Potential of Integrated Nutrient Management to Rehabilitate the Dieback-Affected Mango Cultivar Sammer Bahisht Chaunsa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Identification of Dieback-Affected Plants
2.3. Treatment Plan
2.4. Field Experiment
2.5. Soil and Leaf Sampling and Analysis
2.6. Disease Intensity
2.7. Statistical Analysis
3. Results
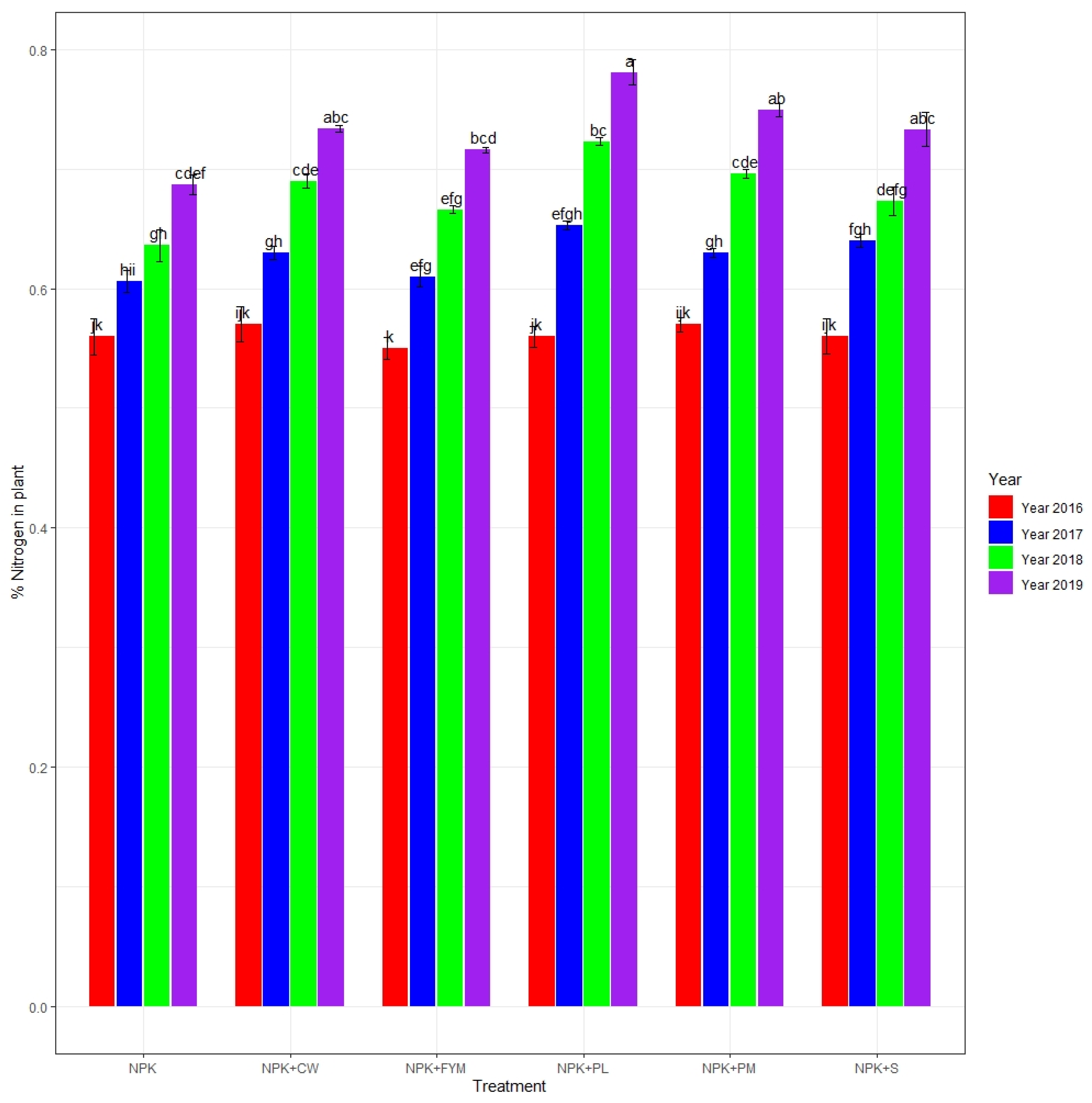
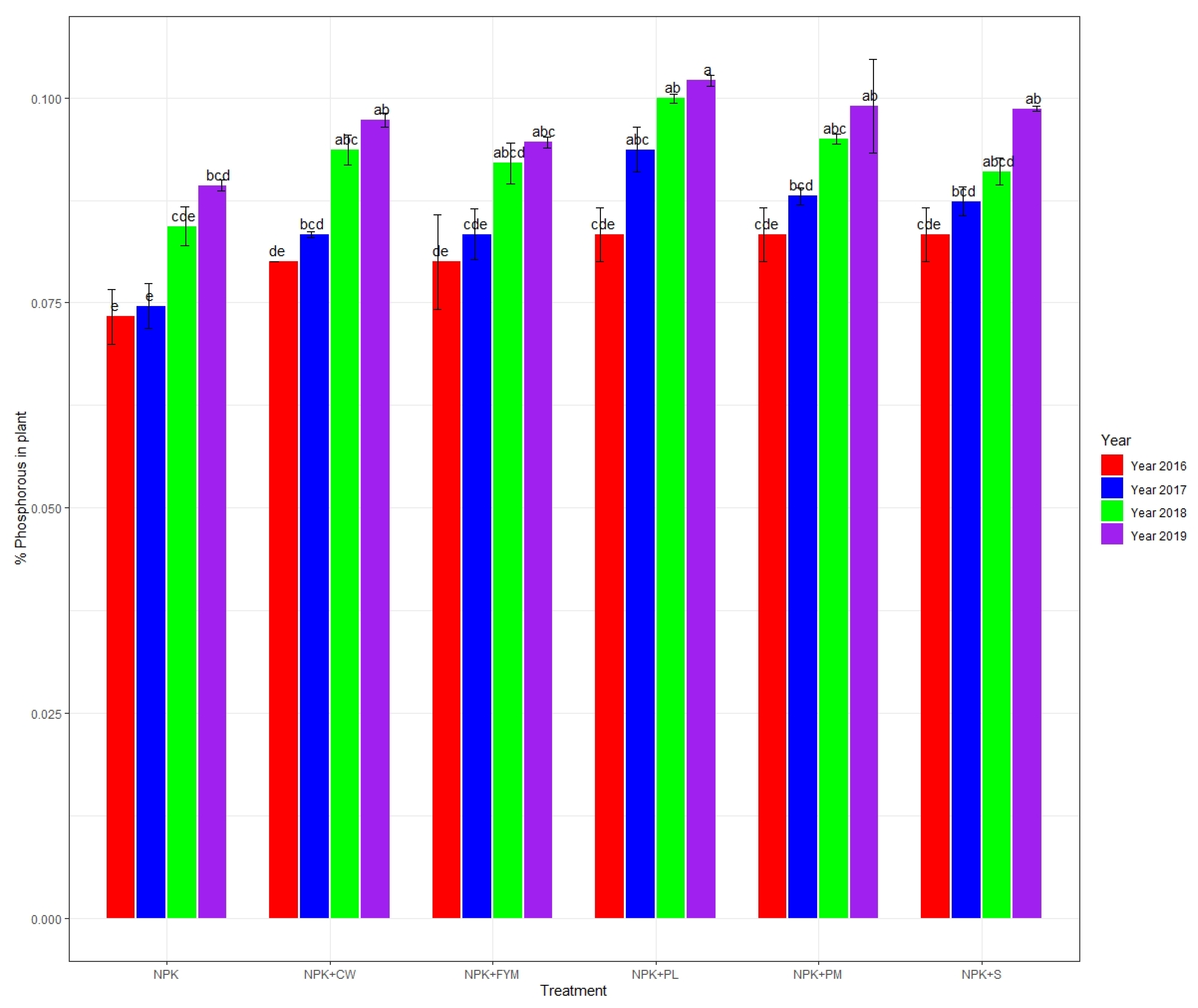
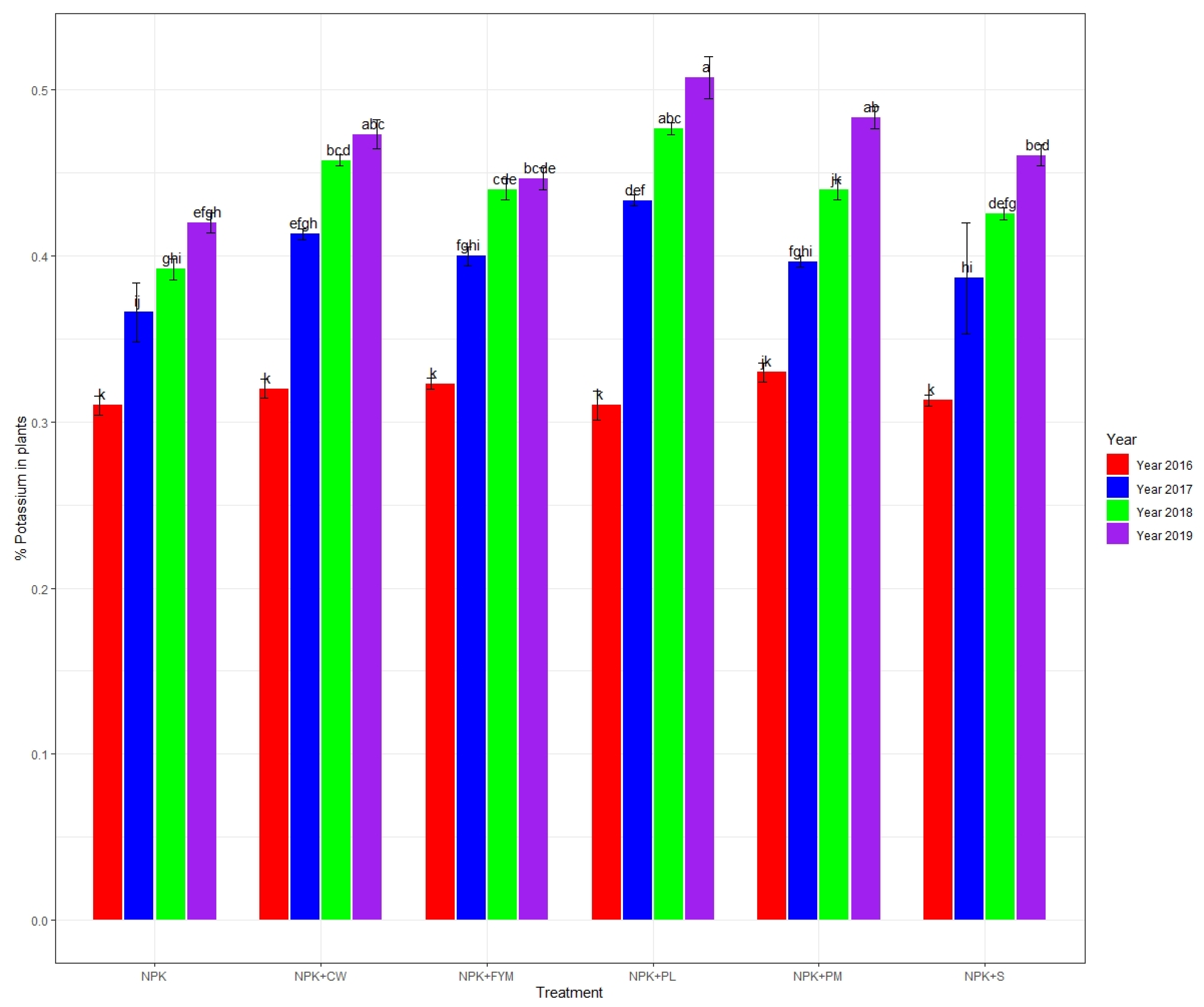
3.1. Plant Tissue Nitrogen, Phosphorus, and Potassium
3.2. Soil pH, EC, Phosphorus, Potassium, and Organic Matter
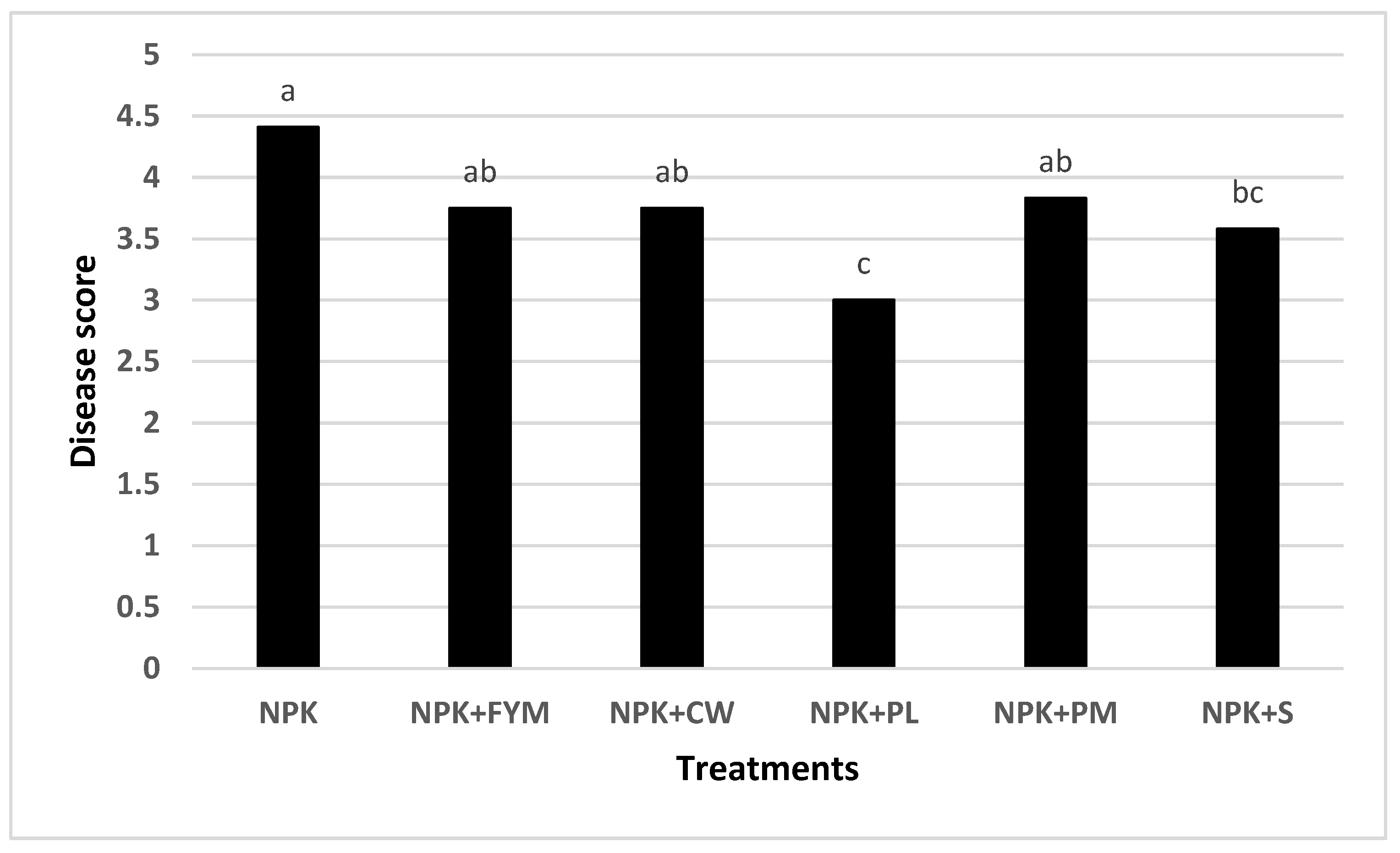
3.3. Plant Tissue Disease Score (Disease Intensity)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatments | Source | Company/Locations |
|---|---|---|
| NPK (control) | CAN, SSP, SOP | |
| NPK + farm yard manure | Manure from cattle farm | |
| NPK + city waste | Municipal waste | |
| NPK + poultry litter | Poultry farm litter | |
| NPK + press mud | Sugar mill waste | Fatima Sugar Mills Limited (30.297787, 70.970250) |
| +NPK + sulfur | Commercial-grade elemental sulfur |
| Parameters | Units | Soil | Farmyard Manure | City Waste | Poultry Litter | Press Mud |
|---|---|---|---|---|---|---|
| pH | 8.49 | 7.70 | 7.59 | 6.03 | 7.89 | |
| EC | dSm−1 | 3.21 | 4.12 | 3.187 | 4.01 | 3.54 |
