Biodiesel Production Using a Banana Peel Extract-Mediated Highly Basic Heterogeneous Nanocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Preparation of Catalyst
2.3. Characterization of Catalyst
2.4. Transesterification of Methanol and Soybean Oil
2.5. Characterization of Product
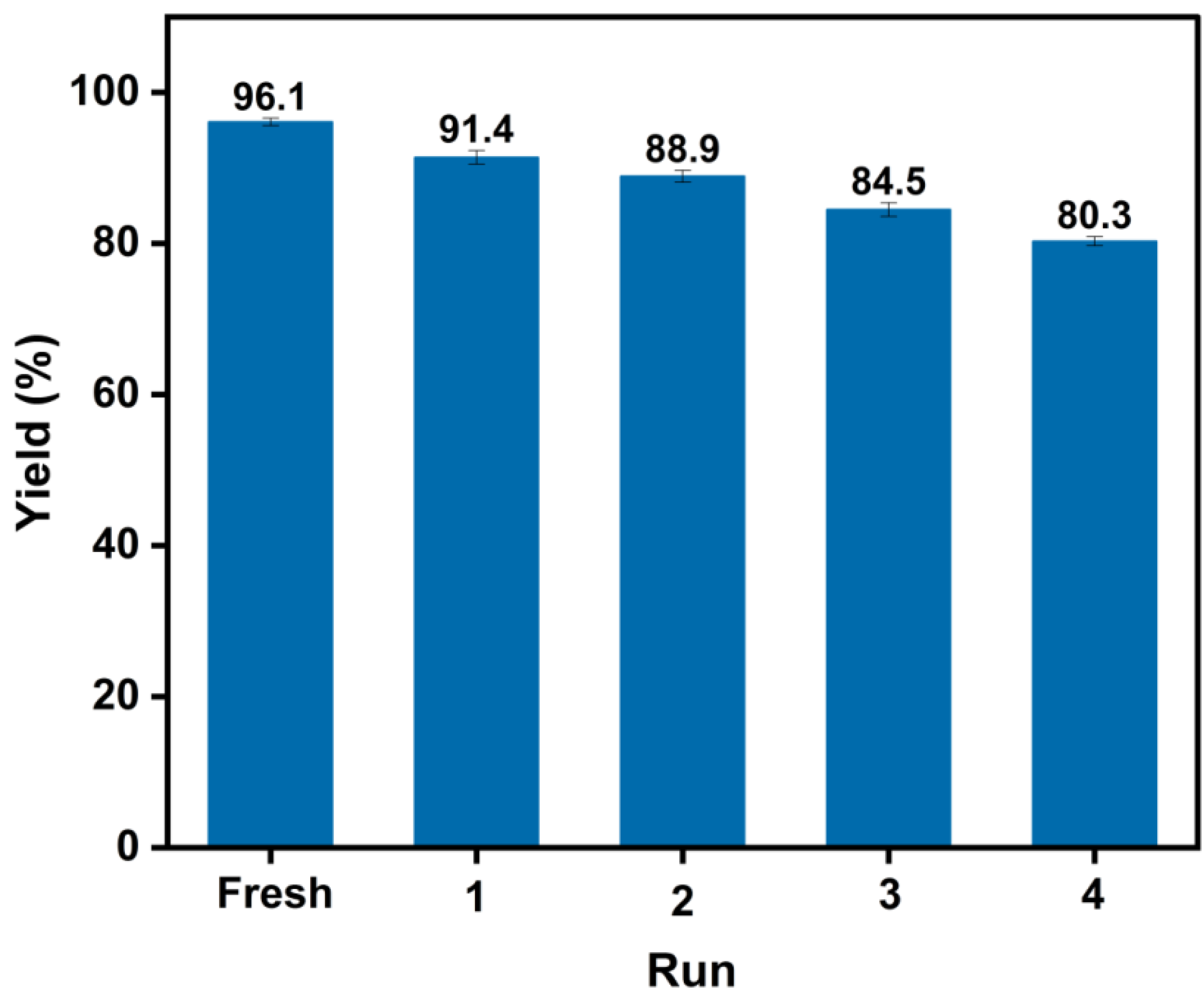
