Enhancing the Immobilization of Hexavalent Chromium by the Interlayer Anion Adsorption of the Brucite-Transformed LDH in the Presence of Aluminum Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Mg(OH)2 Nano-Sheets
2.3. Cr(VI) Adsorption Experiments
2.4. Regeneration Experiment of Cr(VI)
2.5. Analytical Methods
3. Results and Discussion
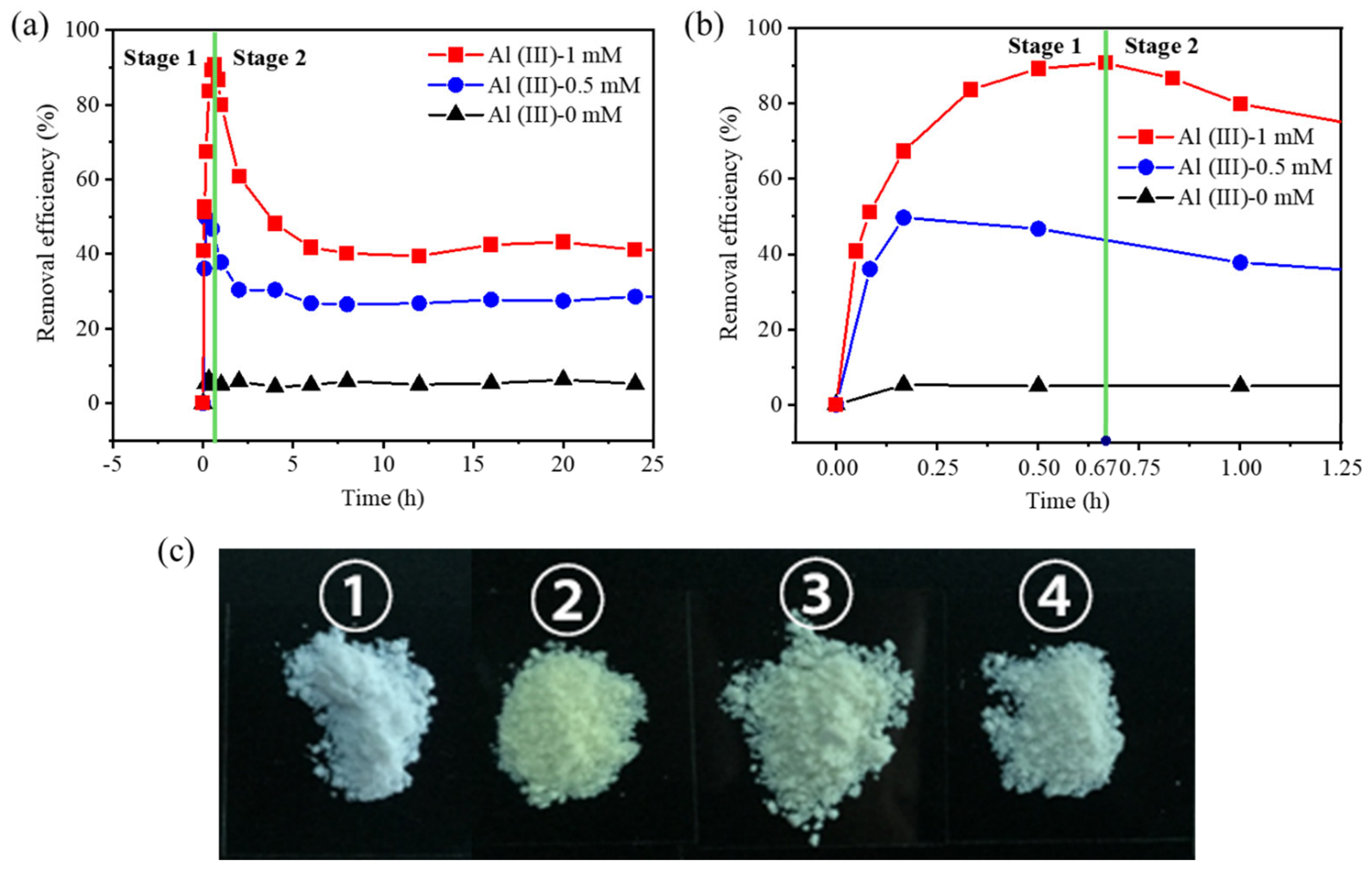
3.1. The Adsorption of Cr(VI) in Mg(OH)2
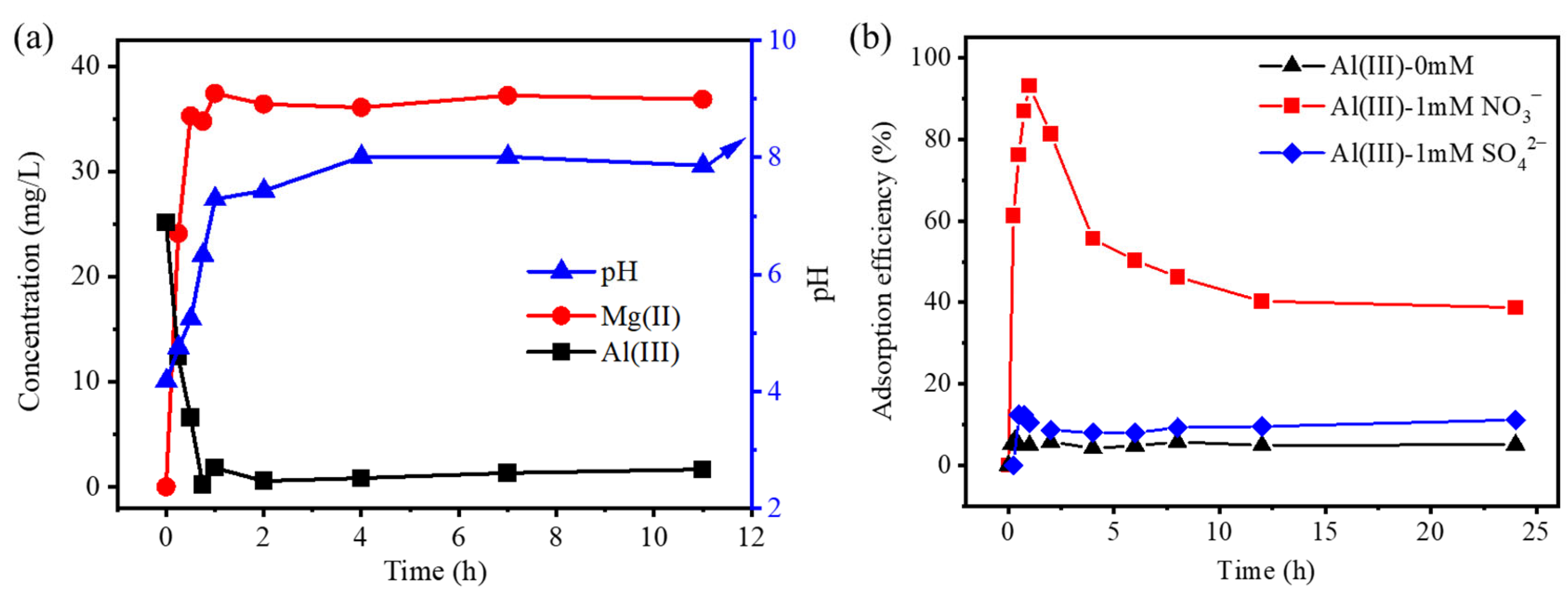
3.2. The Formation of LDH in the Cr(VI) Adsorption
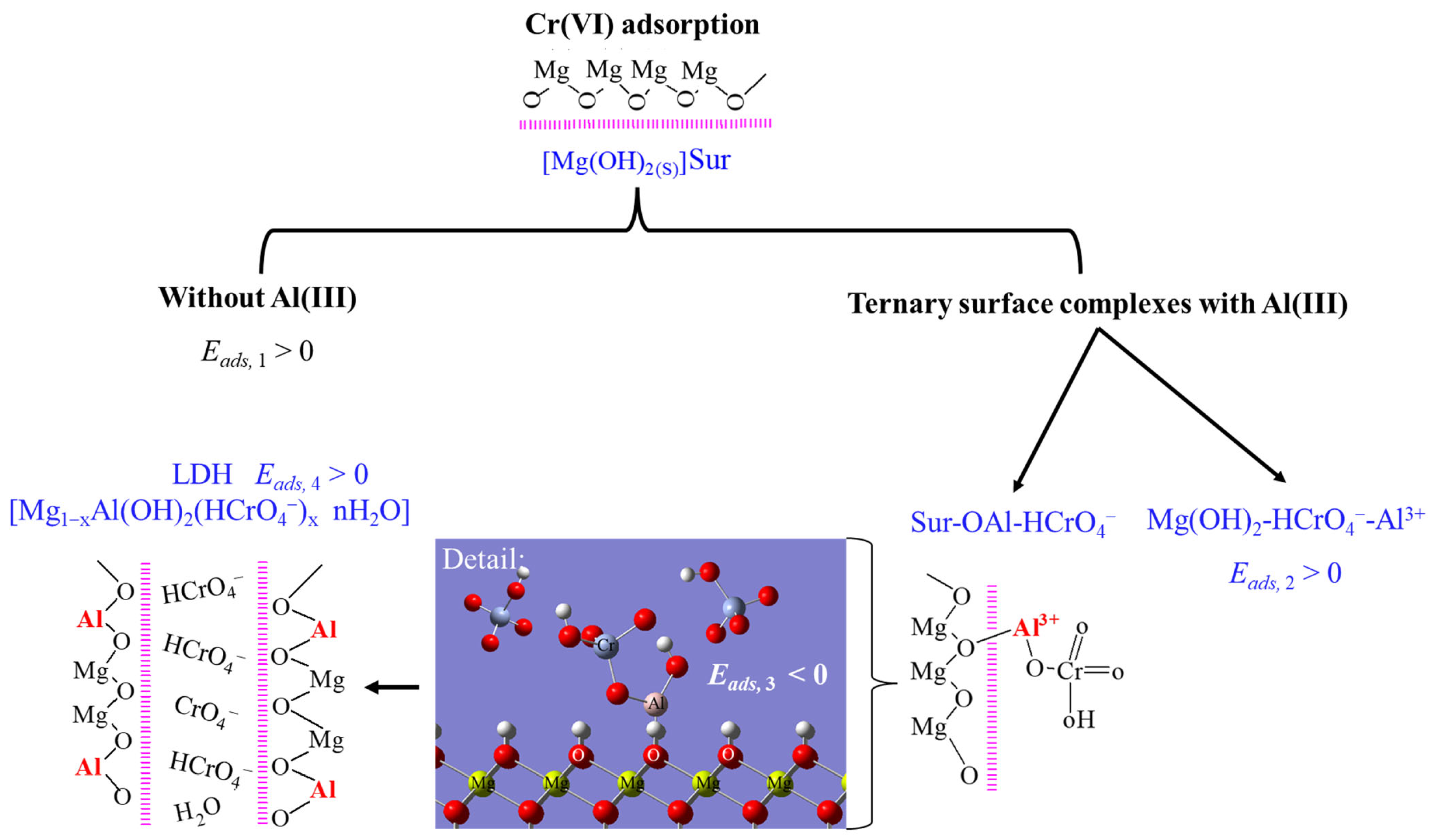
3.3. The Mechanism of the Cr(VI) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, K.; Hu, Q.; Yao, L.; Li, M.; Sun, D.; Shao, Q.; Qiu, B.; Guo, Z. Ultrasonic Pretreated Sludge Derived Stable Magnetic Active Carbon for Cr(VI) Removal from Wastewater. Acs Sustain. Chem. Eng. 2018, 6, 7283–7291. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2018, 276, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yue, Q.; Gao, B.; Xing, X. Equilibrium and kinetic adsorption study of the adsorptive removal of Cr(VI) using modified wheat residue. J. Colloid Interface Sci. 2010, 349, 256–264. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Yang, D.; Shi, P.; Zevenhoven, R. A cleaner method for preparation of chromium oxide from chromite. Process Saf. Environ. Prot. 2016, 105, 91–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, L.; Yan, Y.; Li, H.; Yu, Q.; Jiao, B.; Li, D. Rapid Cr(VI) reduction structure in chromium contaminated soil: The UV-assisted electrokinetic circulation of background iron. Sci. Total Environ. 