| Available Phosphorus | ppm | 7.91 | 0.58 | 0.43 | 1.71 | 0.72 |
| Available Potassium | ppm | 160 | 0.40 | 0.62 | 0.92 | 0.79 |
| Organic Matter | % | 0.65 | 66.22 | 37.02 | 41.75 | 64.32 |
References
- Tharanathan, R.; Yashoda, H.; Prabha, T. Mango (Mangifera indica L.),“The king of fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Amin, M.; Malik, A.; Khalid, M.; Anwar, R. Fruit harvest maturity indicators for mango cultivars ‘Sindhri’ and ‘Samar Bahisht Chaunsa’. In Proceedings of the IX International Mango Symposium, Sanya, China, 8–12 April 2010; pp. 561–567. [Google Scholar]
- Ramay, S.A. Climate change killing agriculture. The Express Tribune, 1 May 2023. [Google Scholar]
- Khan, A.A. Pakistan’s Climate-Induced Water Scarcity and Its Impact on Agriculture. Available online: https://www.hilal.gov.pk/index.php/detail/pakistan%E2%80%99s-climate-induced-water-scarcity-and-its-impact-on-agriculture (accessed on 3 May 2023).
- Ahmed, N.; Umer, A.; Ali, M.A.; Iqbal, J.; Mubashir, M.; Grewal, A.G.; Butt, B.; Rasheed, M.K.; Chaudhry, U.K. Micronutrients status of mango (Mangifera indica) orchards in Multan region, Punjab, Pakistan, and relationship with soil properties. Open Agric. 2020, 5, 271–279. [Google Scholar] [CrossRef]
