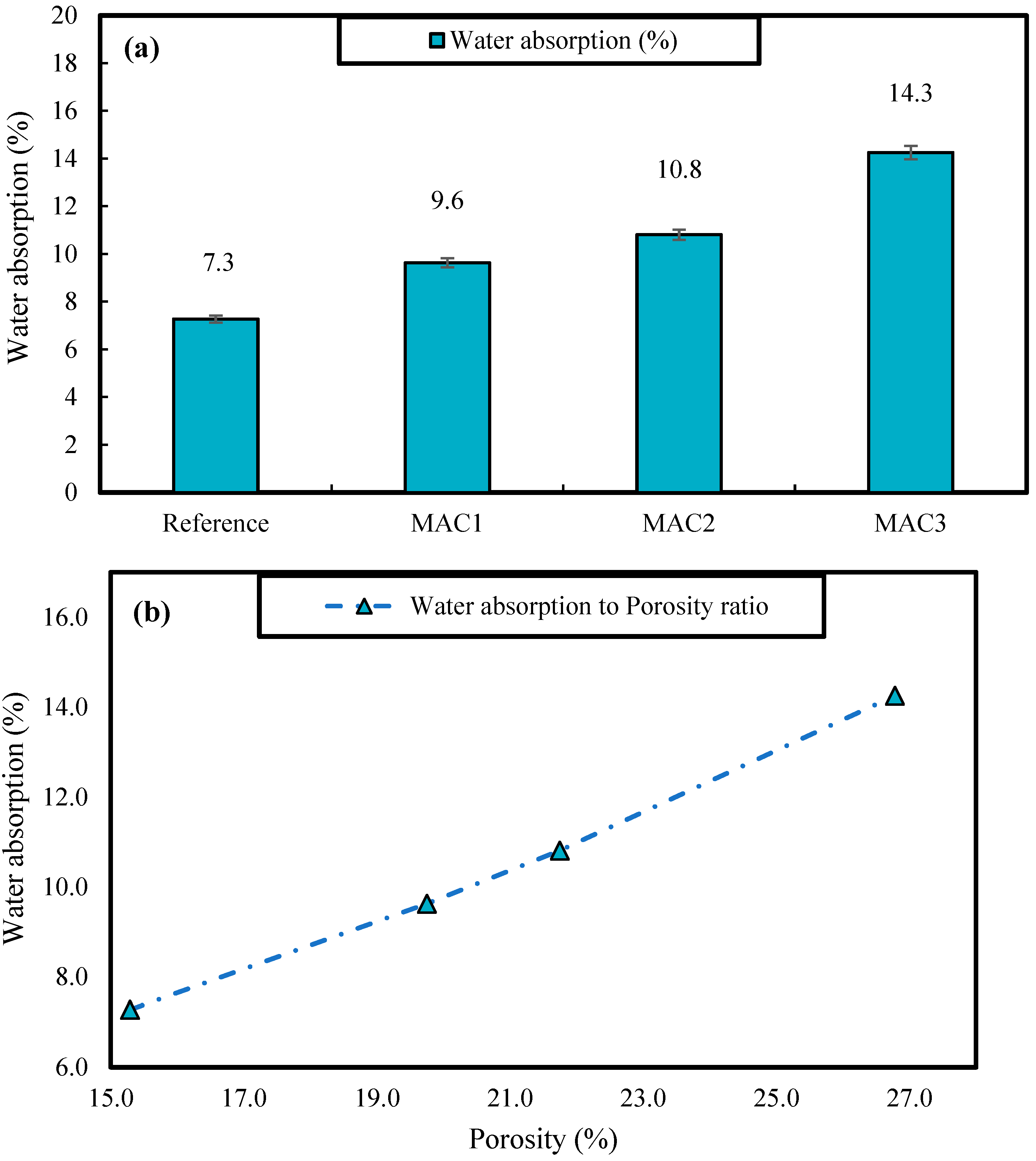
3.2.3. Apparent Porosity
3.2.4. Water Absorption
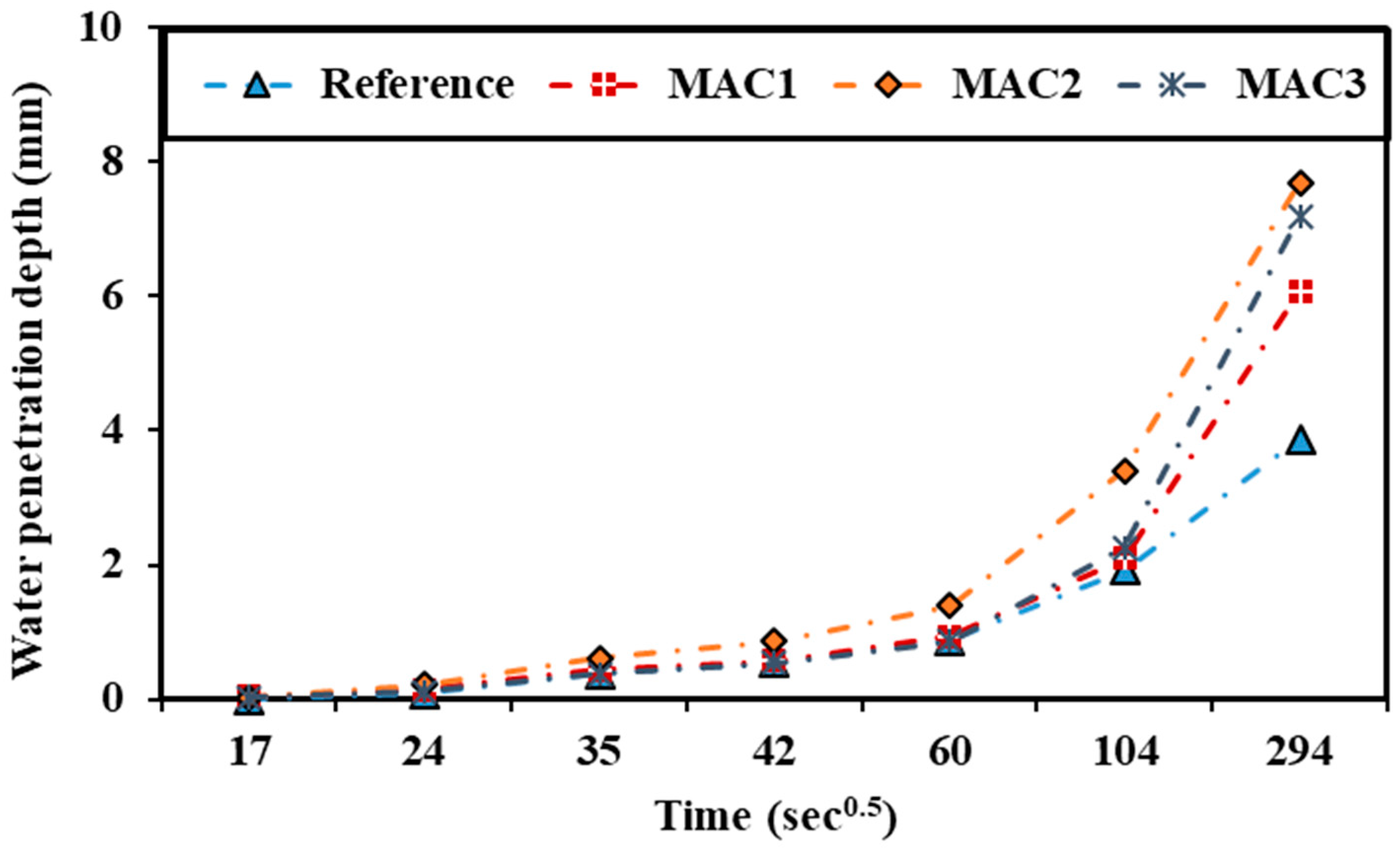
3.2.5. Sorptivity
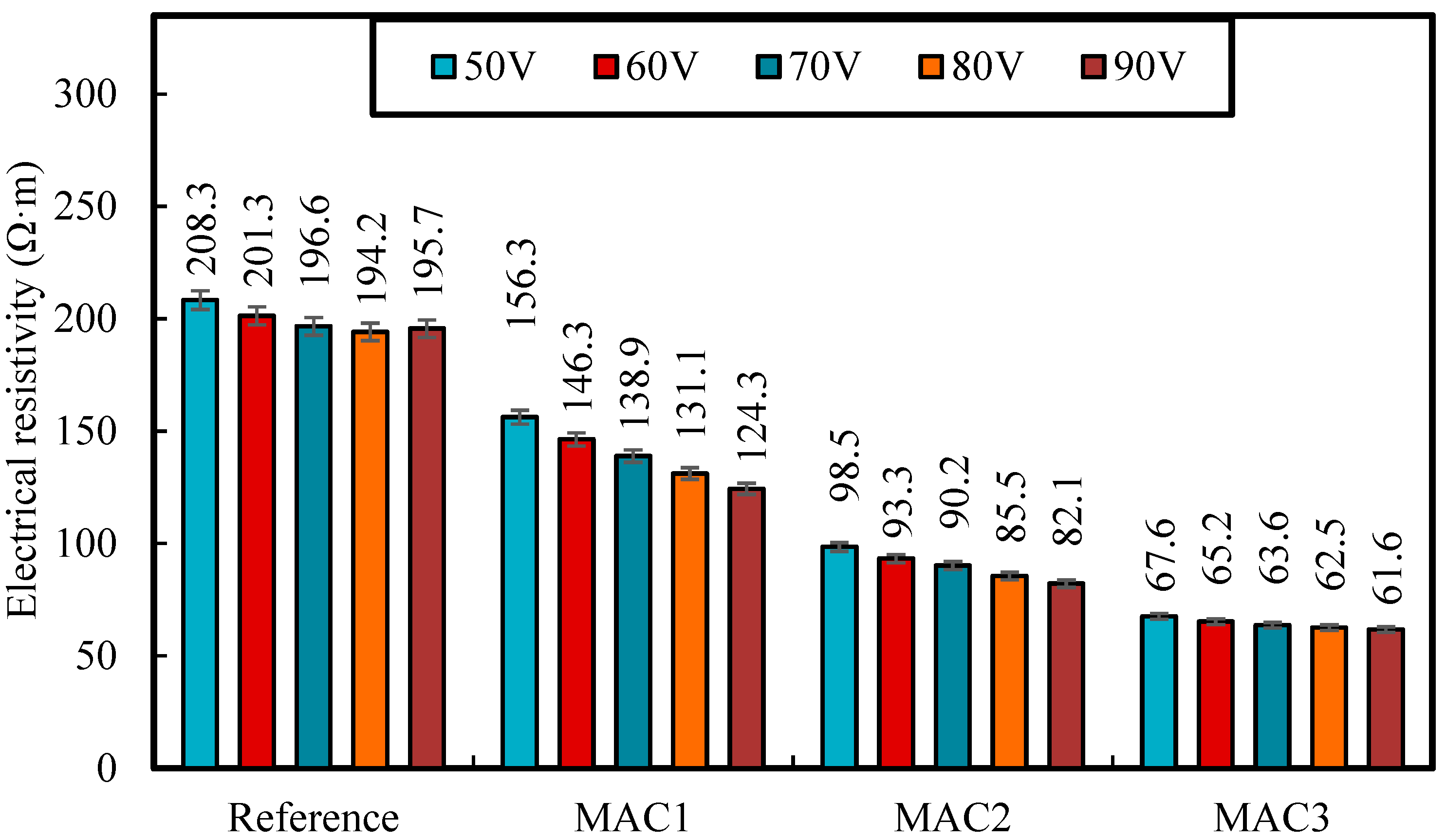
3.2.6. Electrical Resistivity
3.2.7. UPV
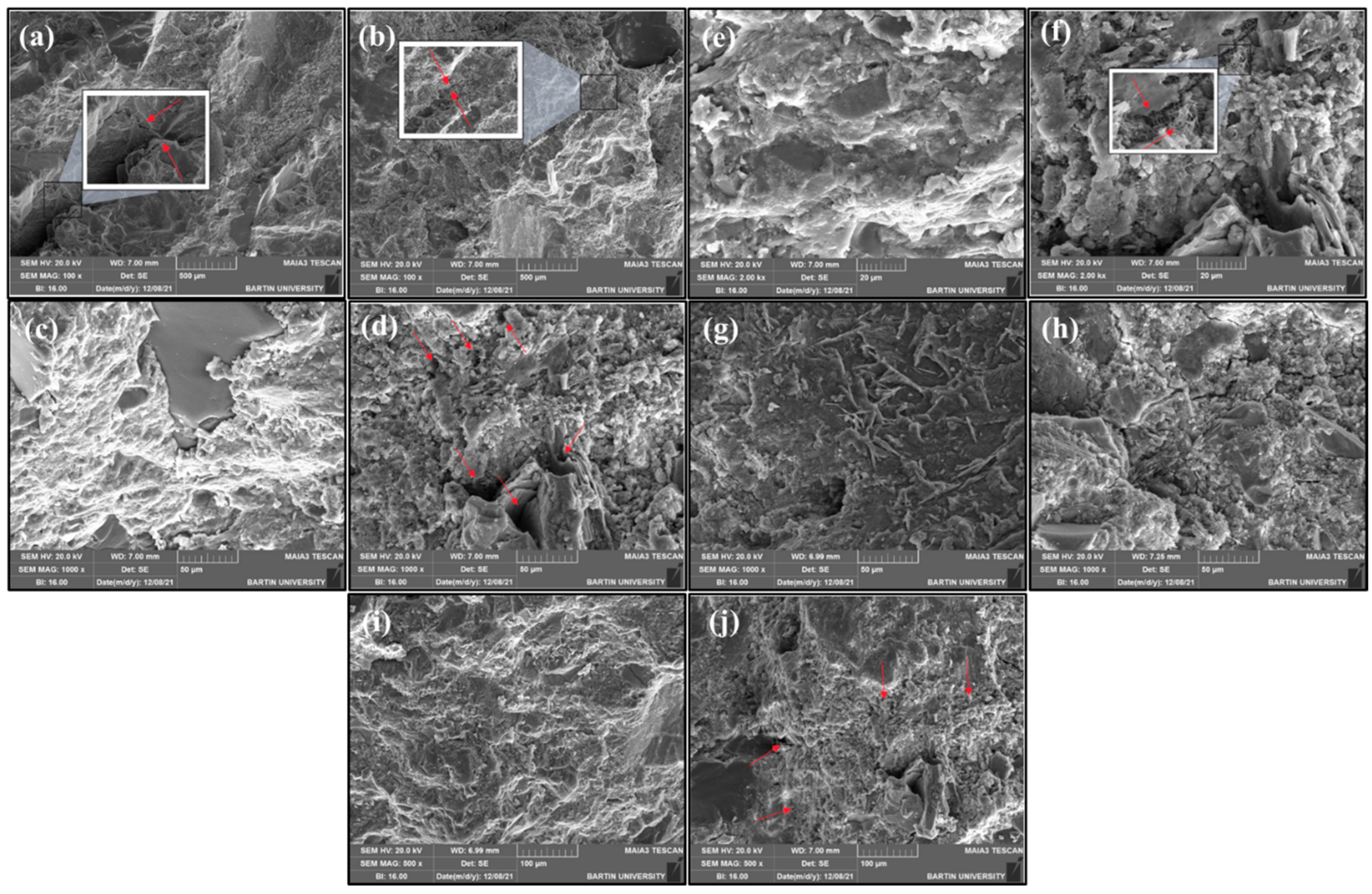
3.2.8. SEM/EDS of Mortars
4. Conclusions
- Based on SEM analysis, larger air-void cavities were embedded in the mortars containing Fe3O4-MWCNTs nanocomposite than that of the unreinforced mortar, which can potentially be due to the adverse early effect of conductive energy/force that creates slight strains in fresh mixtures, developing localized air cavities in the mortar microstructure. Also, it is a possibility that the combined effect of two nano materials (e.g., in this case, Fe3O4 and MWCNTs) is responsible for the coagulation of materials that can reduce the mixing efficiency and result in lower physical-mechanical properties.
- The origin of cracks in mortars containing Fe3O4-MWCNTs nanocomposite was different from that of unreinforced mortars and is potentially caused by the air cavities. This observation was further confirmed by the inhomogenously dispersed C-S-H in the microstructure of the mortars containing a higher content of Fe3O4-MWCNTs nanocomposites. This can cause anisotropic behavior of the produced mortars which, under stress can have variable performance.
- Mortars containing 3% Fe3O4-MWCNTs nanocomposite exhibited ~11, 75, 95, 46, and 50% lower unit weight, apparent porosity, water absorption, sorptivity, and compressive strength at 28 days of curing than unreinforced mortar, respectively. The reason behind that can be the effect of Fe3O4-MWCNTs being exerted on the freshly mixed