2022, 822, 153508. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Song, F.; Zhao, J.; Zhang, L. Facet-Dependent Cr(VI) Adsorption of Hematite Nanocrystals. Environ. Sci. Technol. 2016, 50, 1964. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Li, H.; Liang, X.; Hou, W. Sorption of Cr(VI) on Mg–Al–Fe layered double hydroxides synthesized by a mechanochemical method. RSC Adv. 2014, 4, 46823–46830. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L. In-Situ Remediation of Cr(VI)-Contaminated Groundwater Using Permeable Reactive Walls: Laboratory Studies. Environ. Sci. Technol. 1997, 31, 3348–3357. [Google Scholar] [CrossRef]
- Saad, E.M.; Sun, J.; Chen, S.; Borkiewicz, O.J.; Zhu, M.; Duckworth, O.W.; Tang, Y. Siderophore and Organic Acid Promoted Dissolution and Transformation of Cr(III)-Fe(III)-(oxy)hydroxides. Environ. Sci. Technol. 2017, 51, 3223–3232. [Google Scholar] [CrossRef]
- Al-Jabri, K.; Shoukry, H.; Khalil, I.S.; Nasir, S.; Hassan, H.F. Reuse of Waste Ferrochrome Slag in the Production of Mortar with Improved Thermal and Mechanical Performance. J. Mater. Civ. Eng. 2018, 30, 04018152. [Google Scholar] [CrossRef]