- Mohsin, M.; Jamal, F.; Ajmal, F. Impact of mango orchard diseases on growers economic life in Ahmedpur East, Bahawalpur, Pakistan. Acad. Res. Int. 2014, 5, 196. [Google Scholar]
- Jiskani, M.M.; Pathan, M.A.; Wagan, K.H.; Khaskheli, M. Documentation of identified and unidentified diseases of mango in Sindh, Pakistan. In Proceedings of the International Symposium on Prospects of Horticultural Industry in Pakistan, Institute of Horticultural Science, University of Agriculture, Faisalabad, Pakistan, 28–30 March 2007; pp. 176–190. [Google Scholar]
- Hussain, T.; Ishttiaq, M.; Zafar, B.; Parveen, A.; Maqbool, M.; Khan, F.A. Morphometric characterization of different mango varieties cultivated in tehsil Bernala district Bhimber Azad Kashmir, Pakistan. Pak. J. Bot 2023, 55, 1805–1812. [Google Scholar] [CrossRef]
- Ahmad, F.; Hafiz, I.A.; Asi, A.A.; Ahmad, S.; Khan, M. Mango varietal susceptibility to malformation and its control. Asian J. Plant Sci. 2002, 1, 158–159. [Google Scholar] [CrossRef]
- Akhtar, K.; Asif, M.; Khan, M.; Jaskani, M.; Khan, I. Isolation and Characterization of Fusarium moniliforme var. subglutinans from Malformed Mango. J. Agric. Mar. Sci. 1999, 4, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Masood, A.; Saeed, S.; Silveira, S.; Akem, C.N.; Hussain, N.; Farooq, M. Quick decline of mango in Pakistan: Survey and pathogenicity of fungi isolated from mango tree and bark beetle. Pak. J. Bot. 2011, 43, 1793–1798. [Google Scholar]
