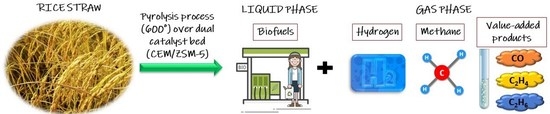
Probing the Pyrolysis Process of Rice Straw over a “Dual-Catalyst Bed” for the Production of Fuel Gases and Value-Added Chemicals
Abstract
1. Introduction
2. Materials and Methods
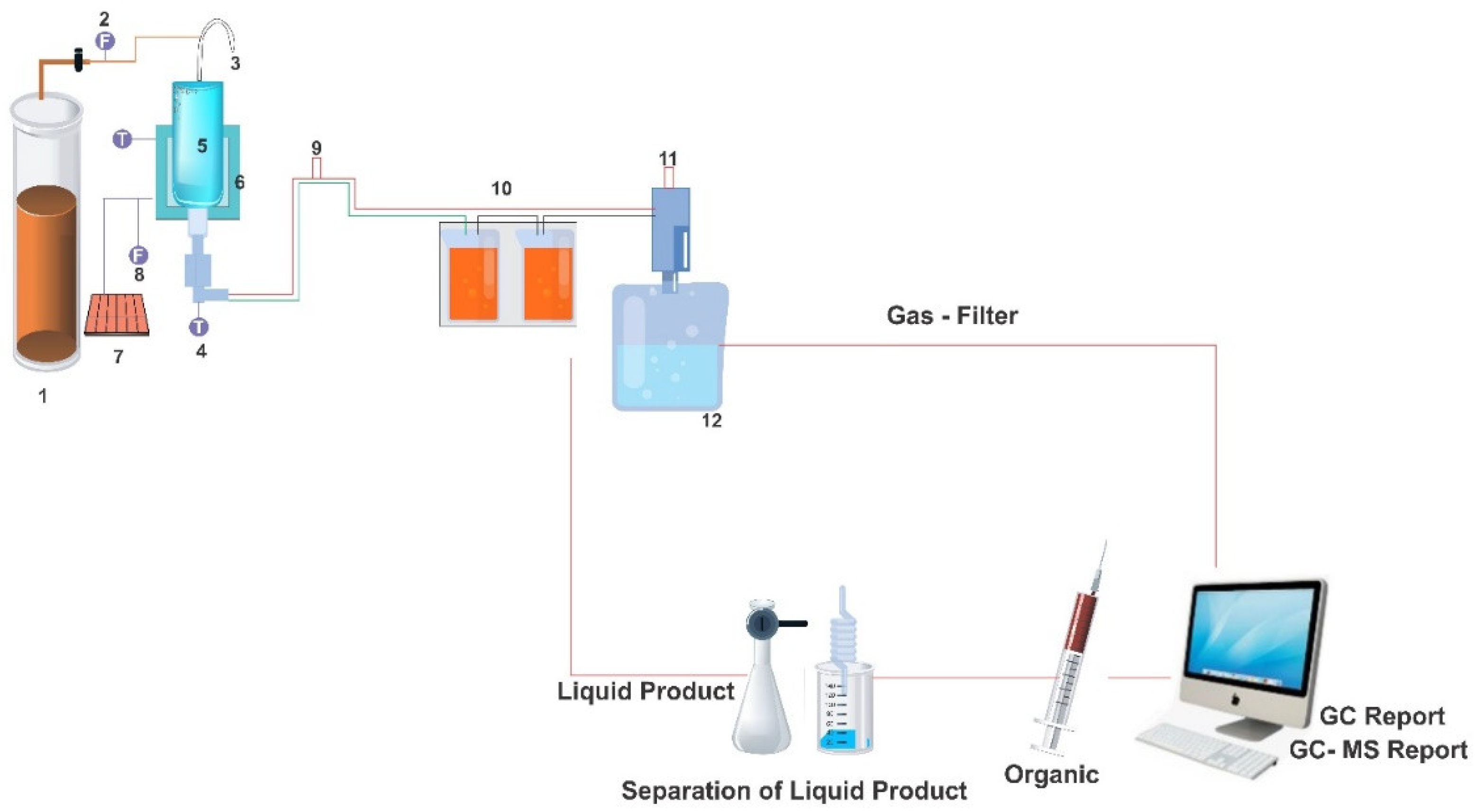
2.1. Materials and Techniques
2.2. The Ultimate, Proximate Analysis and Chemical Composition of the Feedstock
2.3. Determination of Biofuel Output
2.4. ZSM-5 Catalysts
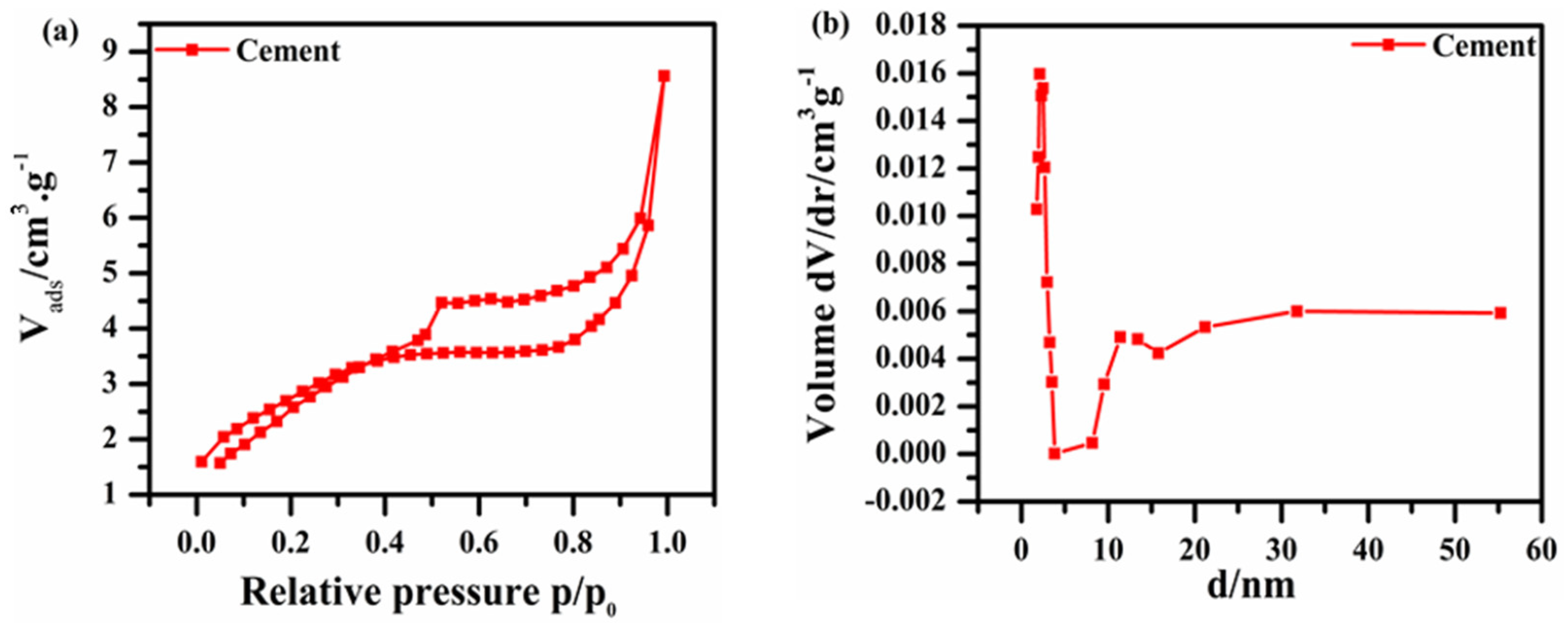
2.5. Detailed Analysis of the Cement Catalyst
3. Results and Discussion
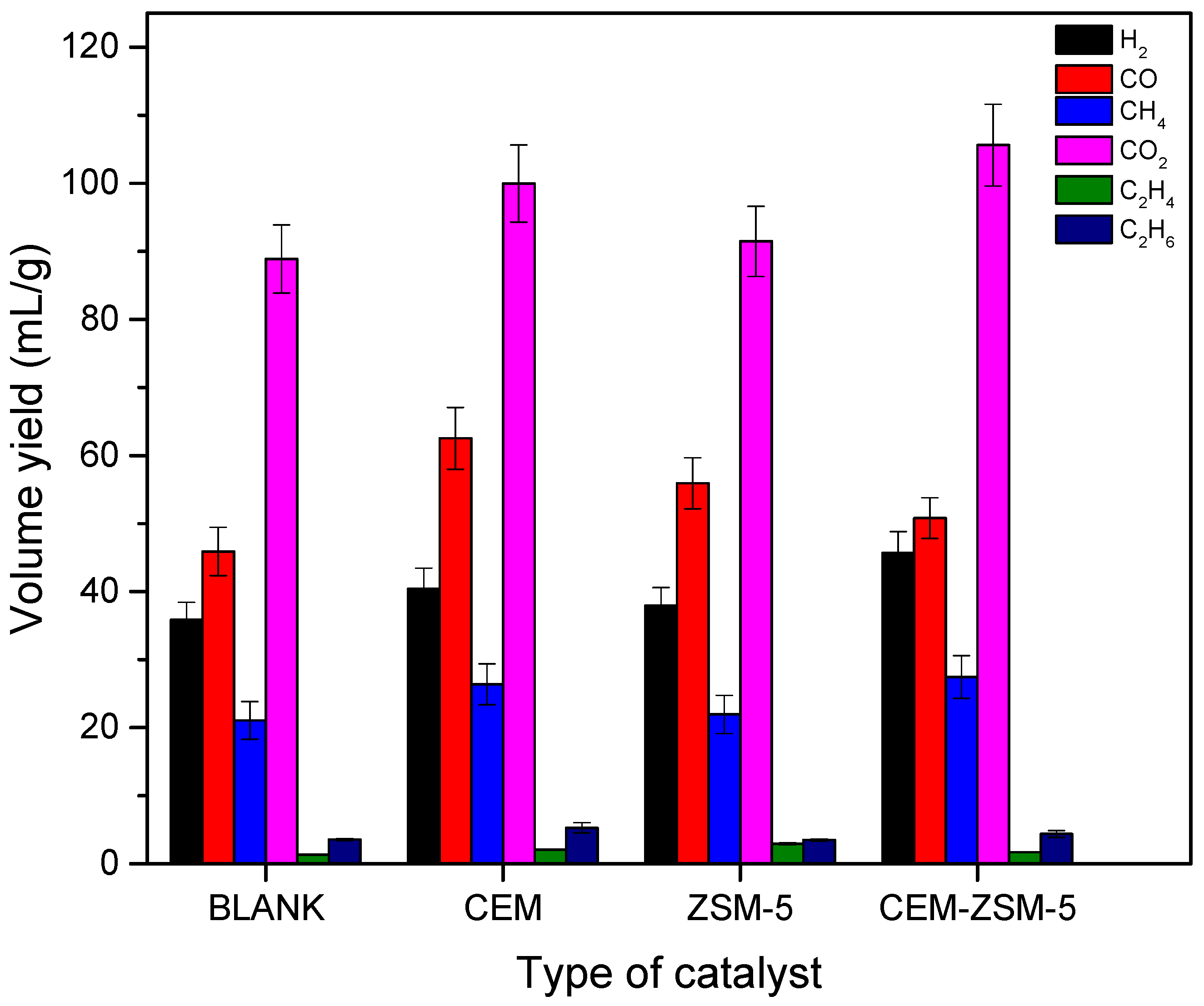
3.1. The Impact of Multiple Catalysts on Product Dispersion and Direct Pyrolysis of the Feedstock
3.2. Carbon Balance Simulation of the Pyrolysis Process
3.3. Constituents Evaluation of Pyrolysis Gas Products
3.4. Analysis of Bio-Oil Composition by GC/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, H.; Hameed, B.H.; Lim, J.K. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: Synergistic effect and product distributions. Energy 2020, 191, 116545. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K.; Lee, L.Y.; Gan, S. Effect of oxide catalysts on the properties of bio-oil from in-situ catalytic pyrolysis of palm empty fruit bunch fiber. J. Environ. Manag. 2019, 247, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Kim, H.J.; Jeong, Y.S.; Na, H.B.; Cha, Y.-L.; Koo, B.-C.; Kim, J.; Yun, H.D.; Lee, J.-K.; Kim, H. Enhanced saccharification of reed and rice straws by the addition of β-1,3-1,4-glucanase with broad substrate specificity and calcium ion. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 29–33. [Google Scholar] [CrossRef]