2.6. Catalyst Reusability
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. X-ray Diffraction Analysis
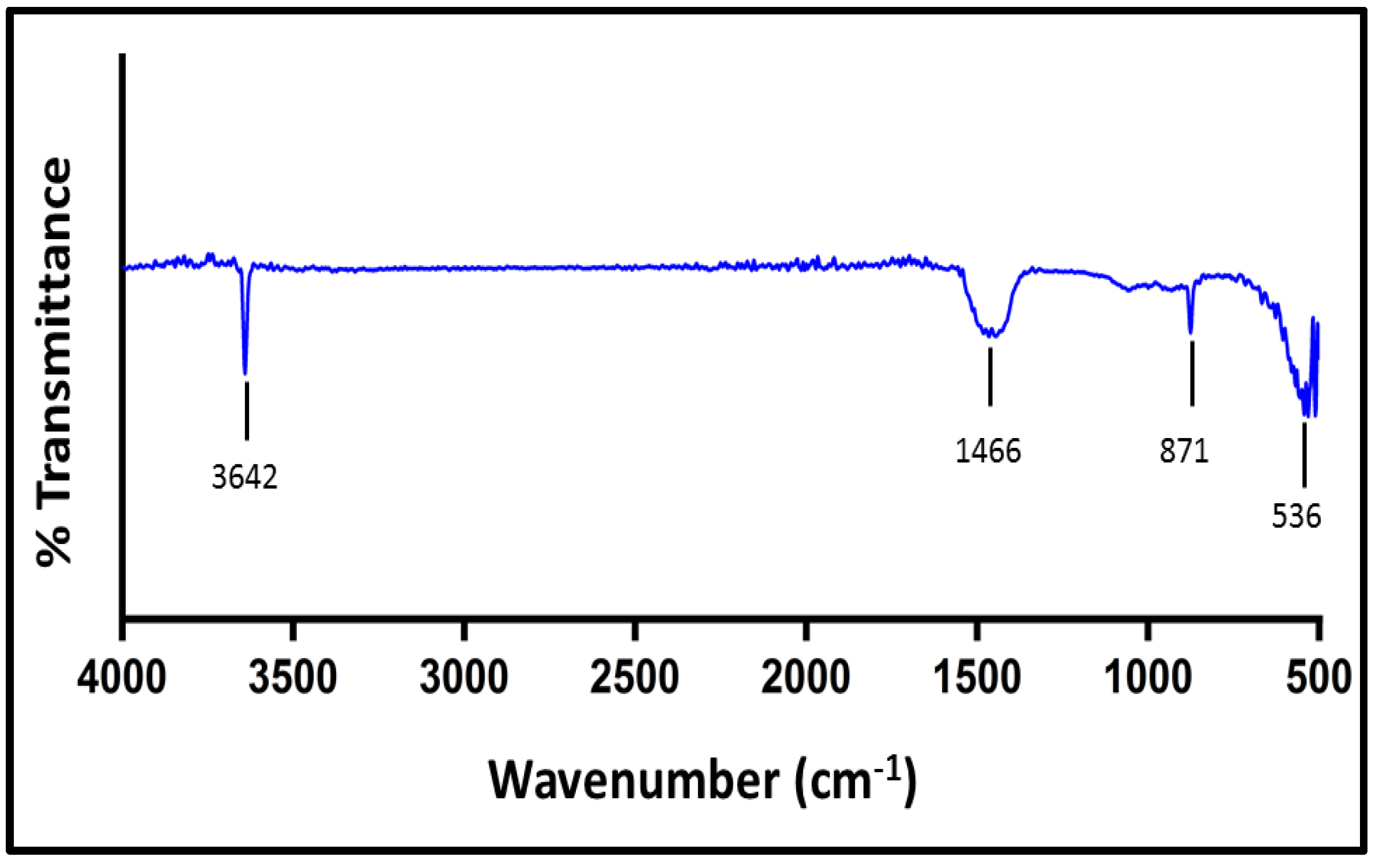