materials that can cause a series of localized flocculation and air cavities within the mixture. This hypothesis is aligned with the observations on SEM micrographs that showed an inhomogeneous microstructure of the mortars produced with Fe3O4-MWCNTs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gencel, O.; Nodehi, M.; Hekimoğlu, G.; Ustaoğlu, A.; Sarı, A.; Kaplan, G.; Bayraktar, O.Y.; Sutcu, M.; Ozbakkaloglu, T. Foam Concrete Produced with Recycled Concrete Powder and Phase Change Materials. Sustainability 2022, 14, 7458. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, L.; Zhang, Q.; Nie, Z.; Hao, L. Enhanced thermoelectric properties of cement-based composites with expanded graphite for climate adaptation and large-scale energy harvesting. Energy Build. 2018, 159, 66–74. [Google Scholar] [CrossRef]
- Ibarra-Sierra, V.G.; Sandoval-Santana, J.C.; Kunold, A.; Herrera, S.A.; Naumis, G.G. Dirac materials under linear polarized light: Quantum wave function time evolution and topological Berry phases as classical charged particles trajectories under electromagnetic fields. J. Phys. Mater. 2022, 5, 014002. [Google Scholar] [CrossRef]
- Lewis, L.H.; Jiménez-Villacorta, F. Perspectives on Permanent Magnetic Materials for Energy Conversion and Power Generation. Metall. Mater. Trans. A 2013, 44, 2–20. [Google Scholar] [CrossRef]
- Dent, P.C. Rare earth elements and permanent magnets (invited). J. Appl. Phys. 2012, 111, 07A721. [Google Scholar] [CrossRef] [Green Version]
- Jiao, D.; Lesage, K.; Yardimci, M.Y.; El Cheikh, K.; Shi, C.; De Schutter, G. Structural evolution of cement paste with nano-Fe3O4 under magnetic field—Effect of concentration and particle size of nano-Fe3O4. Cem. Concr. Compos. 2021, 120, 104036. [Google Scholar] [CrossRef]