- Malik, M.; Ammar, M.; Ranan, M.; Rehman, A.; Bally, I.S. Chemical and cultural management of die back disease of mango in Pakistan. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): IV International Symposium on Papaya, VIII International Pineapple Symposium, and International Symposium on Mango, Brisbane, Australia, 17 August 2014; pp. 363–368. [Google Scholar]
- Umar, U.D.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients foliar and drench application mitigate mango sudden decline disorder and impact fruit yield. Agronomy 2022, 12, 2449. [Google Scholar] [CrossRef]
- Saeed, E.E.; Sham, A.; AbuZarqa, A.; Al Shurafa, K.A.; Al Naqbi, T.S.; Iratni, R.; El-Tarabily, K.; AbuQamar, S.F. Detection and management of mango dieback disease in the United Arab Emirates. Int. J. Mol. Sci. 2017, 18, 2086. [Google Scholar] [CrossRef] [Green Version]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; AbuQamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using streptomycete and non-streptomycete actinobacteria in the United Arab Emirates. Front. Microbiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, A.; Saeed, S.; Mahmood, A.; Malik, S.A.; Hussain, N. Role of nutrients in management of mango sudden death disease in Punjab, Pakistan. Pak. J. Zool. 2012, 44, 675–683. [Google Scholar]