3.1.2. Fourier Transform Infrared Spectroscopy Analysis
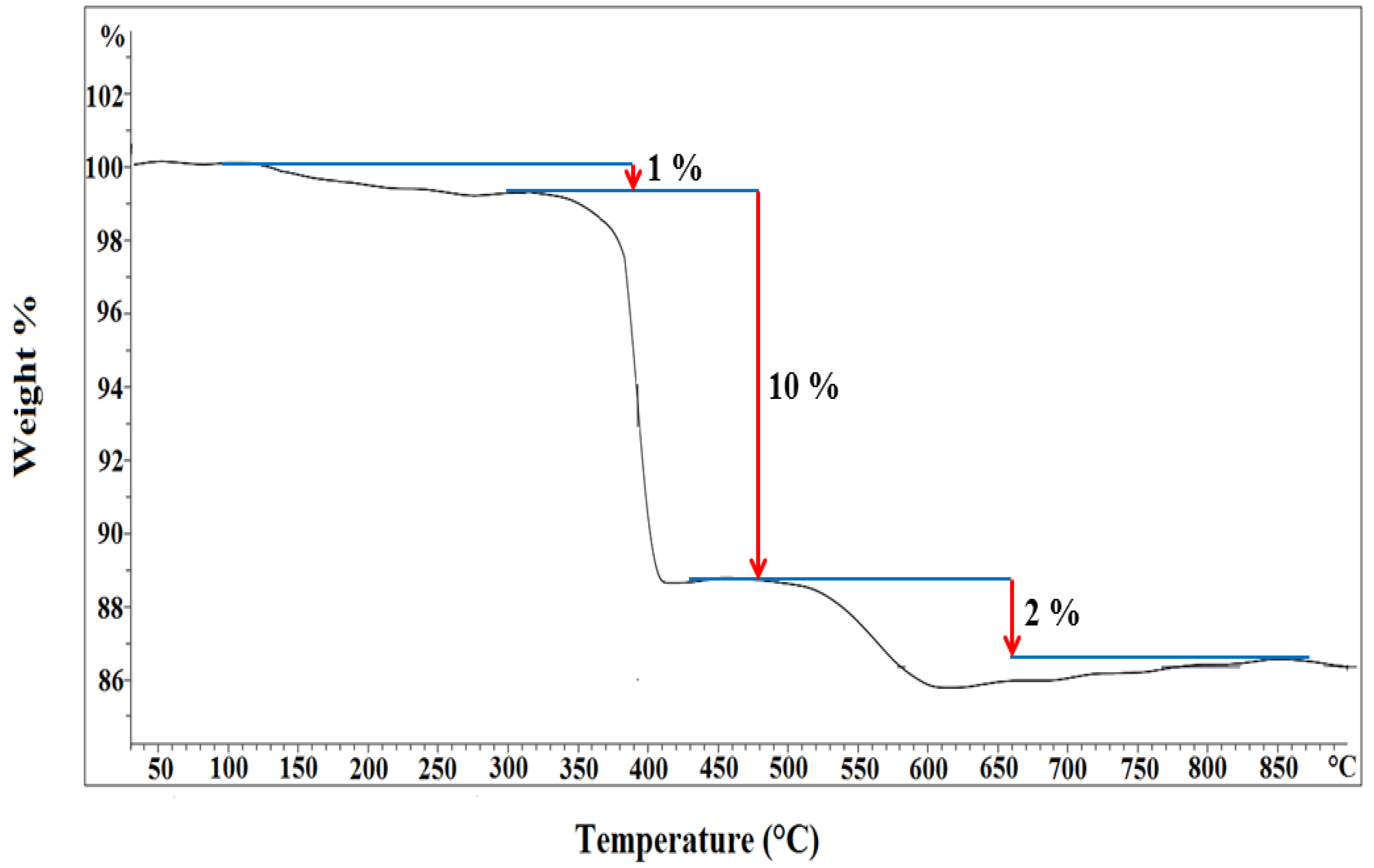
3.1.3. Thermogravimetric Analysis
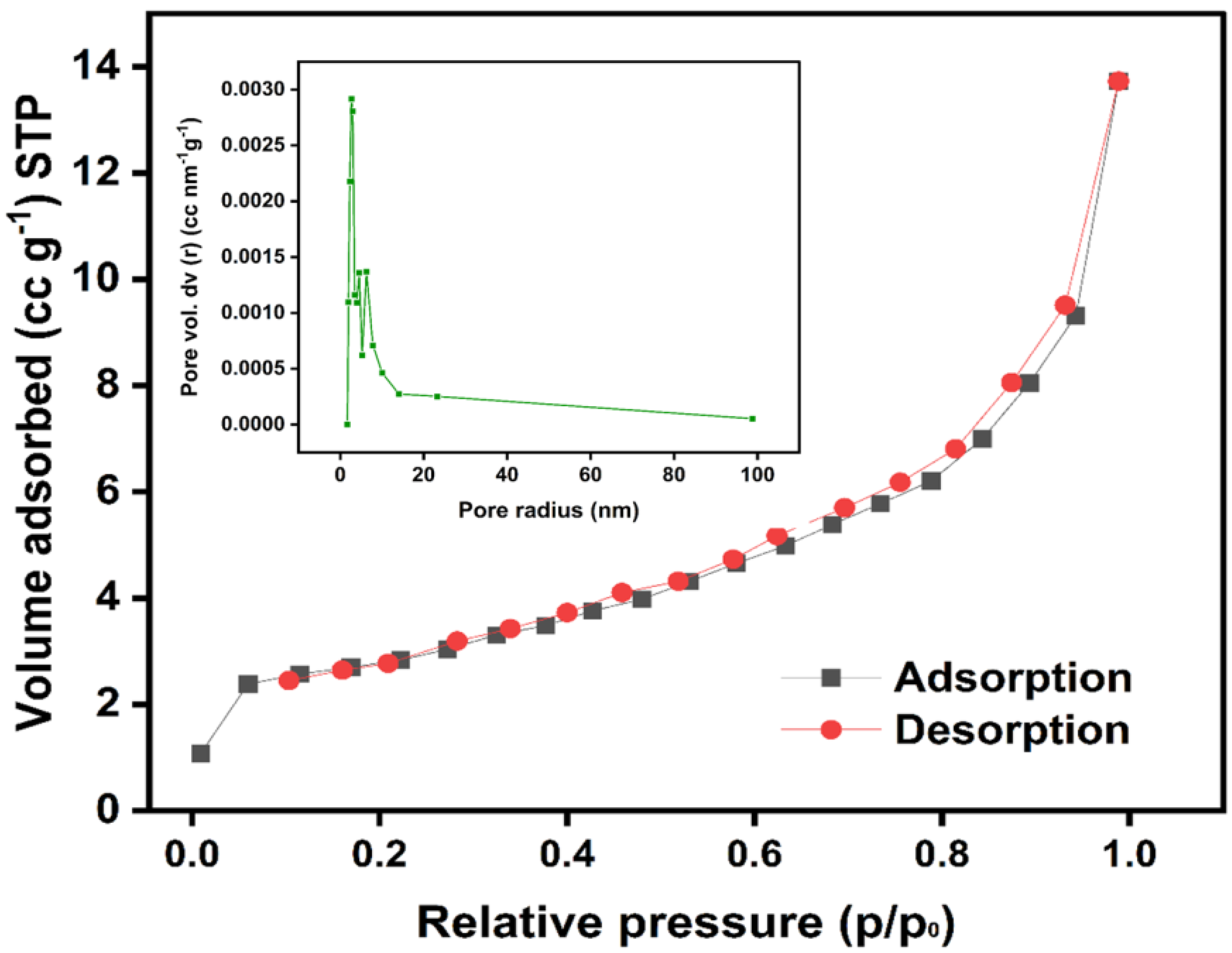