- Tjong, S.C. Carbon Nanotube Reinforced Composites; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Javey, A.; Kong, J. Carbon Nanotube Electronics; Springer: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Madenci, E.; Özkılıç, Y.O.; Aksoylu, C.; Asyraf, M.R.M.; Syamsir, A.; Supian, A.B.M.; Mamaev, N. Buckling Analysis of CNT-Reinforced Polymer Composite Beam Using Experimental and Analytical Methods. Materials 2023, 16, 614. [Google Scholar] [CrossRef]
- Madenci, E.; Özkılıç, Y.O.; Aksoylu, C.; Asyraf, M.R.M.; Syamsir, A.; Supian, A.B.M.; Elizaveta, B. Experimental and Analytical Investigation of Flexural Behavior of Carbon Nanotube Reinforced Textile Based Composites. Materials 2023, 16, 2222. [Google Scholar] [CrossRef]
- Onuralp, Y.; Madenci, E.; Yasin, Ö.; Özkılıç, O.; Hakamy, A.; Hakamy, A.; Tounsi, A. Experimental tensile test and micro mechanic investigation on carbon nanotube reinforced carbon fiber composite beams. Adv. Nano Res. 2023, 14. [Google Scholar]
- Harris, P.J.F. Carbon Nanotube Science; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Samadishadlou, M.; Farshbaf, M.; Annabi, N.; Kavetskyy, T.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A.; Mousavi, S. Magnetic carbon nanotubes: Preparation, physical properties, and applications in biomedicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1314–1330. [Google Scholar] [CrossRef] [Green Version]
- Pantano, A. Carbon Nanotube Based Composites: Processing, Properties, Modelling and Application; Smithers Rapra Technology: Shropshire, UK, 2012. [Google Scholar]
