Electrospun Carboxymethyl Cellulose/Polyvinyl Alcohol Nanofiber Membranes for Enhanced Metal Ion Removal
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
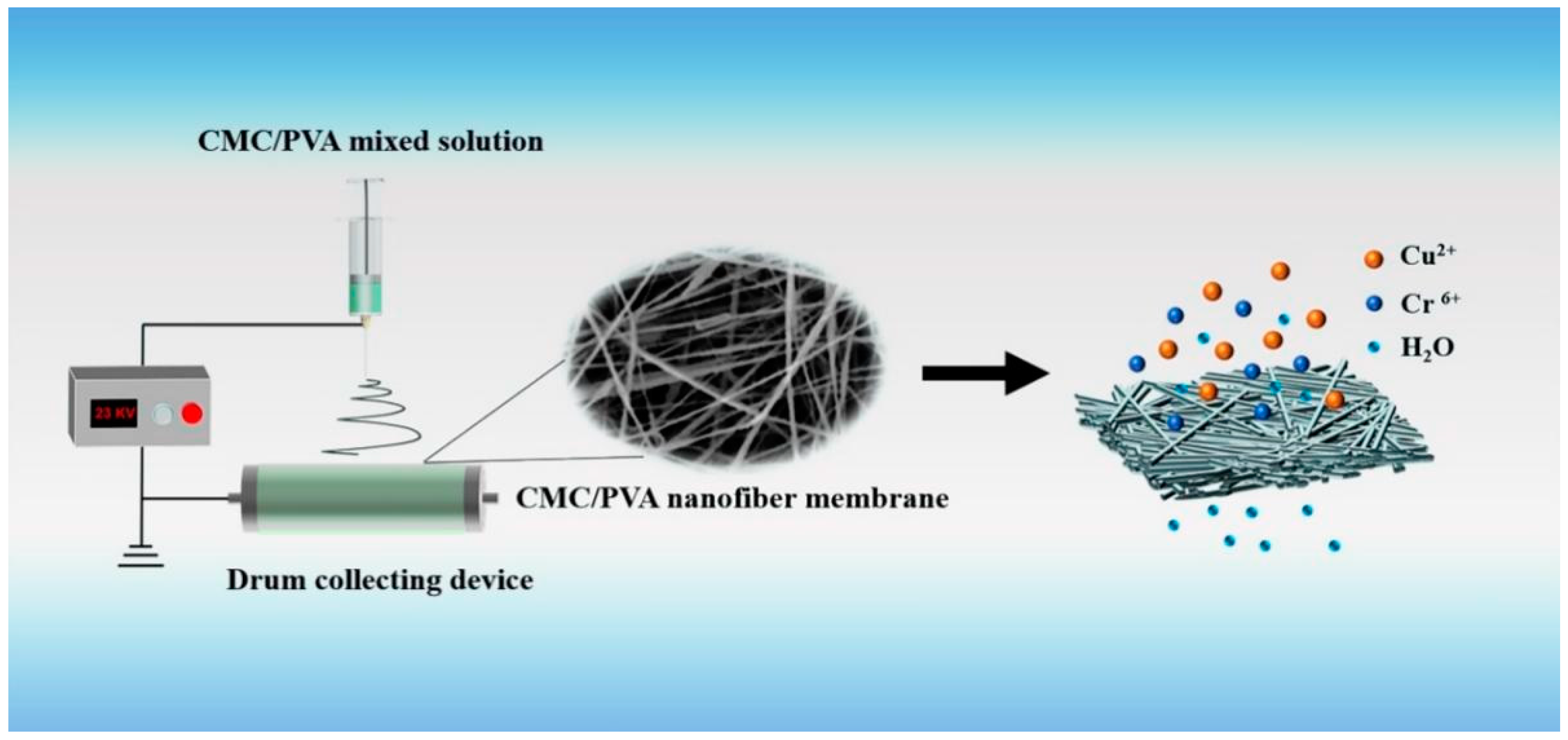
2.2. Preparation of CMC/PVA Nanofiber Membranes
2.3. Characterization of CMC/PVA Nanofiber Membranes
2.4. Preparation and UV Analysis of Cu Reagent and Cr Reagent
2.5. Filtration Tests of CMC/PVA Nanofiber Membranes
2.6. Adsorption Study of CMC/PVA Nanofiber Membranes
3. Results and Discussion
3.1. Characteristics of Nanofiber Membranes
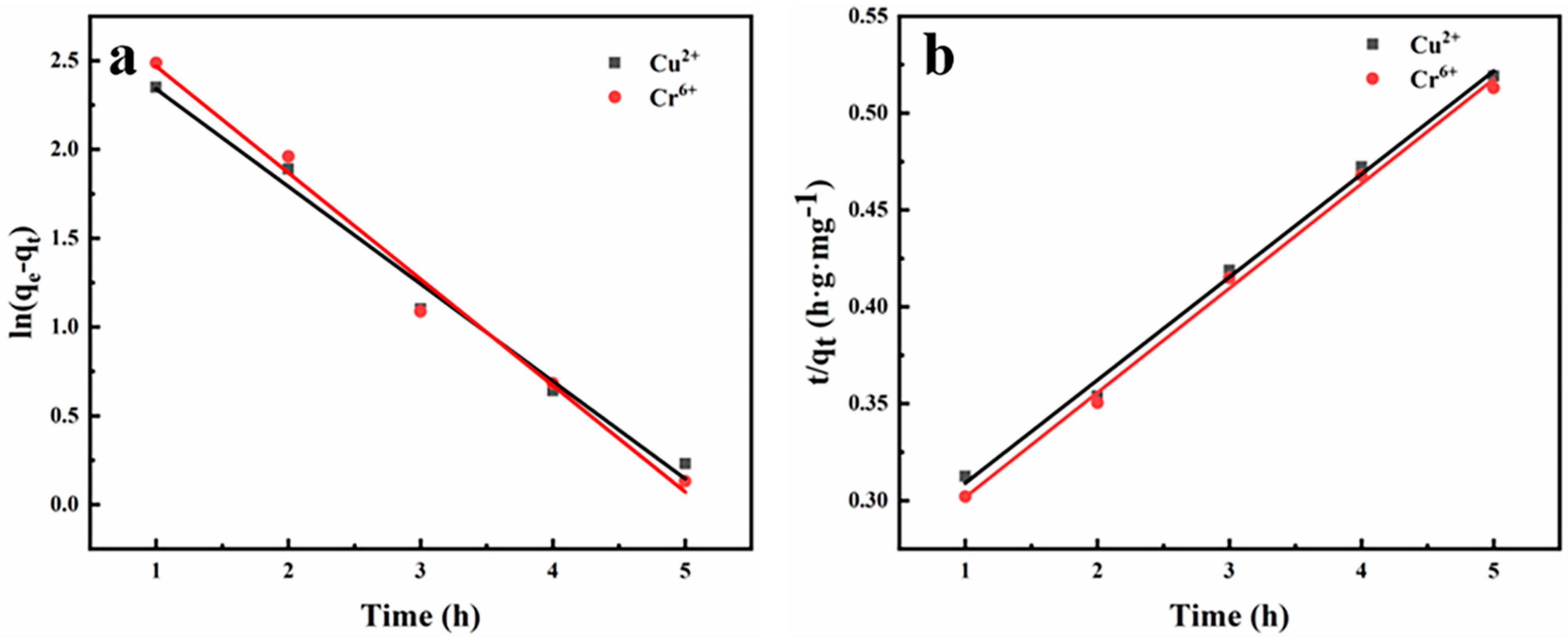
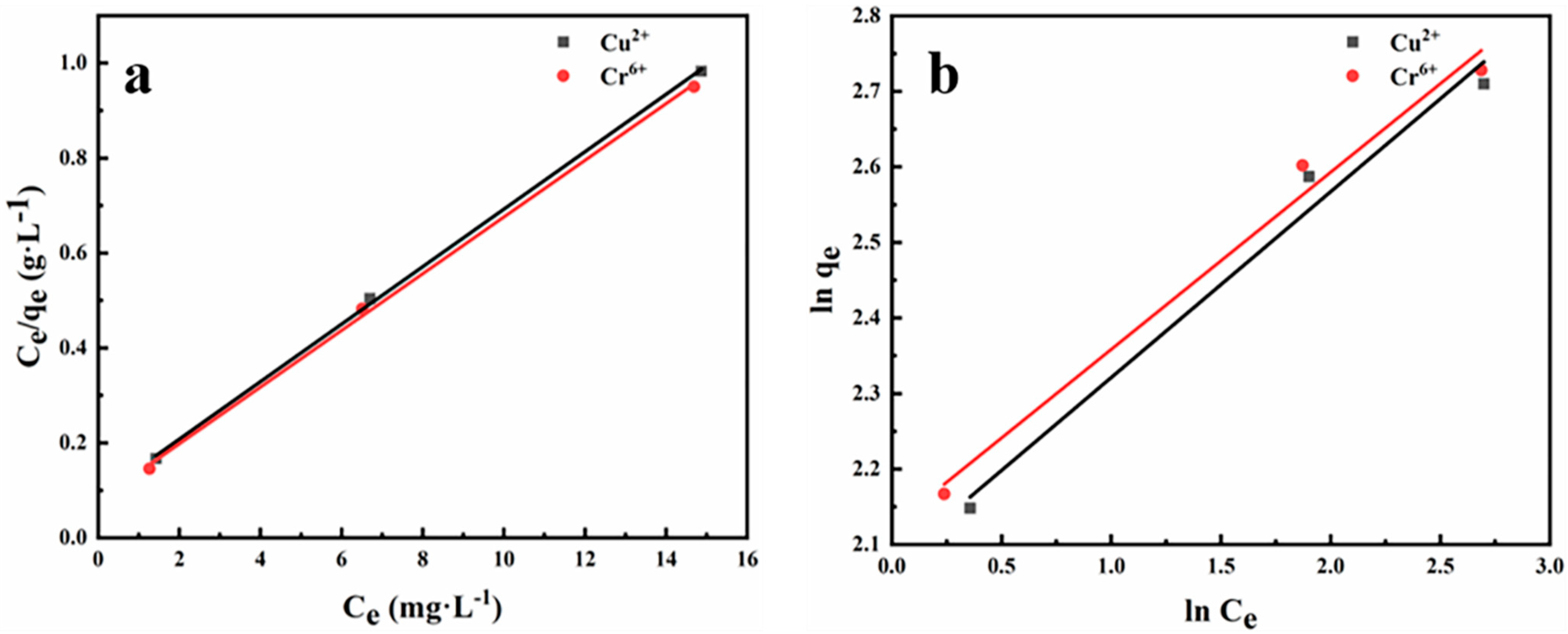
3.2. Adsorption Studies of the CMC/PVA Nanofiber Membranes
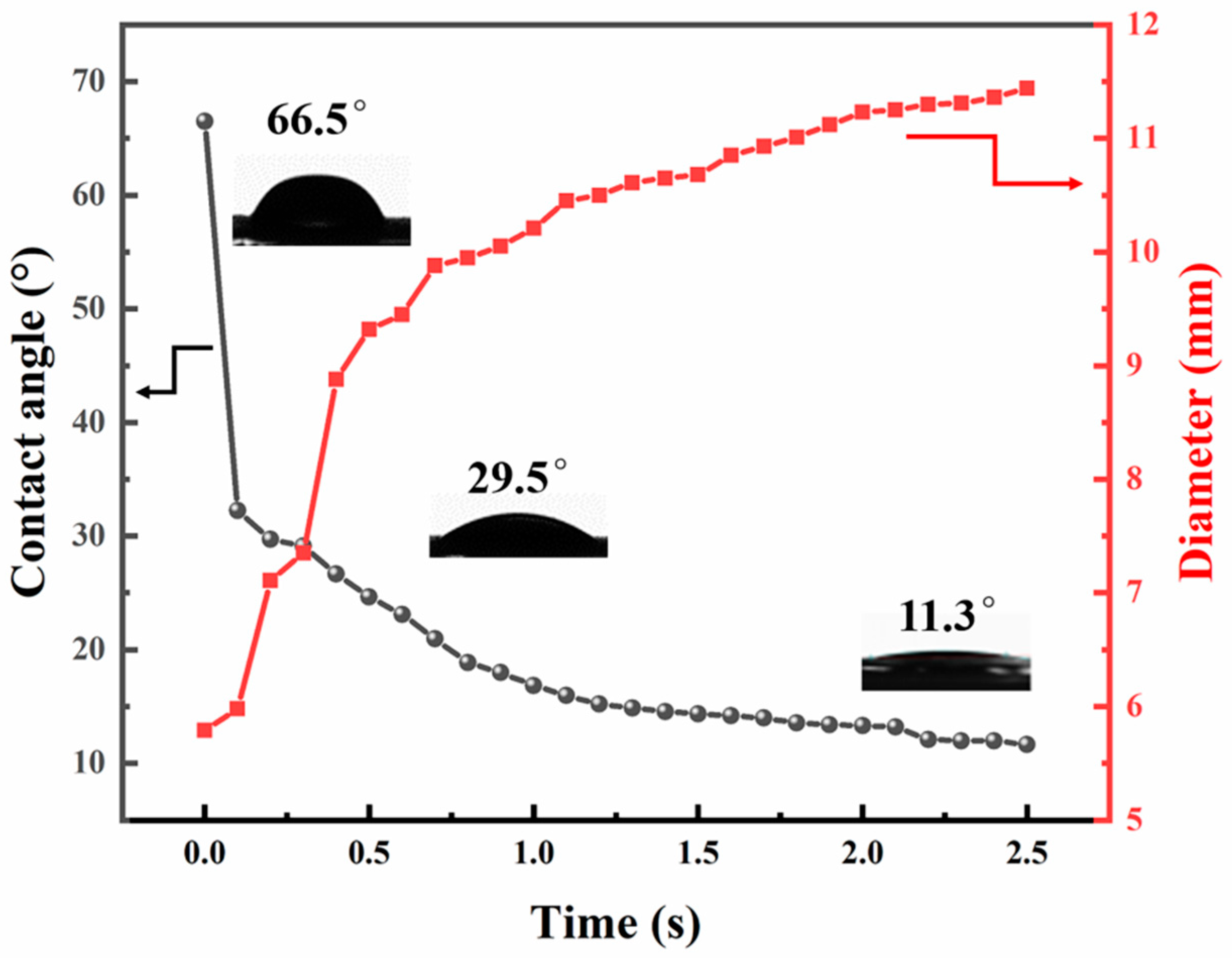
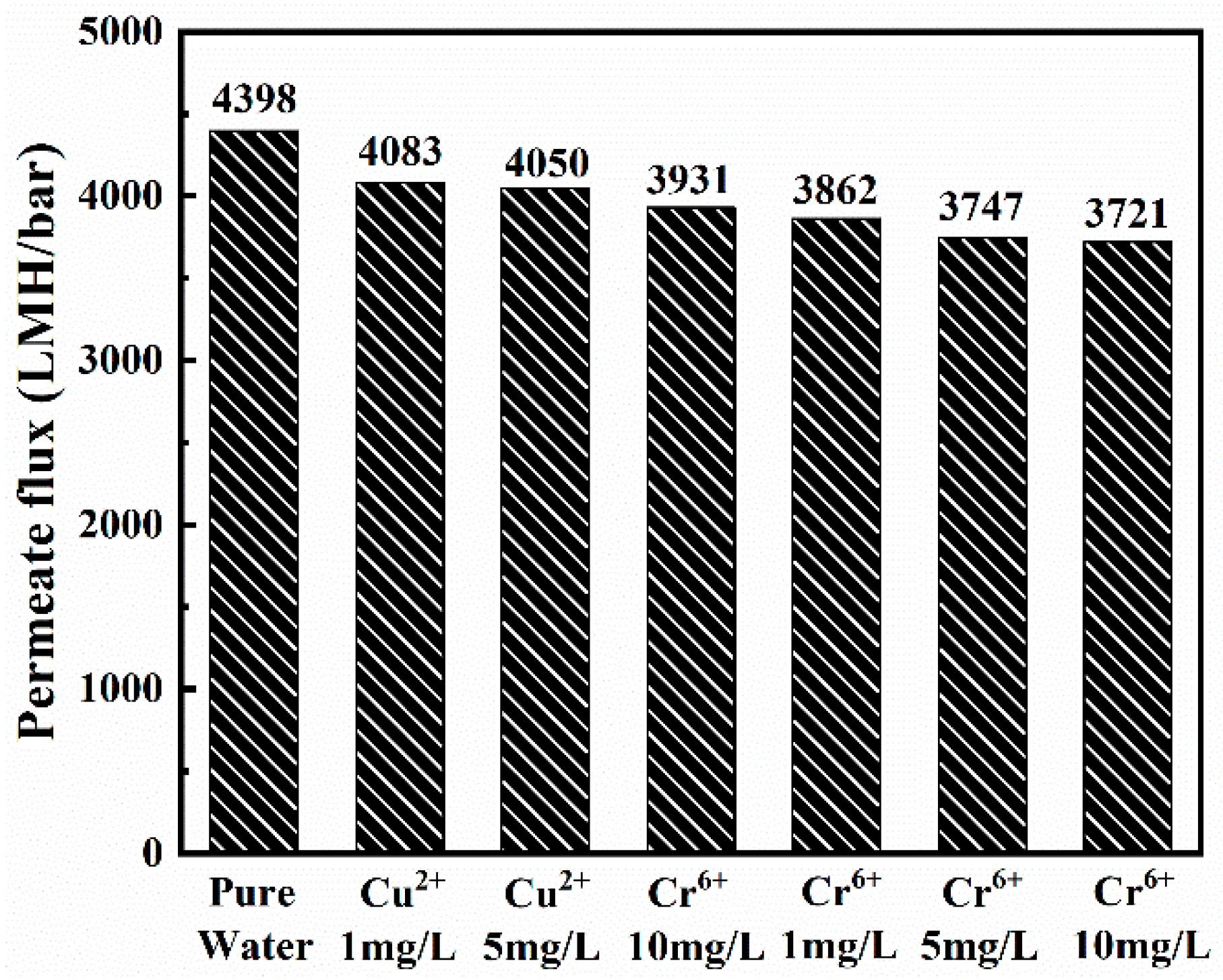
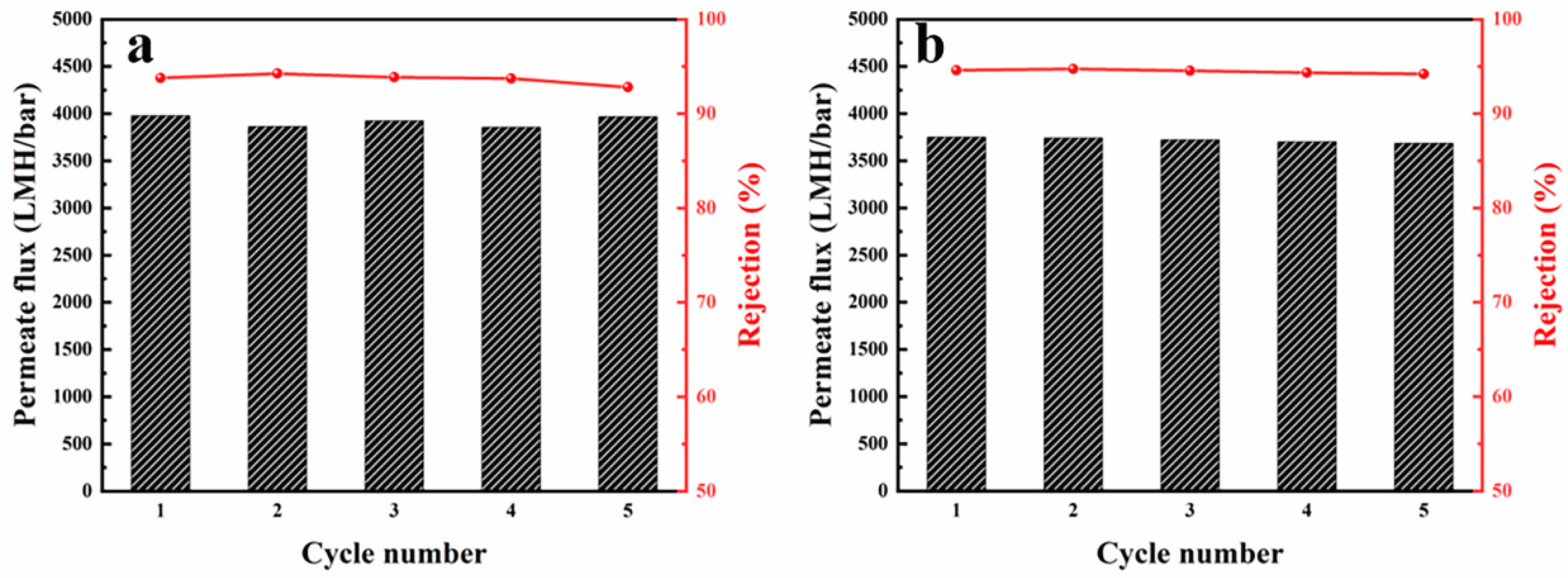
3.3. Nanofiber Membranes Flux and Separation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2018; pp. 103–125. [Google Scholar]
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P. Heavy metal contamination in water and its possible sources. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–189. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Velizarov, S. New insights into the definition of membrane cleaning strategies to diminish the fouling impact in ion exchange membrane separation processes. Sep. Purif. Technol. 