Assessment of Vinca rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Extraction of Total Petroleum Hydrocarbons (TPH)
2.3. TPH Fractionation
2.4. Gas Chromatographic Analysis
2.5. Sulfur Quantification
2.6. Cadmium and Lead Quantification
2.7. Statistical Analysis
3. Results
3.1. Total Petroleum Hydrocarbons
3.2. Saturated Hydrocarbon
3.3. Aromatic Hydrocarbons
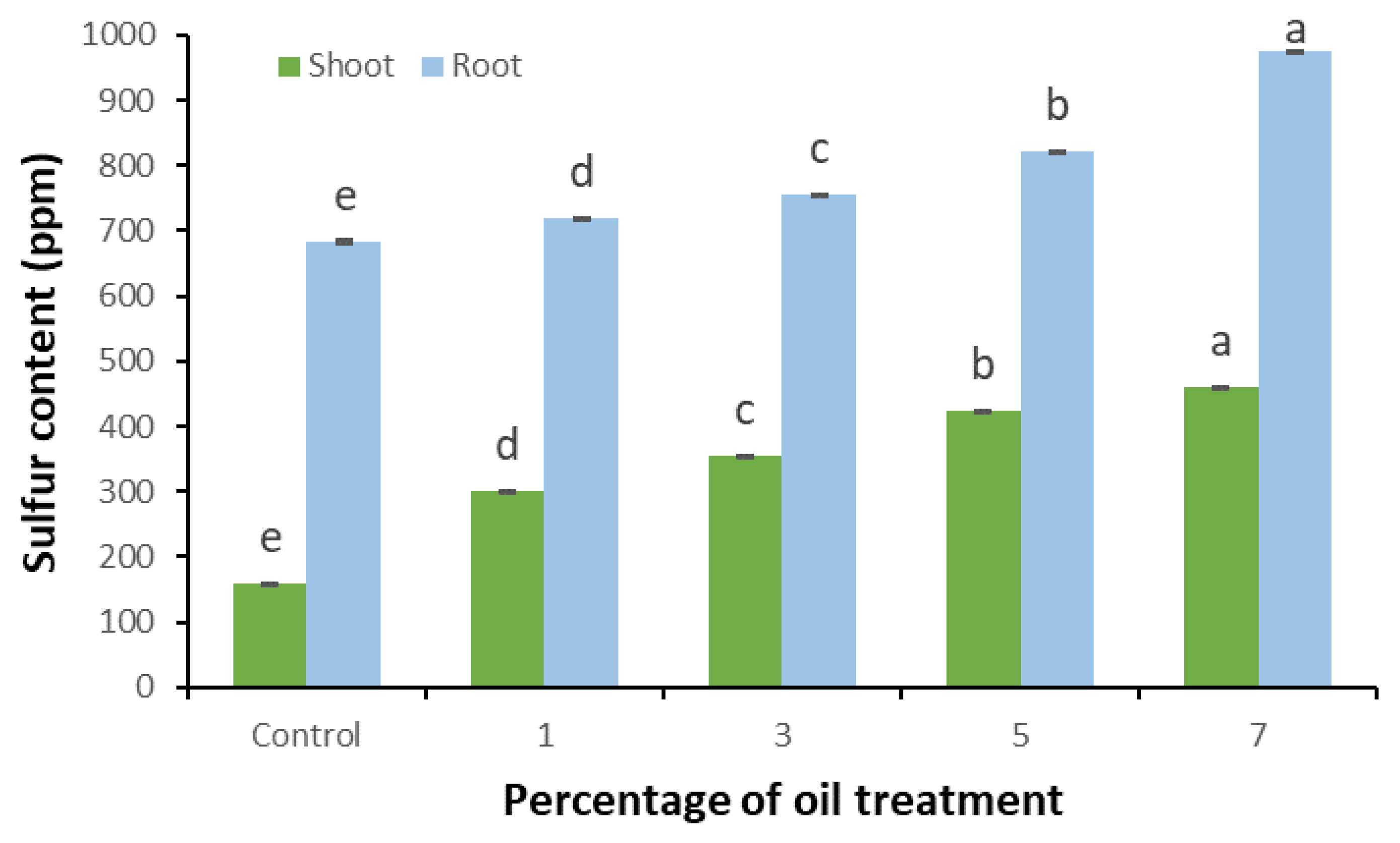
3.4. Sulfur Content
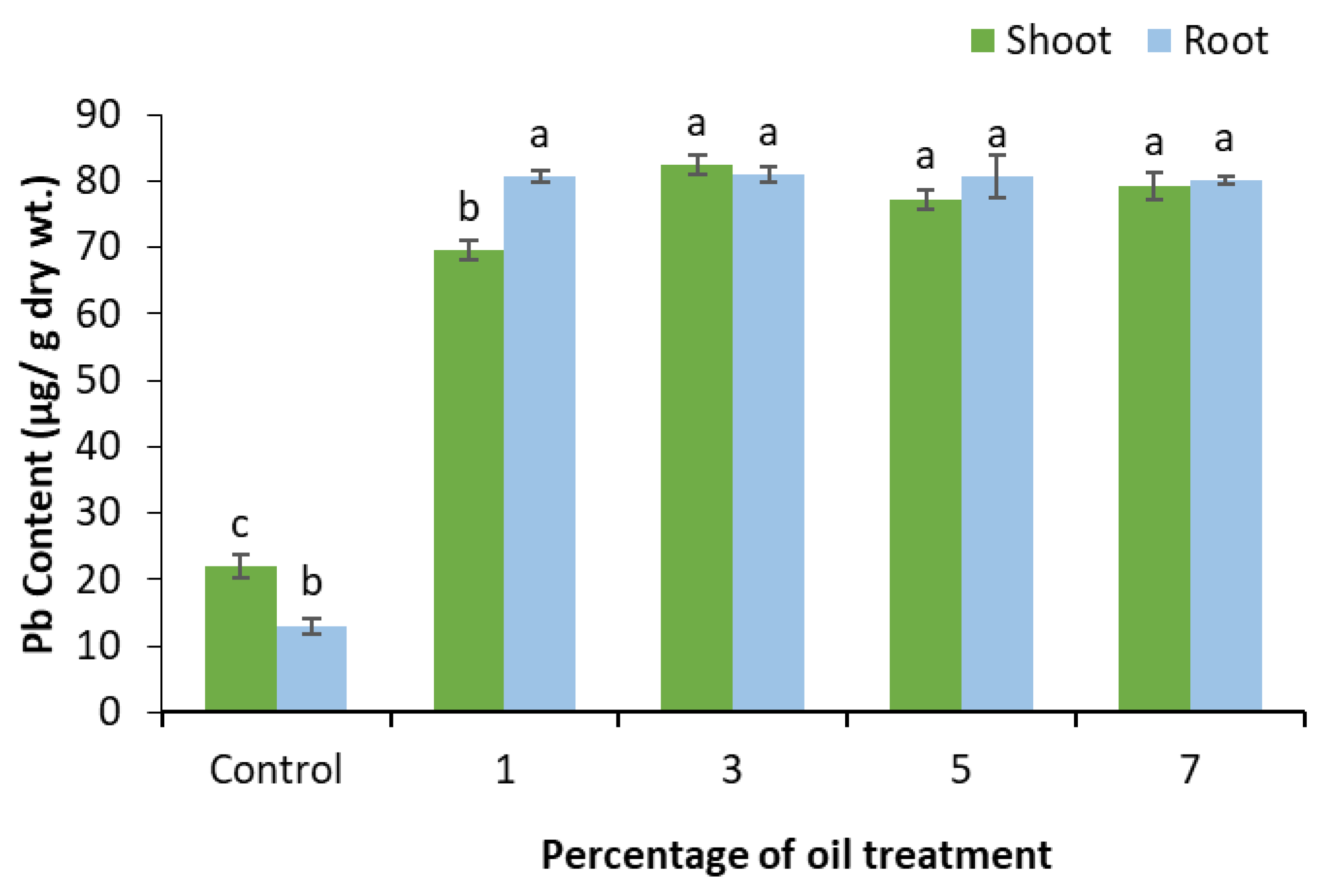
3.5. Lead Content
3.6. Cadmium Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varjani, S.J.; Gnansounou, E. Microbial Dynamics in Petroleum Oilfields and Their Relationship with Physiological Properties of Petroleum Oil Reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Okere, U.V.; Semple, K.T. Biodegradation of PAHs in “Pristine” Soils from Different Climatic Regions. J. Bioremediat. Biodegrad. 2011, s1, 1–11. [Google Scholar] [CrossRef]
- Yap, H.S.; Zakaria, N.N.; Zulkharnain, A.; Sabri, S.; Gomez-Fuentes, C.; Ahmad, S.A. Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation. Biology 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.S.; Hamido, N.; Hegazy, A.K.; El-Dessouky, M.A.; Mohamed, N.H.; Safwat, G. Phytoremediation of Crude Petroleum Oil Pollution: A Review. Egypt. J. Bot. 2022, 62, 611–640. [Google Scholar] [CrossRef]
- Tessarolo, N.S.; Silva, R.C.; Vanini, G.; Pinho, A.; Romão, W.; de Castro, E.V.R.; Azevedo, D.A. Assessing the Chemical Composition of Bio-Oils Using FT-ICR Mass Spectrometry and Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry. Microchem. J. 2014, 117, 68–76. [Google Scholar] [CrossRef]
- Tang, J.; Wang, M.; Wang, F.; Sun, Q.; Zhou, Q. Eco-Toxicity of Petroleum Hydrocarbon Contaminated Soil. J. Environ. Sci. 2011, 23, 845–851. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative Bioremediation of Heavy Metals and Petroleum Hydrocarbons Co-Contaminated Soil by Natural Attenuation, Phytoremediation, Bioaugmentation and Bioaugmentation-Assisted Phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic-inorganic composite modifiers enhance restoration potential of Nerium oleander L. to lead-zinc tailing: Application of phytoremediation. Environ. Sci. Pollut. Res. Int. 2023, 30, 56569–56579. [Google Scholar] [CrossRef]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schöftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of Petroleum Hydrocarbon-Contaminated Soils: Improvement Opportunities and Field Applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Li, Y.; Qie, X.; Zhang, X.; Hu, Z. Reduction of soil contamination by cypermethrin residues using phytoremediation with Plantago major and some surfactants. Environ. Sci. Eur. 2019, 31, 26. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a Green Technology for the Remediation of Petroleum Hydrocarbon-Contaminated Soils. J. Hazard. Mater. 2021, 401, 123282. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-Assisted Remediation of Hydrocarbons in Water and Soil: Application, Mechanisms, Challenges and Opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Kuiper, I.; Bloemberg, G.V.; Lugtenberg, B.J.J. Selection of a Plant-Bacterium Pair as a Novel Tool for Rhizostimulation of Polycyclic Aromatic Hydrocarbon-Degrading Bacteria. Mol. Plant-Microbe Interact. 2001, 14, 1197–1205. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Zuo, Y.; Aioub, A.A.A.; Hu, Z. Biochemical and phytoremediation of Plantago major L. to protect tomato plants from the contamination of cypermethrin pesticide. Environ. Sci. Pollut. Res. 2021, 28, 43992–44001. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Hegazy, A.K.; Mohamed, N.H.; Hafez, R.M.; Azab, E.; Gobouri, A.A.; Saad, H.A.; Fattah, A.M.A.-E.; Mustafa, Y.M. Potentiality of Azolla Pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil. Separations 2021, 8, 39. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Q.; Liu, W.; Zhong, D.; Ge, Y.; Christie, P.; Luo, Y. Soil Microbial Community and Association Network Shift Induced by Several Tall Fescue Cultivars during the Phytoremediation of a Petroleum Hydrocarbon-Contaminated Soil. Sci. Total Environ. 2021, 792, 148411. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Hegazy, A.K.; Moustafa, Y.M.; Mohamed, R.R.; Safwat, G.; Diab, A.A. Green Technology for Remediation of Water Polluted with Petroleum Crude Oil: Using of Eichhornia crassipes (Mart.) Solms Combined with Magnetic Nanoparticles Capped with Myrrh Resources of Saudi Arabia. Nanomaterials 2020, 10, 262. [Google Scholar] [CrossRef]
- Lopez-Echartea, E.; Strejcek, M.; Mukherjee, S.; Uhlik, O.; Yrjälä, K. Bacterial Succession in Oil-Contaminated Soil under Phytoremediation with Poplars. Chemosphere 2020, 243, 125242. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Nwachukwu, E.O.; Sikoki, F.D. Assessing and Modelling the Efficacy of Lemna paucicostata for the Phytoremediation of Petroleum Hydrocarbons in Crude Oil-Contaminated Wetlands. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Nero, B.F. Phytoremediation of Petroleum Hydrocarbon-Contaminated Soils with Two Plant Species: Jatropha curcas and Vetiveria zizanioides at Ghana Manganese Company Ltd. Int. J. Phytoremediat. 2021, 23, 171–180. [Google Scholar] [CrossRef]
- Alanbary, S.R.N.; Abdullah, S.R.S.; Al-Baldawi, I.A.W.; Hassan, H.A.; Anuar, N.; Othman, A.R.; Suja, F. Phytotoxicity of Contaminated Sand Containing Crude Oil Sludge on Ludwigia Octovalvis. Ecol. Eng. 2019, 20, 246–255. [Google Scholar] [CrossRef]
- Jeelani, N.; Yang, W.; Xu, L.; Qiao, Y.; An, S.; Leng, X. Phytoremediation Potential of Acorus calamus in Soils Co-Contaminated with Cadmium and Polycyclic Aromatic Hydrocarbons. Sci. Rep. 2017, 7, 8028. [Google Scholar] [CrossRef] [PubMed]