- Smay, J.E.; Gratson, G.M.; Shepherd, R.F.; Cesarano, J.; Lewis, J.A. Directed Colloidal Assembly of 3D Periodic Structures. Adv. Mater. 2002, 14, 1279–1283. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Bilbao, J. Transformation of Oxygenate Components of Biomass Pyrolysis Oil on a HZSM-5 Zeolite. I. Alcohols and Phenols. Ind. Eng. Chem. Res. 2004, 43, 2610–2618. [Google Scholar] [CrossRef]
- Khan, S.R.; Masood, A.; Zeeshan, M.; Qaisar, S. The influence of dual-catalyst bed system of zeolitic and metal oxide catalysts on the production of valuable hydrocarbons during co-pyrolysis of rice straw and waste tire. Biomass Convers. Biorefinery 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Zheng, Y.; Li, M.; Zhu, X.-F. Arene production by W 2 C/MCM-41-catalyzed upgrading of vapors from fast pyrolysis of lignin. Fuel Process. Technol. 2015, 134, 46–51. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Karakoulia, S.A.; Triantafyllidis, K.S.; van Bokhoven, J.A. Catalytic Fast Pyrolysis of Lignin over High-Surface-Area Mesoporous Aluminosilicates: Effect of Porosity and Acidity. ChemSusChem 2016, 9, 1134–1145. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kang, B.S.; Han, T.U.; Kim, S.; Jung, S.-C.; Kim, S.C.; Jeon, J.-K.; Park, Y.-K. Catalytic Pyrolysis of Organosolv and Klason Lignin Over Al-SBA-15. J. Nanosci. Nanotechnol. 2018, 18, 1423–1426. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Guo, H.; Wei, T.; Dong, D.; Hu, G.; Hu, S.; Xiang, J.; Liu, Q.; Wang, Y. Pyrolysis of poplar, cellulose and lignin: Effects of acidity and alkalinity of the metal oxide catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhong, Z.; Ding, K.