2021, 277, 119445. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2019, 31, 1806090. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Ni, J.; Yuan, C.; Zheng, J.; Liu, Y. Distributions, contamination level and ecological risk of heavy metals in surface sediments from intertidal zone of the Sanmen Bay, East China. J. Sea Res. 2022, 190, 102302. [Google Scholar] [CrossRef]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cai, N.; Chan, V.; Yu, F. Development and applications of MOFs derivative one-dimensional nanofibers via electrospinning: A mini-review. Nanomaterials 2019, 9, 1306. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Chitosan/polyvinylpyrrolidone/polyvinyl alcohol/carbon nanotubes dual layers nanofibrous membrane constructed by electrospinning-electrospray for water purification. Carbohydr. Polym. 2022, 294, 119756. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Qin, L.; Li, X.; Meng, Q.; Shen, C.; Zhang, G. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 2020, 379, 122368. [Google Scholar] [CrossRef]
- Zou, P.; Lee, W.-H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Samal, S.K.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Liao, J.; Lin, H.; Liu, F. PEI modified multiwalled carbon nanotube as a novel additive in PAN nanofiber membrane for enhanced removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122086. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.; Rahman, M.M.; Susan, M.A.B.H.; Shaikh, M.A.A.; Nayeem, J.; Jahan, M.S. A novel approach in increasing carboxymethylation reaction of cellulose. Carbohydr. Polym. Technol. Appl. 2022, 4, 100236. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Ramli, N.A.; Othman, N.A.; Amin, K.N.M.; Adam, F. Characterization and property investigation of microcrystalline cellulose (MCC) and carboxymethyl cellulose (CMC) filler on the carrageenan-based biocomposite film. Mater. Today Proc. 2021, 42, 56–62. [Google Scholar] [CrossRef]
- Mohamadpour, F. Carboxymethyl cellulose (CMC) as a recyclable green catalyst promoted eco-friendly protocol for the solvent-free synthesis of 1H-pyrazolo[1, 2-b]phthalazine-5,10-dione derivatives. Polycycl. Aromat. Compd. 2022, 42, 1091–1102. [Google Scholar] [CrossRef]
- Allafchian, A.; Hosseini, H.; Ghoreishi, S.M. Electrospinning of PVA-carboxymethyl cellulose nanofibers for flufenamic acid drug delivery. Int. J. Biol. Macromol. 2020, 163, 1780–1786. [Google Scholar] [CrossRef]
- Shen, H.; Li, Y.; Yao, W.; Yang, S.; Yang, L.; Pan, F.; Chen, Z.; Yin, X. Solvent-free cellulose nanocrystal fluids for simultaneous enhancement of mechanical properties, thermal conductivity, moisture permeability and antibacterial properties of polylactic acid fibrous membrane. Compos. Part B Eng. 2021, 222, 109042. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Liu, L.; Zhang, P.; Wang, X.; Yu, J.; Ding, B. Transformation of fibrous membranes from opaque to transparent under mechanical pressing. Engineering 2022, 19, 84–92. [Google Scholar] [CrossRef]
- Mazuki, N.; Majeed, A.A.; Nagao, Y.; Samsudin, A. Studies on ionics conduction properties of modification CMC-PVA based polymer blend electrolytes via impedance approach. Polym. Test. 2020, 81, 106234. [Google Scholar] [CrossRef]
- Al-Shamari, A.; Abdelghany, A.; Alnattar, H.; Oraby, A. Structural and optical properties of PEO/CMC polymer blend modified with gold nanoparticles synthesized by laser ablation in water. J. Mater. Res. Technol. 2021, 12, 1597–1605. [Google Scholar] [CrossRef]
- Behdarvand, F.; Valamohammadi, E.; Tofighy, M.A.; Mohammadi, T. Polyvinyl alcohol/polyethersulfone thin-film nanocomposite membranes with carbon nanomaterials incorporated in substrate for water treatment. J. Environ. Chem. Eng. 2021, 9, 104650. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Raksa, A.; Numpaisal, P.-o.; Ruksakulpiwat, Y. The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Mater. Today Proc. 2021, 47, 3458–3461. [Google Scholar] [CrossRef]