- Al-Sbani, N.H.; Abdullah, S.R.S.; Idris, M.; Hasan, H.A.; Jehawi, O.H.; Ismail, N. Sub-Surface Flow System for PAHs Removal in Water Using Lepironia articulate under Greenhouse Conditions. Ecol. Eng. 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of Soils Polluted with Crude Petroleum Oil Using Bassia scoparia and Its Associated Rhizosphere Microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon Degradation, Plant Colonization and Gene Expression of Alkane Degradation Genes by Endophytic Enterobacter ludwigii Strains. Environ. Pollut. 2011, 159, 2675–2683. [Google Scholar] [CrossRef]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of Contaminated Soils Using Ornamental Plants. Environ. Rev. 2018, 26, 43–54. [Google Scholar] [CrossRef]
- Ramana, S.; Biswas, A.K.; Singh, A.B.; Ajay, A.; Ahirwar, N.K.; Subba Rao, A. Tolerance of Ornamental Succulent Plant Crown of Thorns (Euphorbia Milli) to Chromium and Its Remediation. Int. J. Phytoremediat. 2015, 17, 363–368. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [CrossRef]
- Subhashini, V.; Swamy, A.V.V.S. Potential of Catharanthus Roseus (L.) in Phytoremediation of Heavy Metals. In Catharanthus Roseus: Current Research and Future Prospects; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 349–364. [Google Scholar] [CrossRef]
- Zafar, N.; Athar, M.; Zafar, I.M.; Shafiq, M. Effect of Diesel Generator Exhaust Pollutants on Growth of Vinca Rosea and Ruellia Tuberosa. J. Appl. Sci. Environ. Manag. 2017, 20, 1191–1197. [Google Scholar] [CrossRef]
- Hussein, Z.S.; Hegazy, A.K.; Mohamed, N.H.; El-Desouky, M.A.; Ibrahim, S.D.; Safwat, G. Eco-Physiological Response and Genotoxicity Induced by Crude Petroleum Oil in the Potential Phytoremediator Vinca Rosea L. J. Genet. Eng. Biotechnol. 2022, 20, 135. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of Petroleum Contaminated Soils by Mirabilis Jalapa L. in a Greenhouse Plot Experiment. J. Hazard. Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef]