- Nayfeh, M.H. Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines; Elsevier: Amsterdam, The Netherlands, 2008; pp. 287–309. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Carbon Nanotubes for Sustainable Energy Applications. ChemSusChem 2011, 4, 913–925. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Bankole, M.T.; Abdulkareem, A.S.; Ochigbo, S.S.; Afolabi, A.S.; Abubakre, O.K. Full factorial design approach to carbon nanotubes synthesis by CVD method in argon environment. S. Afr. J. Chem. Eng. 2017, 24, 17–42. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y. (Ed.) Carbon Nanotube and Related Field Emitters; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Li, H.; Ou, J.P. Smart Concrete, Sensors and Self-Sensing Concrete Structures. Key Eng. Mater. 2008, 400–402, 69–80. [Google Scholar] [CrossRef]
- Jung, M.; Lee, Y.S.; Hong, S.G. Effect of Incident Area Size on Estimation of EMI Shielding Effectiveness for Ultra-High Performance Concrete with Carbon Nanotubes. IEEE Access 2019, 7, 183105–183117. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.; Park, S.; Chung, W. Enhanced detection systems of filling rates using carbon nanotube cement grout. Nanomaterials 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çınar, E.; Uygunoğlu, T.; Şimşek, B.; Topçu, İ.B. Effect of carbon black on electrical curing of fresh concrete for cold regions. Constr. Build. Mater. 2020, 247. [Google Scholar] [CrossRef]
- Ahmed, H.I. Behavior of magnetic concrete incorporated with Egyptian nano alumina. Constr. Build. Mater. 2017, 150, 404–408. [Google Scholar] [CrossRef]
- Chuewangkam, N.; Pinitsoontorn, S.; Chindaprasirt, P. Properties of NdFeB magnetic cement. Cem. Concr. Compos. 2019, 103, 204–212. [Google Scholar] [CrossRef]