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Catalytic fast co-pyrolysis of bamboo residual and waste lubricating oil over an ex-situ dual catalytic beds of MgO and HZSM-5: Analytical PY-GC/MS study. Energy Convers. Manag. 2017, 139, 222–231. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Two-stage ex-situ catalytic pyrolysis of lignocellulose for the production of gasoline-range chemicals. J. Anal. Appl. Pyrolysis 2018, 134, 454–464. [Google Scholar] [CrossRef]
- Zhang, H.; Likun, P.K.W.; Xiao, R. Improving the hydrocarbon production via co-pyrolysis of bagasse with bio-plastic and dual-catalysts layout. Sci. Total Environ. 2018, 618, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Z.; Ding, K.; Li, M.; Hao, N.; Meng, X.; Ruan, R.; Ragauskas, A.J. Catalytic fast co-pyrolysis of bamboo sawdust and waste tire using a tandem reactor with cascade bubbling fluidized bed and fixed bed system. Energy Convers. Manag. 2019, 180, 60–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Khan, A.; Ullah, S.; Karim, A.; Perveen, S. The conversion of biomass into liquid hydrocarbon fuel by two step pyrolysis using cement as catalyst. J. Anal. Appl. Pyrolysis 2013, 101, 90–95. [Google Scholar] [CrossRef]
- Snellings, R.; Schepper, M.; Buysser, K.; Driessche, I.; Belie, N. Clinkering Reactions During Firing of Recyclable Concrete. J. Am. Ceram. Soc. 2012, 95, 1741–1749. [Google Scholar] [CrossRef]
- Miller, J.E.; Ambrosini, A.; Coker, E.N.; Allendorf, M.D.; McDaniel, A.H. Advancing Oxide Materials for Thermochemical Production of Solar Fuels. Energy Procedia 2014, 49, 2019–2026. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Shende, R.V.; Puszynski, J.A. Thermochemical water-splitting for H2 generation using sol-gel derived Mn-ferrite in a packed bed reactor. Int. J. Hydrogen Energy 2012, 37, 2924–2934. [Google Scholar] [CrossRef]