- Mishra, S.; Jyot, J.; Kuhad, R.C.; Lal, B. In Situ Bioremediation Potential of an Oily Sludge-Degrading Bacterial Consortium. Curr. Microbiol. 2001, 43, 328–335. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Lin, Z.; Zhang, J.; Norbu, N.; Liu, W. The Harm of Petroleum-Polluted Soil and Its Remediation Research. In AIP Conference Proceedings; American Institute of Physics Inc.: New York, NY, USA, 2017; Volume 1864, p. 020222. [Google Scholar] [CrossRef]
- Chen, G.; Lin, J.; Hu, W.; Cheng, C.; Gu, X.; Du, W.; Zhang, J.; Qu, C. Characteristics of a Crude Oil Composition and Its in Situ Waxing Inhibition Behavior. Fuel 2018, 218, 213–217. [Google Scholar] [CrossRef]
- Stephen, C. Phytoremediation and Crude Oil Bioaccumulation Potential of Zea mays L. IOSR J. Environ. Sci. 2013, 7, 24–26. [Google Scholar]
- Kuo, H.C.; Juang, D.F.; Yang, L.; Kuo, W.C.; Wu, Y.M. Phytoremediation of Soil Contaminated by Heavy Oil with Plants Colonized by Mycorrhizal Fungi. Int. J. Environ. Sci. Technol. 2014, 11, 1661–1668. [Google Scholar] [CrossRef]
- Liu, R.; Jadeja, R.N.; Zhou, Q.; Liu, Z. Treatment and Remediation of Petroleum-Contaminated Soils Using Selective Ornamental Plants. Environ. Eng. Sci. 2012, 29, 494–501. [Google Scholar] [CrossRef]
- Singer, A.C.; Crowley, D.E.; Thompson, I.P. Secondary Plant Metabolites in Phytoremediation and Biotransformation. Trends Biotechnol. 2003, 21, 123–130. [Google Scholar] [CrossRef]
- Sun, M.; Fu, D.; Teng, Y.; Shen, Y.; Luo, Y.; Li, Z.; Christie, P. In Situ Phytoremediation of PAH-Contaminated Soil by Intercropping Alfalfa (Medicago sativa L.) with Tall Fescue (Festuca arundinacea Schreb.) and Associated Soil Microbial Activity. J. Soils Sediments 2011, 11, 980–989. [Google Scholar] [CrossRef]