- He, Y.; Lu, L.; Sun, K.; Wang, F.; Hu, S. Electromagnetic wave absorbing cement-based composite using Nano-Fe3O4 magnetic fluid as absorber. Cem. Concr. Compos. 2018, 92, 1–6. [Google Scholar] [CrossRef]
- Assi, L.; Alsalman, A.; Bianco, D.; Ziehl, P.; El-Khatib, J.; Bayat, M.; Hussein, F.H. Multiwall carbon nanotubes (MWCNTs) dispersion & mechanical effects in OPC mortar & paste: A review. J. Build. Eng. 2021, 43, 102512. [Google Scholar] [CrossRef]
- ASTM C150/C150M-19a; Standard Specification for Portland Cement. Book of ASTM Standards. 04.01. ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–10. [CrossRef]
- Choi, H.; Gong, J.; Lim, Y.; Im, K.H.; Jeon, M. Effects of the electrical conductivity and orientation of silicon substrate on the synthesis of multi-walled carbon nanotubes by thermal chemical vapor deposition. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Yamashita, M.; Ata, S.; Futaba, D.N.; Yamada, T.; Hata, K. Controlling exfoliation in order to minimize damage during dispersion of long SWCNTs for advanced composites. Sci. Rep. 2015, 4, 3907. [Google Scholar] [CrossRef] [Green Version]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Verma, M.; Brar, S.K. Biopolymer-Based Nanomaterials: Potential Applications in Bioremediation of Contaminated Wastewaters and Soils, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef] [Green Version]
- Bayazit, Ş.S.; Kerkez, Ö. Hexavalent chromium adsorption on superparamagnetic multi-wall carbon nanotubes and activated carbon composites. Chem. Eng. Res. Des. 2014, 92, 2725–2733. [Google Scholar] [CrossRef]
- ASTM ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–3.