- Jena, S.; Panigrahi, R. Performance assessment of geopolymer concrete with partial replacement of ferrochrome slag as coarse aggregate. Constr. Build. Mater. 2019, 220, 525–537. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Fakra, S.C.; Marcus, M.A.; Moon, D.H.; Dermatas, D. Microstructural analyses of Cr(VI) speciation in chromite ore processing residue (COPR). Am. Chem. Soc. 2009, 43, 5461–5466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, P.; Liu, F.; Liu, W.; Zhang, J.; Lin, Z. Simultaneous oxidation of Cr(III) and extraction of Cr(VI) from chromite ore processing residue by silicate-assisted hydrothermal treatment. Chem. Eng. J. 2019, 371, 565–574. [Google Scholar] [CrossRef]
- Gomez, M.A.; Hendry, M.J.; Koshinsky, J.; Essilfie-Dughan, J.; Paikaray, S.; Chen, J. Mineralogical controls on aluminum and magnesium in uranium mill tailings: Key Lake, Saskatchewan, Canada. Environ. Sci. Technol. 2013, 47, 7883–7891. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuang, Z.; Cao, Q.; Pan, X.; Guan, X.; Lin, Z. Adsorption-Induced Crystallization of U-Rich Nanocrystals on Nano-Mg(OH)(2) and the Aqueous Uranyl Enrichment. ACS Appl. Mater. Interfaces 2014, 6, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, H.; Wang, Y.; Zou, T.; Zhang, L. Recycling Mg(OH)(2) Nanoadsorbent during Treating the Low Concentration of Cr-VI. Environ. Sci. Technol. 2011, 45, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Bochkarev, G.R.; Pushkareva, G.I. Intensification of sorption properties of brucite. J. Min. Sci. 2005, 41, 380–384. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Tao, L.; Liu, F.; Zhang, J. Enhanced removal of antimony by acid birnessite with doped iron ions: Companied by the structural transformation. Chemosphere 2019, 226, 834–840. [Google Scholar] [CrossRef]