- Kratky, L.; Jirout, T. Biomass Size Reduction Machines for Enhancing Biogas Production. Chem. Eng. Technol. 2011, 34, 391–399. [Google Scholar] [CrossRef]
- Uddin, I.; Wang, G.; Gao, D.; Li, N.; Hussain, Z.; Hou, B. Cement Catalyzed Valorization of Rice Straw into Upgraded Bio-Oil and Fuel Gases Using Pyrolysis Reactions. Combust. Sci. Technol. 2022, 194, 3094–3108. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.D.; Duc Vu, C.; Viet Nguyen, H. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar] [CrossRef]
- Wang, Z.; Saleem, J.; Barford, J.P.; McKay, G. Preparation and characterization of modified rice husks by biological delignification and acetylation for oil spill cleanup. Environ. Technol. 2020, 41, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Uddin, I.; Ali, H.; Al-Sehemi, A.G.; Muhammad, S.; Hamad, N.; Taha, T.A.; AlSalem, H.S.; Alenad, A.M.; Palamanit, A.; Hayat, A.; et al. Efficient pyrolysis process of lignin over dual catalyst bed for the production of Phenols and Aromatics. S. Afr. J. Bot. 2022, 149, 109–116. [Google Scholar] [CrossRef]
- Hussain, Z.; Sulaiman, S.A.; Khan, A.; Khan, K.M.; Perveen, S.; Naz, M.Y. Two-Step Pyrolysis of Spirogyra for Fuels Using Cement Catalytic. Waste Biomass Valoriz. 2016, 7, 1481–1489. [Google Scholar] [CrossRef]
- Li, J.; Kwak, J.-H.; Chen, J.; An, Z.; Gong, X.; Chang, S.X. Canola straw biochars produced under different pyrolysis temperatures and nitrapyrin independently affected cropland soil nitrous oxide emissions. Biol. Fertil. Soils 2021, 57, 319–328. [Google Scholar] [CrossRef]