- Ayyaz, S.; Shad, M.; Nawaz, H.; Ahmad, F. Influence of Dieback Disease on Soil Chemistry and Bacterial Diversity in the Rhizosphere of Mango Plants. Proc. Bulg. Acad. Sci. 2023, 76, 486–494. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wakeel, A.; Shahzad, M.N.; Kiran, A.; Li, X. Severity of zinc and iron malnutrition linked to low intake through a staple crop: A case study in east-central Pakistan. Environ. Geochem. Health 2021, 43, 4219–4233. [Google Scholar] [CrossRef]
- Quereshi, A.S.; Sarwar, A. Managing salinity in the Indus Basin of Pakistan. Int. J. River Basin Manag. 2009, 7, 111–117. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; Khaliq, A.; Cheema, S.A.; Alaraidh, I.A.; Hirotsu, N.; Wakeel, A. Zinc biofortified wheat cultivar lessens grain cadmium accumulation under cadmium contaminated conditions. Int. J. Agric. Biol. 2018, 20, 2842–2846. [Google Scholar]
- Fageria, N.; Gheyi, H.; Moreira, A. Nutrient bioavailability in salt affected soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Saleem, I.; Javid, S.; Ehsan, S.; Danish, S.; Ahmad, I. Phosphorus release kinetics of applied phosphate is influenced by time and organic sources in clay loam and sandy clay loam soils. Soil Environ. 2018, 37, 136–142. [Google Scholar]