- Yue, X.; Liu, W.; Chen, Z.; Lin, Z. Simultaneous removal of Cu(II) and Cr(VI) by Mg–Al–Cl layered double hydroxide and mechanism insight. J. Environ. Sci. 2016, 53, 16. [Google Scholar] [CrossRef]
- Ou, X.; Zhuang, Z.; Li, J.; Huang, F.; Lin, Z. Mechanism of adsorption affinity and capacity of Mg(OH)2 to uranyl revealed by molecular dynamics simulation. RSC Adv. 2016, 6, 31507–31513. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Bao, Q. In situ reactive zone with modified Mg(OH)2 for remediation of heavy metal polluted groundwater: Immobilization and interaction of Cr(III), Pb(II) and Cd(II). J. Contam. Hydrol. 2017, 199, 50–57. [Google Scholar] [CrossRef]
- Pishtshev, A.; Karazhanov, S.Z.; Klopov, M. Materials properties of magnesium and calcium hydroxides from first-principles calculations. Comput. Mater. Sci. 2014, 95, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Hota, G. Amine-Functionalized GO Decorated with ZnO-ZnFe2O4 Nanomaterials for Remediation of Cr(VI) from Water. ACS Appl. Nano Mater. 2019, 2, 983–996. [Google Scholar] [CrossRef]
- GB 7467-87; Water Quality-Determination of Chromiun(VI)-1,5 Dtphenylcarbohydrazide Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1987. Available online: https://www.mee.gov.cn/image20010518/3602.pdf (accessed on 5 June 2023).