- ASTM C349-08; Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–4.
- C1760/1876, A. ASTM Standard C1760; Standard Test Method for Bulk Electrical Conductivity of Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–5.
- Hekmatara, H.; Seifi, M.; Forooraghi, K. Microwave absorption property of aligned MWCNT/Fe3O4. J. Magn. Magn. Mater. 2013, 346, 186–191. [Google Scholar] [CrossRef]
- Jiao, Z.; Qiu, J. Microwave absorption performance of iron oxide/multiwalled carbon nanotubes nanohybrids prepared by electrostatic attraction. J. Mater. Sci. 2018, 53, 3640–3646. [Google Scholar] [CrossRef]
- Nas, M.S.; Kuyuldar, E.; Demirkan, B.; Calimli, M.H.; Demirbaş, O.; Sen, F. Magnetic nanocomposites decorated on multiwalled carbon nanotube for removal of Maxilon Blue 5G using the sono-Fenton method. Sci. Rep. 2019, 9, 10850. [Google Scholar] [CrossRef] [Green Version]
- Tolba, A.; Gar Alalm, M.; Elsamadony, M.; Mostafa, A.; Afify, H.; Dionysiou, D.D. Modeling and optimization of heterogeneous Fenton-like and photo-Fenton processes using reusable Fe3O4-MWCNTs. Process Saf. Environ. Prot. 2019, 128, 273–283. [Google Scholar] [CrossRef]
- Reddy, P.N.; Kavyateja, B.V.; Jindal, B.B. Structural health monitoring methods, dispersion of fibers, micro and macro structural properties, sensing, and mechanical properties of self-sensing concrete—A review. Struct. Concr. 2021, 22, 793–805. [Google Scholar] [CrossRef]
- Florez, R.; Colorado, H.A.; Alajo, A.; Giraldo, C.H.C. The material characterization and gamma attenuation properties of Portland cement-Fe3O4 composites for potential dry cask applications. Prog. Nucl. Energy 2019, 111, 65–73. [Google Scholar] [CrossRef]
- Gao, F.; Tian, W.; Cheng, X. Investigation of moisture migration of MWCNTs concrete after different heating-cooling process by LF-NMR. Constr. Build. Mater. 2021, 288, 123146. [Google Scholar] [CrossRef]
- Jiao, D.; Lesage, K.; Yardimci, M.Y.; El Cheikh, K.; Shi, C.; De Schutter, G. Rheological behavior of cement paste with nano-Fe3O4 under magnetic field: Magneto-rheological responses and conceptual calculations. Cem. Concr. Compos. 2021, 120, 104035. [Google Scholar] [CrossRef]
- Cwirzen, A.; Habermehl-Cwirzen, K.; Penttala, V. Surface decoration of carbon nanotubes and mechanical properties of cement/carbon nanotube composites. Adv. Cem. Res. 2008, 20, 65–73. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Li, Q. Mechanical properties and microstructure of multi-walled carbon nanotube-reinforced cement paste. Constr. Build. Mater. 2015, 76, 16–23. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Madhavi, T.C.; Pavithra, P.; Singh, S.B.; Raj, S.V.; Paul, S. Effect of Multiwalled Carbon Nanotubes On Mechanical Properties of Concrete. Int. J. Sci. Res. 2012, 2, 166–168. [Google Scholar] [CrossRef]