- Antil, R.S.; Singh, M. Effects of organic manures and fertilizers on organic matter and nutrients status of the soil. Arch. Agron. Soil Sci. 2007, 53, 519–528. [Google Scholar] [CrossRef]
- Awasthi, L. Recent Advances in the Diagnosis and Management of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 93–125. [Google Scholar]
- Jat, L.K.; Singh, Y.; Meena, S.K.; Meena, S.K.; Parihar, M.; Jatav, H.; Meena, R.K.; Meena, V.S. Does integrated nutrient management enhance agricultural productivity. J. Pure Appl. Microbiol. 2015, 9, 1211–1221. [Google Scholar]
- Rowell, D.L. Soil Science: Methods & Applications; Routledge: London, UK, 2014. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Mason, J. Flame Photometric Determination of Potassium in Unashed Plant Leaves. Anal. Chem. 1963, 35, 874–875. [Google Scholar] [CrossRef]
- Gupta, A.; Neue, H.; Singh, V. Phosphorus determination in rice plants containing variable manganese content by the phospho-molybdo-vanadate (yellow) and phosphomolybdate (blue) colorimetric methods. Commun. Soil Sci. Plant Anal. 1993, 24, 1309–1318. [Google Scholar] [CrossRef]
- Amin, M.; Flowers, T. Evaluation of Kjeldahl digestion method. J. Res. Sci. 2004, 15, 159–179. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Packag, Version 1.0. 2017. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 17 November 2022).