- Borriss, R. Phytostimulation and biocontrol by the plant-associated Bacillus amyloliquefaciens FZB42: An Update. In Phyto-Microbiome in Stress Regulation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–20. [Google Scholar]
- Balasubramaniyam, A. The Influence of Plants in the Remediation of Petroleum Hydrocarbon- Contaminated Sites. Pharm. Anal. Chem. 2015, 1, 105. [Google Scholar] [CrossRef]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef]
- Wasoh, H.; Veeraswamy, K.; Gunasekaran, B.; Shukor, M.Y. Biodegradation of Hydrocarbon Sludge by Pseudomonas Sp. Strain UPM-KV. J. Environ. Microbiol. Toxicol. 2019, 7, 10–15. [Google Scholar] [CrossRef]
- Vieira, P.A.; Vieira, R.B.; Faria, S.; Ribeiro, E.J.; Cardoso, V.L. Biodegradation of Diesel Oil and Gasoline Contaminated Effluent Employing Intermittent Aeration. J. Hazard. Mater. 2009, 168, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Su, S.M.; Luo, Y.J.; Lu, M. Improvement of Natural Microbial Remediation of Petroleum-Polluted Soil Using Graminaceous Plants. Water Sci. Technol. 2009, 59, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, N.S.; Ali, H.R.; El-Nady, M.M.; Deriase, S.F.; Moustafa, Y.M.; Roushdy, M.I. Effect of Different Bioremediation Techniques on Petroleum Biomarkers and Asphaltene Fraction in Oil-Polluted Sea Water. Desalination Water Treat. 2014, 52, 7484–7494. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Kučera, T.; Horáková, H.; Šonská, A. Toxic Metal Ions in Photoautotrophic Organisms. Photosynthetica 2008, 46, 481–489. [Google Scholar] [CrossRef]
- Bertrand, M.; Poirier, I. Photosynthetic Organisms and Excess of Metals. Photosynthetica 2005, 43, 345–353. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Hafez, R.M.; Hegazy, A.K.; Abd-El Fattah, A.M.; Mohamed, N.H.; Mustafa, Y.M.; Gobouri, A.A.; Azab, E. Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions. Sustainability 2021, 13, 2142. [Google Scholar] [CrossRef]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead Responses and Tolerance Mechanisms of Koelreuteria paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef]