- Stutzman, P.E.; Feng, P.; Bullard, J.W. Phase Analysis of Portland Cement by Combined Quantitative X-Ray Powder Diffraction and Scanning Electron Microscopy. J. Res. Natl. Inst. Stand. Technol. 2016, 121, 47. [Google Scholar] [CrossRef]
- Uddin, I.; Wang, G.; Gao, D.; Hussain, Z.; Naz, M.Y.; Hou, B.; Hayat, A. Conventional and cement-catalyzed co-pyrolysis of rice straw and waste polyethylene into liquid and gaseous fuels by using a fixed bed reactor. Biomass Convers. Biorefin. 2023, 13, 5307–5316. [Google Scholar] [CrossRef]
- Buska, A.; Mačiulaitis, R. The compressive strength properties of mineral wool slabs: Influence of structure anisotropy and methodical factors. J. Civ. Eng. Manag. 2007, 13, 97–106. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Zhu, C.; Lin, Y.; Chen, L.; Fan, J.; Zhang, H. Synthesis, characterization, structures and magnetic property of chiral oxalate-bridged dicopper(II) complexes. Sci. China Chem. 2010, 53, 1255–1260. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, H.; Xiao, R.; Shen, D.; Zheng, J. Comparison of Catalytic Characteristics of Biomass Derivates with Different Structures Over ZSM-5. BioEnergy Res. 2013, 6, 1173–1182. [Google Scholar] [CrossRef]
- Li, R.; Zhong, Z.; Jin, B.; Zheng, A. Selection of Temperature for Bio-oil Production from Pyrolysis of Algae from Lake Blooms. Energy Fuels 2012, 26, 2996–3002. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.-F.; Dong, C.-Q.; Zhu, X.-F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
| 2 ϴ (°) | Phase | d-Spacing (nm) |
|---|---|---|
| 11.59 | Gypsum (G) (100) | 0.763 |
| 29.91 | alite, (A) triclinic (25) | 0.299 |
| 33.03 | belite, (B) α form (100) | 0.271 |
| 22.77 | aluminate, cubic (12) | 0.408 |