- Saúco, V.G. Nutrition and Fertilization in Mango. Literature Review; Spain. 2018; pp. 1–74. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwify8mozYuAAxVugf0HHQgjAIQQFnoECBIQAQ&url=https%3A%2F%2Fwww.mango.org%2Fwp-content%2Fuploads%2F2020%2F02%2FNutrition_Fertilization_ENG.pdf&usg=AOvVaw0Es7Btfmjm0gro7qwWzS4y&opi=89978449 (accessed on 10 November 2022).
- Van Averbeke, W.; Chabalala, M.; Okorogbona, A.; Rumania, T.; Azeez, J.; Slabbert, M. Plant nutrient requirements of African leafy vegetables. In Nutritional Value and Water Use of African Leafy Vegetables for Improved Livelihoods; WRC TT535/12; Water Research Commission: Gezina, South Africa, 2012; pp. 1–302. [Google Scholar]
- Brautigan, D.; Rengasamy, P.; Chittleborough, D. Amelioration of alkaline phytotoxicity by lowering soil pH. Crop Pasture Sci. 2014, 65, 1278–1287. [Google Scholar] [CrossRef]
- Azeez, J.; Van Averbeke, W. Dynamics of soil pH and electrical conductivity with the application of three animal manures. Commun. Soil Sci. Plant Anal. 2012, 43, 865–874. [Google Scholar] [CrossRef]
- Poulsen, P.H.; Al-Soud, W.A.; Bergmark, L.; Magid, J.; Hansen, L.H.; Sørensen, S.J. Effects of fertilization with urban and agricultural organic wastes in a field trial–Prokaryotic diversity investigated by pyrosequencing. Soil Biol. Biochem. 2013, 57, 784–793. [Google Scholar] [CrossRef]
- Dewes, T.; Hünsche, E. Composition and microbial degradability in the soil of farmyard manure from ecologically-managed farms. Biol. Agric. Hortic. 1998, 16, 251–268. [Google Scholar] [CrossRef]
- Dey, A.; Srivastava, P.C.; Pachauri, S.P.; Shukla, A.K. Time-dependent release of some plant nutrients from different organic amendments in a laboratory study. Int. J. Recycl. Org. Waste Agric. 2019, 8, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Zhang, X.; Zhang, S.; Chen, Y. Organic manure input improves soil water and nutrients use for sustainable maize (Zea mays L.) productivity on the Loess Plateau. PLoS ONE 2020, 15, e0238042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T. Carbon science in 2016: Status, challenges and perspectives. Carbon 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Hasnain, M.; Chen, J.; Ahmed, N.; Memon, S.; Wang, L.; Wang, Y.; Wang, P. The effects of fertilizer type and application time on soil properties, plant traits, yield and quality of tomato. Sustainability 2020, 12, 9065. [Google Scholar] [CrossRef]
- McMahon, P. Effect of nutrition and soil function on pathogens of tropical tree crops. Plant Pathol. 2012, 4, 243–272. [Google Scholar]