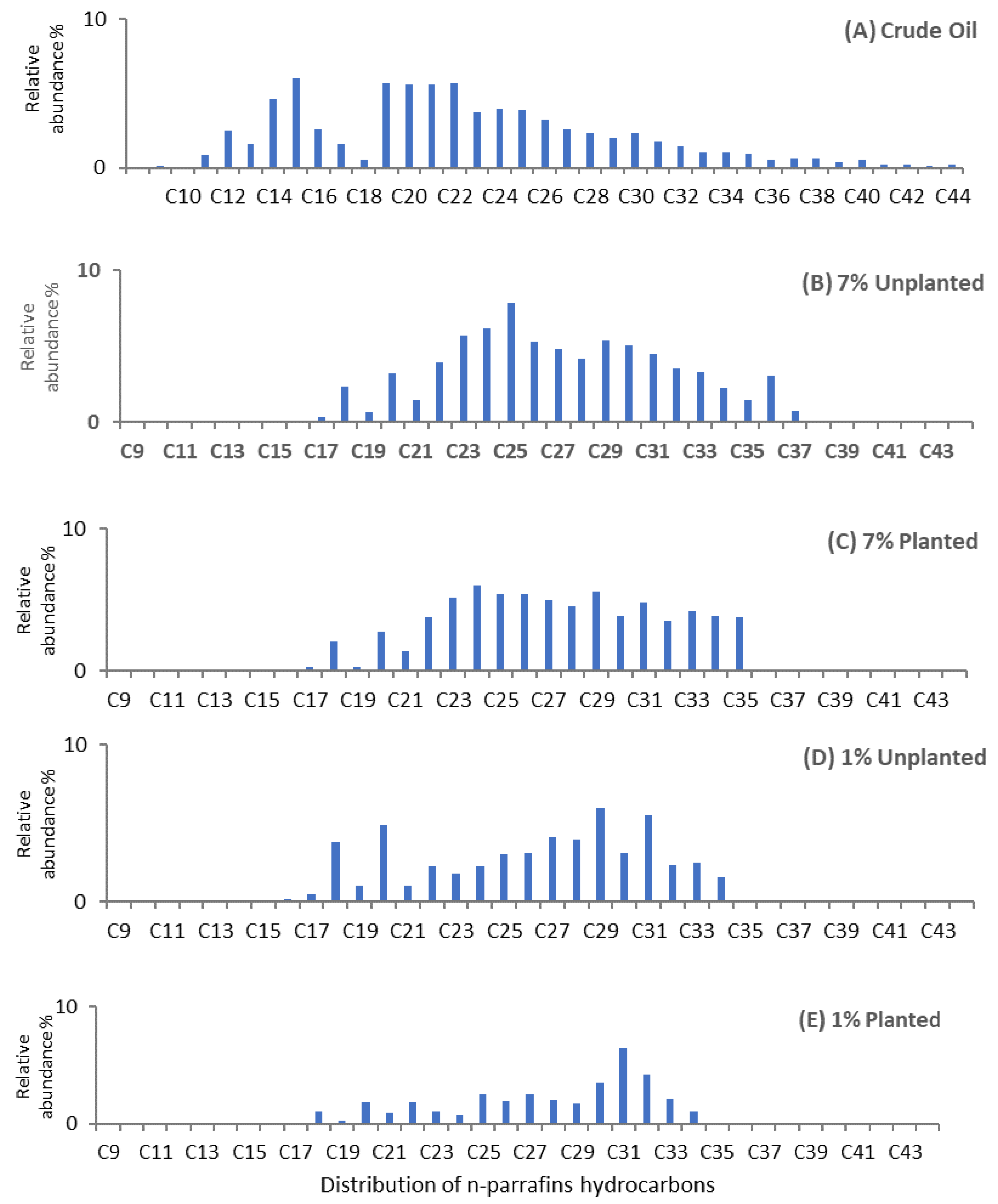


| Carbon Number | Crude Oil | 1% | 7% | ||
|---|---|---|---|---|---|
| Unplanted | Planted | Unplanted | Planted | ||
| C9 | 0.096 | 0.00 | 0.00 | 0.00 | 0.00 |
| C10 | 0.048 | 0.00 | 0.00 | 0.00 | 0.00 |
| C11 | 0.856 | 0.00 | 0.00 | 0.00 | 0.00 |
| C12 | 2.48 | 0.00 | 0.00 | 0.00 | 0.00 |
| C13 | 1.619 | 0.00 | 0.00 | 0.00 | 0.00 |
| C14 | 4.622 | 0.00 | 0.00 | 0.00 | 0.00 |
| C15 | 6.039 | 0.00 | 0.00 | 0.00 | 0.00 |
| C16 | 2.6 | 0.21 | 0.00 | 0.07 | 0.00 |
| C17 | 1.578 | 0.50 | 0.00 | 0.30 | 0.29 |
| C18 | 0.568 | 3.85 | 1.10 | 2.30 | 2.12 |
| C19 | 5.652 | 1.04 | 0.32 | 0.64 | 0.26 |
| C20 | 5.562 | 4.94 | 1.89 | 3.19 | 2.74 |
| C21 | 5.599 | 1.06 | 0.95 | 1.47 | 1.39 |
| C22 | 5.654 | 2.30 | 1.89 | 3.96 | 3.84 |
| C23 | 3.753 | 1.78 | 1.04 | 5.71 | 5.14 |
| C24 | 3.987 | 2.29 | 0.77 | 6.19 | 6.06 |
| C25 | 3.906 | 3.04 | 2.55 | 7.84 | 5.45 |
| C26 | 3.21 | 3.14 | 1.93 | 5.27 | 5.46 |
| C27 | 2.614 | 4.12 | 2.57 | 4.84 | 4.98 |
| C28 | 2.364 | 3.97 | 2.10 | 4.19 | 4.54 |
| C29 | 2.041 | 6.00 | 1.79 | 5.38 | 5.62 |
| C30 | 2.345 | 3.10 | 3.52 | 5.06 | 3.85 |
| C31 | 1.764 | 5.56 | 6.48 | 4.48 | 4.85 |
| C32 | 1.4 | 2.36 | 4.21 | 3.56 | 3.58 |
| C33 | 1.006 | 2.49 | 2.12 | 3.30 | 4.26 |
| C34 | 0.992 | 1.55 | 1.10 | 2.27 | 3.86 |
| C35 | 0.981 | 0.00 | 0.00 | 1.48 | 3.76 |
| C36 | 0.508 | 0.00 | 0.00 | 3.04 | 0.00 |
| C37 | 0.602 | 0.00 | 0.00 | 0.73 | 0.00 |
| C38 | 0.608 | 0.00 | 0.00 | 0.00 | 0.00 |
| C39 | 0.346 | 0.00 | 0.00 | 0.00 | 0.00 |
| C40 | 0.502 | 0.00 | 0.00 | 0.00 | 0.00 |
| C41 | 0.233 | 0.00 | 0.00 | 0.00 | 0.00 |
| C42 | 0.223 | 0.00 | 0.00 | 0.00 | 0.00 |
| C43 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| C44 | 0.172 | 0.00 | 0.00 | 0.00 | 0.00 |
| n-paraffins % | 76.68 | 53.28 | 36.35 | 75.29 | 72.07 |