- Unal, B.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. An electrospun nanofiber matrix based on organo-clay for biosensors: PVA/PAMAM-Montmorillonite. Appl. Surf. Sci. 2018, 444, 542–551. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Ullah, S.; Waqas, M.; Abbasi, A.M.R.; Saito, Y.; Zhu, C.; Kim, I.S. Self-cleaning properties of electrospun PVA/TiO2 and PVA/ZnO nanofibers composites. Nanomaterials 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, M.; Ullah, S.; Ullah, A.; Saito, Y.; Haider, M.K.; Bie, X.; Wada, K.; Kim, I.S. Carboxymethyl Cellulose (CMC) Based Electrospun Composite Nanofiber Mats for Food Packaging. Polym. 2021, 13, 302. [Google Scholar] [CrossRef]
- Duran-Guerrero, J.G.; Martinez-Rodriguez, M.A.; Garza-Navarro, M.A.; Gonzalez-Gonzalez, V.A.; Torres-Castro, A.; De La Rosa, J.R. Magnetic nanofibrous materials based on CMC/PVA polymeric blends. Carbohydr. Polym. 2018, 200, 289–296. [Google Scholar] [CrossRef]
- Angel, N.; Guo, L.; Yan, F.; Wang, H.; Kong, L. Effect of processing parameters on the electrospinning of cellulose acetate studied by response surface methodology. J. Agric. Food Res. 2020, 2, 100015. [Google Scholar] [CrossRef]