| 33.88 | ferrite (100) | 0.264 |
| 37.36 | free lime (100) | 0.241 |
| Type of Catalyst | % Gas | % Liquid | % Char | Conversion Efficiency |
|---|---|---|---|---|
| CEM-ZSM-5 (bed) | 29.9 ± 0.3 | 42.1 ± 0.3 | 28.0 ± 0.3 | 72.0 ± 0.3 |
| Cement 10 wt.% | 30.5 ± 0.2 | 40.4 ± 0.2 | 29.1 ± 0.2 | 70.9 ± 0.2 |
| ZSM-5 10 wt.% | 27.7 ± 0.2 | 42.8 ± 0.2 | 29.5 ± 0.2 | 70.5 ± 0.2 |
| Without Catalyst | 24.7 ± 0.4 | 40.8 ± 0.4 | 33.5 ± 0.4 | 66.5 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, I.; Sohail, M.; Hussain, M.I.; Alhokbany, N.; Amaro-Gahete, J.; Estévez, R. Probing the Pyrolysis Process of Rice Straw over a “Dual-Catalyst Bed” for the Production of Fuel Gases and Value-Added Chemicals. Sustainability 2023, 15, 11057. https://doi.org/10.3390/su151411057
Uddin I, Sohail M, Hussain MI, Alhokbany N, Amaro-Gahete J, Estévez R. Probing the Pyrolysis Process of Rice Straw over a “Dual-Catalyst Bed” for the Production of Fuel Gases and Value-Added Chemicals. Sustainability. 2023; 15(14):11057. https://doi.org/10.3390/su151411057
Chicago/Turabian StyleUddin, Ikram, Muhammad Sohail, Muhammad Ijaz Hussain, Norah Alhokbany, Juan Amaro-Gahete, and Rafael Estévez. 2023. "Probing the Pyrolysis Process of Rice Straw over a “Dual-Catalyst Bed” for the Production of Fuel Gases and Value-Added Chemicals" Sustainability 15, no. 14: 11057. https://doi.org/10.3390/su151411057
APA StyleUddin, I., Sohail, M., Hussain, M. I., Alhokbany, N., Amaro-Gahete, J., & Estévez, R. (2023). Probing the Pyrolysis Process of Rice Straw over a “Dual-Catalyst Bed” for the Production of Fuel Gases and Value-Added Chemicals. Sustainability, 15(14), 11057. https://doi.org/10.3390/su151411057