| Iso-paraffins % | 23.32 | 46.72 | 63.65 | 24.71 | 27.93 |
| n-paraffins/iso-paraffins | 3.29 | 1.14 | 0.57 | 3.05 | 2.58 |
| Aromatic Constituent | ||||
|---|---|---|---|---|
| Monoaromatic | Diaromatics | Polyaromatics | ||
| (Wt. %) | (Wt. %) | (Wt. %) | ||
| Crude oil | 5.06 ± 0.51 | 8.13 ± 1.51 | 45.22 ± 3.51 | |
| Planted Soil | 1% | 19.26 ± 0.22 aA | 10.02 ± 0.91 aA | 10.01 ± 0.31 aA |
| 3% | 15.74 ± 0.91 bA | 15.08 ± 0.31 bB | 13.81 ± 0.28 bB | |
| 5% | 15.11 ± 0.44 bA | 17.23 ± 0.74 cC | 15.28 ± 0.51 cC | |
| 7% | 13.23 ± 0.21 cA | 19.48 ± 0.51 dD | 21.87 ± 0.35 dE | |
| Unplanted Soil | 1% | 11.60 ± 0.64 aB | 10.21 ± 0.85 aA | 27.44 ± 0.37 aB |
| 3% | 10.21 ± 0.33 aB | 12.15 ± 0.27 bC | 30.29 ± 0.49 bC | |
| 5% | 07.90 ± 0.48 bB | 15.50 ± 0.65 cD | 31.55 ± 0.68 bD | |
| 7% | 06.71 ± 0.43 bB | 16.68 ± 0.87 cE | 33.24 ± 0.24 cD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazy, A.K.; Hussein, Z.S.; Mohamed, N.H.; Safwat, G.; El-Dessouky, M.A.; Imbrea, I.; Imbrea, F. Assessment of Vinca rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil. Sustainability 2023, 15, 11046. https://doi.org/10.3390/su151411046
Hegazy AK, Hussein ZS, Mohamed NH, Safwat G, El-Dessouky MA, Imbrea I, Imbrea F. Assessment of Vinca rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil. Sustainability. 2023; 15(14):11046. https://doi.org/10.3390/su151411046
Chicago/Turabian StyleHegazy, Ahmad K., Zahra S. Hussein, Nermen H. Mohamed, Gehan Safwat, Mohamed A. El-Dessouky, Ilinca Imbrea, and Florin Imbrea. 2023. "Assessment of Vinca rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil" Sustainability 15, no. 14: 11046. https://doi.org/10.3390/su151411046
APA StyleHegazy, A. K., Hussein, Z. S., Mohamed, N. H., Safwat, G., El-Dessouky, M. A., Imbrea, I., & Imbrea, F. (2023). Assessment of Vinca rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil. Sustainability, 15(14), 11046. https://doi.org/10.3390/su151411046