- Bakar, S.S.S.; Fong, K.C.; Eleyas, A.; Nazeri, M.F.M. Effect of Voltage and Flow Rate Electrospinning Parameters on Polyacrylonitrile Electrospun Fibers. IOP Conf. Ser.Mater. Sci. Eng. 2018, 318, 012076. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, D.; Soomro, R.A.; Fu, F.; Qiao, N.; Yu, Y.; Wang, R.; Xu, B. Ceramic supported attapulgite-graphene oxide composite membrane for efficient removal of heavy metal contamination. J. Membr. Sci. 2019, 591, 117323. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Gouda, M.H.; Elnouby, M.; Ali, S.M.; Salerno, M.; Youssef, M.E. Sustainable Microbial and Heavy Metal Reduction in Water Purification Systems Based on PVA/IC Nanofiber Membrane Doped with PANI/GO. Polymers 2022, 14, 1558. [Google Scholar] [CrossRef]
- Hezarjaribi, M.; Bakeri, G.; Sillanpaa, M.; Chaichi, M.J.; Akbari, S.; Rahimpour, A. Novel adsorptive PVC nanofibrous/thiol-functionalized TNT composite UF membranes for effective dynamic removal of heavy metal ions. J. Environ. Manag. 2021, 284, 111996. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Li, H.; Liu, X.; Du, Z.; Yu, W. Electrospun Polyurethane/Zeolitic Imidazolate Framework Nanofibrous Membrane with Superior Stability for Filtering Performance. ACS Appl. Polym. Mater. 2020, 3, 710–719. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, P.; Xu, D.; Zheng, J.; Zhan, Z.-M.; Gao, Q.; Yuan, S.; Xu, Z.-L.; Van der Bruggen, B. Triethanolamine modification produces ultra-permeable nanofiltration membrane with enhanced removal efficiency of heavy metal ions. J. Membr. Sci. 2022, 644, 120127. [Google Scholar] [CrossRef]