- GB/T 15555.4-1995; Solid Waste-Determination of Chromium(VI)-1,5-Diphenylcarbohydrazide Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1995. Available online: https://www.mee.gov.cn/image20010518/1934.pdf (accessed on 5 June 2023).
- Chen, Z.; Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Multifunctional kaolinite-supported nanoscale zero-valent iron used for the adsorption and degradation of crystal violet in aqueous solution. J. Colloid Interface Sci. 2013, 398, 59–66. [Google Scholar] [CrossRef]
- Manzoor, K. Synthesis, Characterization, Kinetics, and Thermodynamics of EDTA-Modified Chitosan-Carboxymethyl Cellulose as Cu(II) Ion Adsorbent. ACS Omega 2019, 4, 17425–17437. [Google Scholar] [CrossRef] [Green Version]
- Choong, C.E.; Wong, K.T.; Jang, S.B.; Saravanan, P.; Park, C.; Kim, S.H.; Jeon, B.H.; Choi, J.; Yoon, Y.; Jang, M. Granular Mg-Fe layered double hydroxide prepared using dual polymers: Insights into synergistic removal of As(Ⅲ) and As(Ⅴ). J. Hazard. Mater. 2021, 403, 123883. [Google Scholar] [CrossRef]
- Zhang, B.; Luan, L.; Gao, R.; Li, F.; Li, Y.; Wu, T. Rapid and effective removal of Cr(VI) from aqueous solution using exfoliated LDH nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 399–408. [Google Scholar] [CrossRef]
- Chen, W.; Qu, B.J. Structural characteristics and thermal properties of PE-g-MA/MgAl-LDH exfoliation nanocomposites synthesized by solution intercalation. Chem. Mater. 2003, 15, 3208–3213. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Mills, C.T.; Bern, C.R.; Wolf, R.E.; Foster, A.L.; Morrison, J.M.; Benzel, W.M. Modifications to EPA Method 3060A to Improve Extraction of Cr(VI) from Chromium Ore Processing Residue-Contaminated Soils. Environ. Sci. Technol. 2017, 51, 11235–11243. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Avila, M.; Burks, T.; Akhtar, F.; Göthelid, M.; Lansåker, P.C.; Toprak, M.S.; Muhammed, M.; Uheida, A. Surface functionalized nanofibers for the removal of chromium(VI) from aqueous solutions. Chem. Eng. J. 2014, 245, 201–209. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Wang, H.; Li, H.; Zeng, G. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Achari, G.; Yu, J.; Cai, W. Cr(vi) removal from aqueous solutions by hydrothermal synthetic layered double hydroxides: Adsorption performance, coexisting anions and regeneration studies. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 33–40. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Huang, L.; Li, Z.; Chen, B.; Zhang, M.; Wu, C.; Ma, P.; Zhang, W. Enhancing the Immobilization of Hexavalent Chromium by the Interlayer Anion Adsorption of the Brucite-Transformed LDH in the Presence of Aluminum Ions. Sustainability 2023, 15, 11173. https://doi.org/10.3390/su151411173
Chen X, Huang L, Li Z, Chen B, Zhang M, Wu C, Ma P, Zhang W. Enhancing the Immobilization of Hexavalent Chromium by the Interlayer Anion Adsorption of the Brucite-Transformed LDH in the Presence of Aluminum Ions. Sustainability. 2023; 15(14):11173. https://doi.org/10.3390/su151411173
Chicago/Turabian StyleChen, Xiaoduo, Lianyang Huang, Zheng Li, Binfeng Chen, Menglu Zhang, Chunshan Wu, Pengchen Ma, and Weifang Zhang. 2023. "Enhancing the Immobilization of Hexavalent Chromium by the Interlayer Anion Adsorption of the Brucite-Transformed LDH in the Presence of Aluminum Ions" Sustainability 15, no. 14: 11173. https://doi.org/10.3390/su151411173