- Sikora, P.; Horszczaruk, E.; Cendrowski, K.; Mijowska, E. The Influence of Nano-Fe3O4 on the Microstructure and Mechanical Properties of Cementitious Composites. Nanoscale Res. Lett. 2016, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuya, T. Electrical Resistance of Ferromagnetic Metals. Prog. Theor. Phys. 1956, 16, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Goodings, D.A. Electrical Resistivity of Ferromagnetic Metals at Low Temperatures. Phys. Rev. 1963, 132, 542–558. [Google Scholar] [CrossRef]
- Heaney, M.B. Electrical conductivity and resistivity. Electr. Meas. Signal Process. Disp. 2003, 1983, 7-1–7-14. [Google Scholar] [CrossRef]
- García, Á.; Schlangen, E.; van de Ven, M.; Liu, Q. Electrical conductivity of asphalt mortar containing conductive fibers and fillers. Constr. Build. Mater. 2009, 23, 3175–3181. [Google Scholar] [CrossRef]
- Le, J.-L.; Du, H.; Dai Pang, S. Use of 2D Graphene Nanoplatelets (GNP) in cement composites for structural health evaluation. Compos. Part B Eng. 2014, 67, 555–563. [Google Scholar] [CrossRef]
- Chacko, R.M.; Banthia, N.; Mufti, A.A. Carbon-fiber-reinforced cement-based sensors. Can. J. Civ. Eng. 2007, 34, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Chung, D.D.L. A comparative study of steel-and carbon-fibre cement as piezoresistive strain sensors. Adv. Cem. Res. 2003, 15, 119–128. [Google Scholar] [CrossRef]
- Lübeck, A.; Gastaldini, A.L.G.; Barin, D.S.; Siqueira, H.C. Compressive strength and electrical properties of concrete with white Portland cement and blast-furnace slag. Cem. Concr. Compos. 2012, 34, 392–399. [Google Scholar] [CrossRef]
- Lavagna, L.; Musso, S.; Ferro, G.; Pavese, M. Cement-based composites containing functionalized carbon fibers. Cem. Concr. Compos. 2018, 88, 165–171. [Google Scholar] [CrossRef]
- Solís-Carcaño, R.; Moreno, E.I. Evaluation of concrete made with crushed limestone aggregate based on ultrasonic pulse velocity. Constr. Build. Mater. 2008, 22, 1225–1231. [Google Scholar] [CrossRef]
- Güneyli, H.; Karahan, S.; Güneyli, A.; Yapιcι, N. Water content and temperature effect on ultrasonic pulse velocity of concrete. Russ. J. Nondestruct. Test. 2017, 53, 159–166. [Google Scholar] [CrossRef]
- Zhang, J.; Ju, S.; Jiang, D.; Peng, H.-X. Reducing dispersity of mechanical properties of carbon fiber/epoxy composites by introducing multi-walled carbon nanotubes. Compos. Part B Eng. 2013, 54, 371–376. [Google Scholar] [CrossRef]