- Abdulkarem, E.; Ibrahim, Y.; Kumar, M.; Arafat, H.A.; Naddeo, V.; Banat, F.; Hasan, S.W. Polyvinylidene fluoride (PVDF)-α-zirconium phosphate (α-ZrP) nanoparticles based mixed matrix membranes for removal of heavy metal ions. Chemosphere 2021, 267, 128896. [Google Scholar] [CrossRef]
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2021, 406, 124746. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. High–performance nanofiltration membrane blended by Fe3O4@SiO2–CS bionanocomposite for efficient simultaneous rejection of salts/heavy metals ions/dyes with high permeability, retention increase and fouling decline. Chem. Eng. J. 2021, 417, 127930. [Google Scholar] [CrossRef]






| Nanofibrous Membrane | |||||||
|---|---|---|---|---|---|---|---|
| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
| qexp | qe | k1 | R2 | qe | k2 (×10−2) | R2 | |
| Cu2+ | 15.13 | 17.81 | 0.5486 | 0.9869 | 18.80 | 1.10 | 0.9961 |
| Cr6+ | 16.87 | 19.68 | 0.5991 | 0.9875 | 18.53 | 1.18 | 0.9967 |
| Nanofibrous Membrane | ||||||
|---|---|---|---|---|---|---|
| Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
| qmax | KL | R2 | n | KF | R2 | |
| Cu2+ | 26.34 | 0.4364 | 0.9994 | 4.06 | 7.965 | 0.9828 |