- Majhi, P.; Rout, K.; Nanda, G.; Singh, M. Soil quality for rice productivity and yield sustainability under long-term fertilizer and manure application. Commun. Soil Sci. Plant Anal. 2019, 50, 1330–1343. [Google Scholar] [CrossRef]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef] [Green Version]

| Treatment | pH | EC (dS m−1) | Phosphorus (mg kg−1) | Potassium (mg kg−1) | Organic Matter (%) |
|---|---|---|---|---|---|
| NPK | 8.38 ± 0.03 a | 4.2 ± 0.08 a | 7.38 ± 0.18 b | 149.70 ± 5.13 ab | 0.608 ± 0.003 c |
| NPK + CW | 8.32 ± 0.02 a | 3.85 ± 0.21 ab | 7.80 ± 0.07 ab | 149.79 ± 3.19 ab | 0.637 ± 0.009 a |
| NPK + FYM | 8.38 ± 0.04 a | 3.80 ± 0.06 ab | 7.76 ± 0.16 ab | 148.29 ± 4.17 ab | 0.629 ± 0.006 ab |
| NPK + PL | 8.14 ± 0.07 b | 3.58 ± 0.05 b | 7.95 ± 0.05 a | 159.37 ± 4.53 a | 0.64 ± 0.015 a |
| NPK + PM | 8.38 ± 0.05 a | 3.93 ± 0.1 ab | 7.47 ± 0.11 ab | 150.12 ± 4.22 ab | 0.61 ± 0.009 bc |
| NPK + S | 8.34 ± 0.01 a | 3.88 ± 0.09 ab | 7.70 ± 0.09 ab | 146.29 ± 3.81 b | 0.60 ± 0.005 c |
| p-value | <0.01 | <0.05 | <0.05 | ≤0.05 | <0.01 |
| F-value | 5.98 | 3.02 | 2.62 | 2.11 | 10.87 |
| DF | 5 | 5 | 5 | 5 | 5 |
| Year | pH | EC (dS m−1) | Phosphorus (mg kg−1) | Potassium (mg kg−1) | Organic Matter (%) |
|---|---|---|---|---|---|
| 2016 | 8.47 ± 0.03 a | 4.08 ± 0.15 a | 7.51 ± 0.13 a | 133.33 ± 3.35 c | 0.59 ± 0.004 c |
| 2017 | 8.29 ± 0.03 b | 3.81 ± 0.06 a | 7.60 ± 0.10 a | 150.61 ± 2.16 b | 0.60 ±0.004 c |
| 2018 | 8.27 ± 0.03 b | 3.81 ± 0.06 a | 7.76 ± 0.09 a | 157.33 ± 2.17 ab | 0.62 ± 0.007 b |
| 2019 | 8.27 ± 0.03 b | 3.79 ± 0.06 a | 7.83 ± 0.08 a | 161.11 ± 1.67 a | 0.65 ± 0.008 a |
| p-value | <0.01 | >0.05 | >0.05 | <0.05 | <0.01 |
| F-value | 515.39 | 2.21 | 1.84 | 2.11 | 35.74 |
| DF | 3 | 3 | 3 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, F.; Hameed, A.; Muhammad, N.; Shahzad, K.; Ahmad, I.; Shah, T.A.; Z. Gaafar, A.-R.; Hodhod, M.S.; Bourhia, M.; Nafidi, H.-A. Potential of Integrated Nutrient Management to Rehabilitate the Dieback-Affected Mango Cultivar Sammer Bahisht Chaunsa. Sustainability 2023, 15, 11118. https://doi.org/10.3390/su151411118
Bibi F, Hameed A, Muhammad N, Shahzad K, Ahmad I, Shah TA, Z. Gaafar A-R, Hodhod MS, Bourhia M, Nafidi H-A. Potential of Integrated Nutrient Management to Rehabilitate the Dieback-Affected Mango Cultivar Sammer Bahisht Chaunsa. Sustainability. 2023; 15(14):11118. https://doi.org/10.3390/su151411118
Chicago/Turabian StyleBibi, Fatma, Asifa Hameed, Noor Muhammad, Khurram Shahzad, Iftikhar Ahmad, Tawaf Ali Shah, Abdel-Rhman Z. Gaafar, Mohamed S. Hodhod, Mohammed Bourhia, and Hiba-Allah Nafidi. 2023. "Potential of Integrated Nutrient Management to Rehabilitate the Dieback-Affected Mango Cultivar Sammer Bahisht Chaunsa" Sustainability 15, no. 14: 11118. https://doi.org/10.3390/su151411118