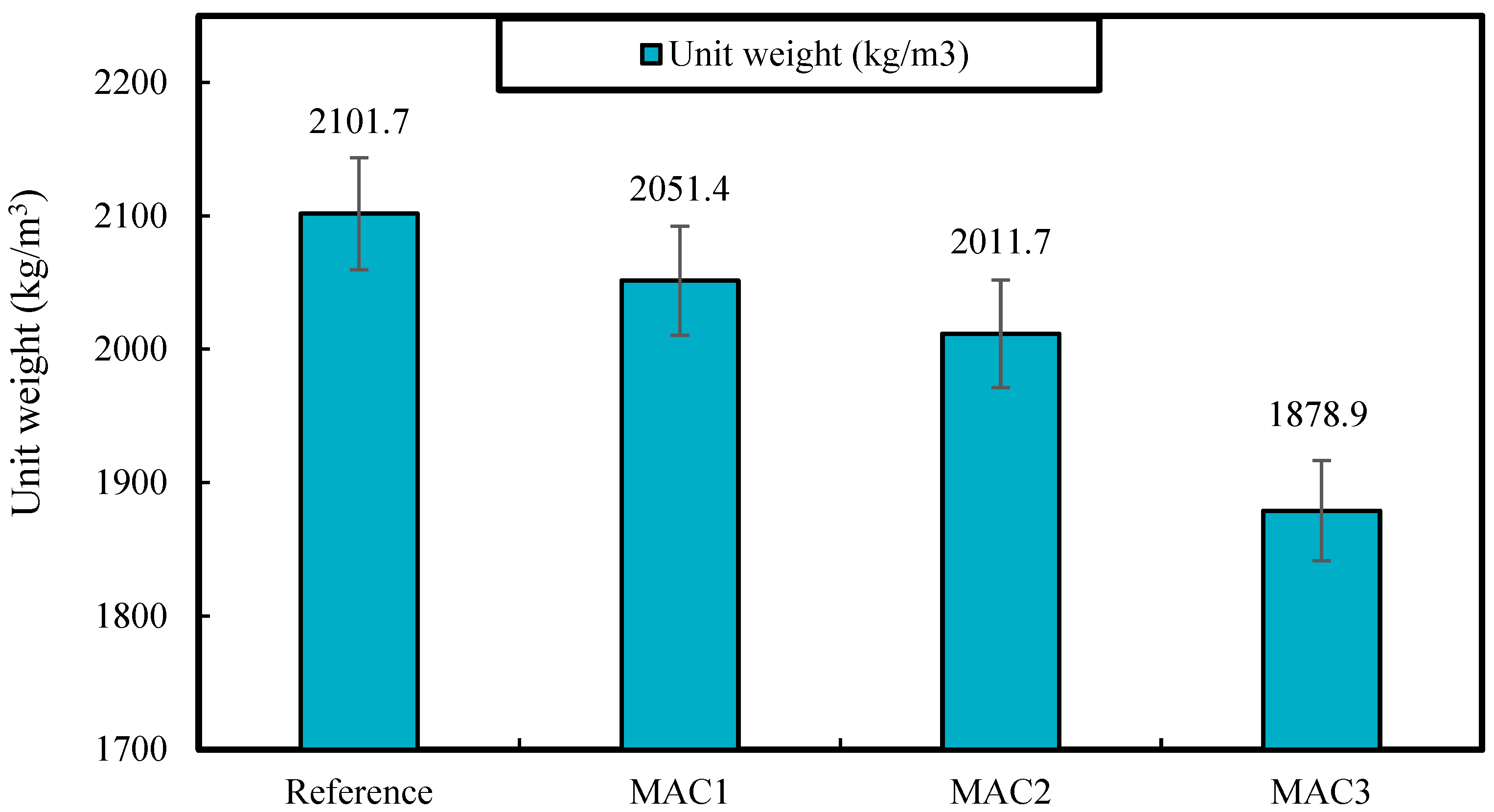


| SiO2 (%) | 18.8 |
| Al2O3 (%) | 5.3 |
| Fe2O3 (%) | 3.4 |
| CaO (%) | 63.7 |
| MgO (%) | 1.7 |
| SO3 (%) | 2.7 |
| Na2O(eqv) (%) | 0.8 |
| Specific gravity | 3.15 |
| Setting time (Initial/Final) | 165/205 |
| Blaine fineness (m2/kg) | 333 |
| Loss on ignition (LOI) | <4% |
| Setting time | >45 min (initial) and <6.5 h (final). |
| Specimen | Sand (g) | Cement (g) | MAC (g) | MAC (Binder wt %) | Water (g) |
|---|---|---|---|---|---|
| Reference | 1350.0 | 450 | 0 | 0 | 225 |
| MAC1 | 1345.5 | 450 | 4.5 | 1 | 225 |
| MAC2 | 1341.0 | 450 | 9.0 | 2 | 225 |
| MAC3 | 1336.5 | 450 | 13.5 | 3 | 225 |
| Matrix | Additive | Electrical Resistivity (Ω·m) | Reference |
|---|---|---|---|
| Mortar | Graphite | 75 | [55] |
| Mortar | Graphene | 2500 | [56] |
| Cement | Carbon fibre | 6.75 | [57] |
| Cement | Steel fiber | 0.57 | [58] |
| Cement | Blast-furnace slag | 596.7 | [59] |
| Cement | Pristine carbon fiber | 35 | [60] |
| Cement | Carbon fiber treated with piranha solution | 5 | [60] |
| Cement | Fe3O4-MWCNTs nanocomposite | 61.6 | This study |
| Mix | Ca | Si | Na | Al | K | Fe |
|---|---|---|---|---|---|---|
| Reference | 63.28 | 24.61 | 3.70 | 5.45 | 6.46 | 4.17 |
| MAC1 | 63.85 | 21.96 | 5.90 | 8.57 | 6.20 | 3.63 |
| MAC2 | 68.28 | 16.64 | 0 | 5.41 | 10.4 | 6.30 |
| MAC3 | 72.78 | 19.01 | 3.40 | 3.26 | 3.20 | 7.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selen, V.; Guler, O.; Nodehi, M.; Sarı, A.; Yaras, A.; Gencel, O.; Gholampour, A.; Ozbakkaloglu, T. Physical-Mechanical and Electrical Resistivity Properties of Cementitious Mortars Containing Fe3O4-MWCNTs Nanocomposite. Sustainability 2023, 15, 11045. https://doi.org/10.3390/su151411045
Selen V, Guler O, Nodehi M, Sarı A, Yaras A, Gencel O, Gholampour A, Ozbakkaloglu T. Physical-Mechanical and Electrical Resistivity Properties of Cementitious Mortars Containing Fe3O4-MWCNTs Nanocomposite. Sustainability. 2023; 15(14):11045. https://doi.org/10.3390/su151411045
Chicago/Turabian StyleSelen, Veyis, Omer Guler, Mehrab Nodehi, Ahmet Sarı, Ali Yaras, Osman Gencel, Aliakbar Gholampour, and Togay Ozbakkaloglu. 2023. "Physical-Mechanical and Electrical Resistivity Properties of Cementitious Mortars Containing Fe3O4-MWCNTs Nanocomposite" Sustainability 15, no. 14: 11045. https://doi.org/10.3390/su151411045
APA StyleSelen, V., Guler, O., Nodehi, M., Sarı, A., Yaras, A., Gencel, O., Gholampour, A., & Ozbakkaloglu, T. (2023). Physical-Mechanical and Electrical Resistivity Properties of Cementitious Mortars Containing Fe3O4-MWCNTs Nanocomposite. Sustainability, 15(14), 11045. https://doi.org/10.3390/su151411045