| Cr6+ | 28.93 | 0.4381 | 0.9989 | 4.26 | 8.364 | 0.9864 |
| Membrane Type | Heavy Metal Ion | Rejection Rate | Reference |
|---|---|---|---|
| GO/Attapulgite/Al2O3 | Cu2+ | 99.9% | [43] |
| PVA/IC/PANI/GO | Pb2+, Cd2+ | 97.19%, 91.4% | [44] |
| PVC/TNT | Cu2+, Ni2+ | 90%, 86.7% | [45] |
| PU/ZIF | Cr6+ | 85% | [46] |
| PEI/TMC | Zn2+, Cd2+, Ni2+, Cu2+ | 97% | [47] |
| PVDF/α-ZrP | Cu2+, Pb2+ | 93.1%, 91.2% | [48] |
| CA/P(MA-co-AA)/PEI | Cu2+ | 97.4% | [49] |
| CS/Fe3O4@SiO2 | Cu2+, Pb2+ | 98.61%, 98.11% | [50] |
| CMC/PVA | Cu2+, Cr2+ | 97.2%, 98.8% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Cai, J.; Yang, Y.; Xu, C.; Lu, J.; Wu, S. Electrospun Carboxymethyl Cellulose/Polyvinyl Alcohol Nanofiber Membranes for Enhanced Metal Ion Removal. Sustainability 2023, 15, 11331. https://doi.org/10.3390/su151411331
Shi W, Cai J, Yang Y, Xu C, Lu J, Wu S. Electrospun Carboxymethyl Cellulose/Polyvinyl Alcohol Nanofiber Membranes for Enhanced Metal Ion Removal. Sustainability. 2023; 15(14):11331. https://doi.org/10.3390/su151411331
Chicago/Turabian StyleShi, Weijian, Jiawei Cai, Yuan Yang, Chao Xu, Jianwei Lu, and Shuping Wu. 2023. "Electrospun Carboxymethyl Cellulose/Polyvinyl Alcohol Nanofiber Membranes for Enhanced Metal Ion Removal" Sustainability 15, no. 14: 11331. https://doi.org/10.3390/su151411331