3.1.4. Brunauer–Emmett-Teller Analysis
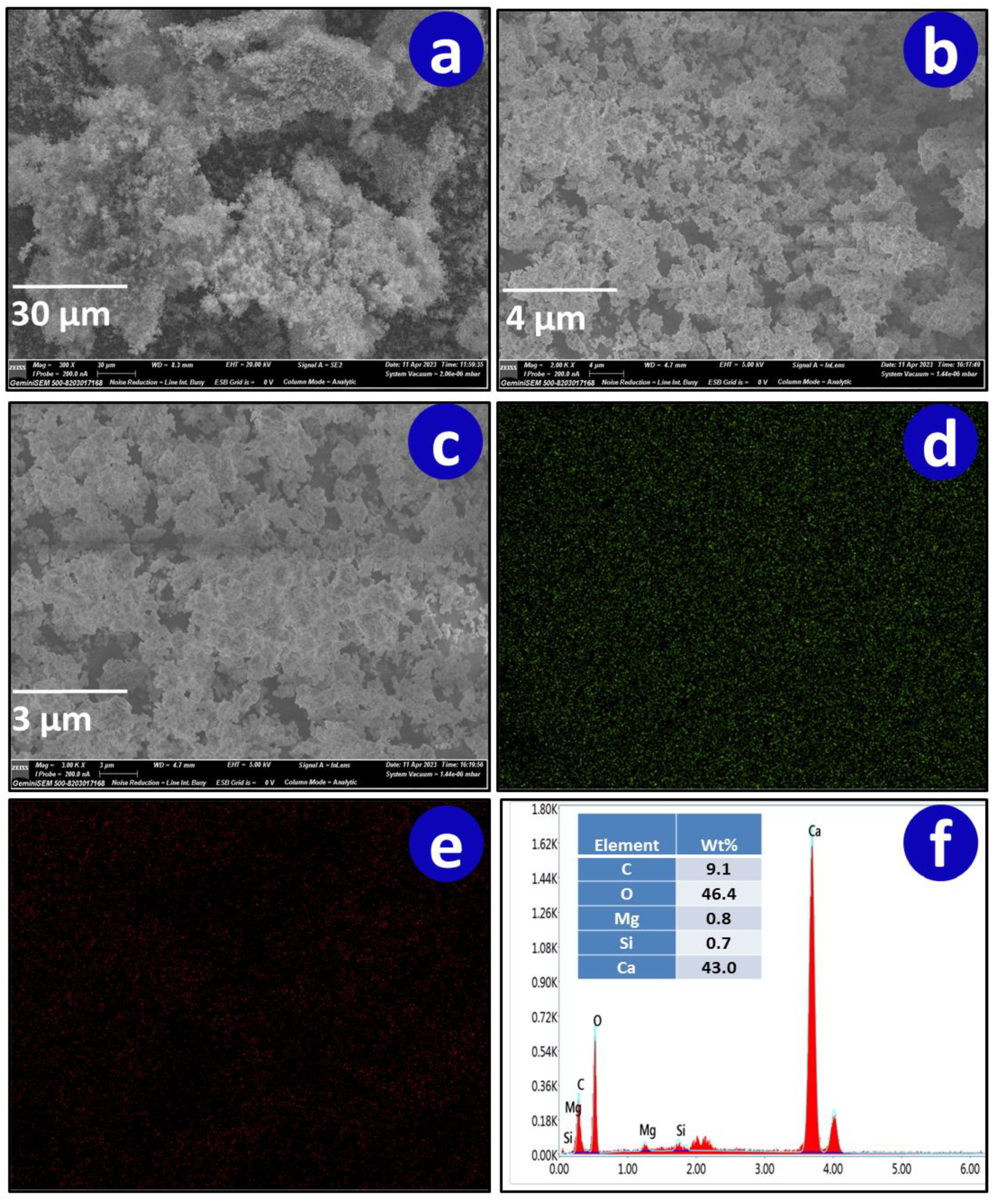
3.1.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
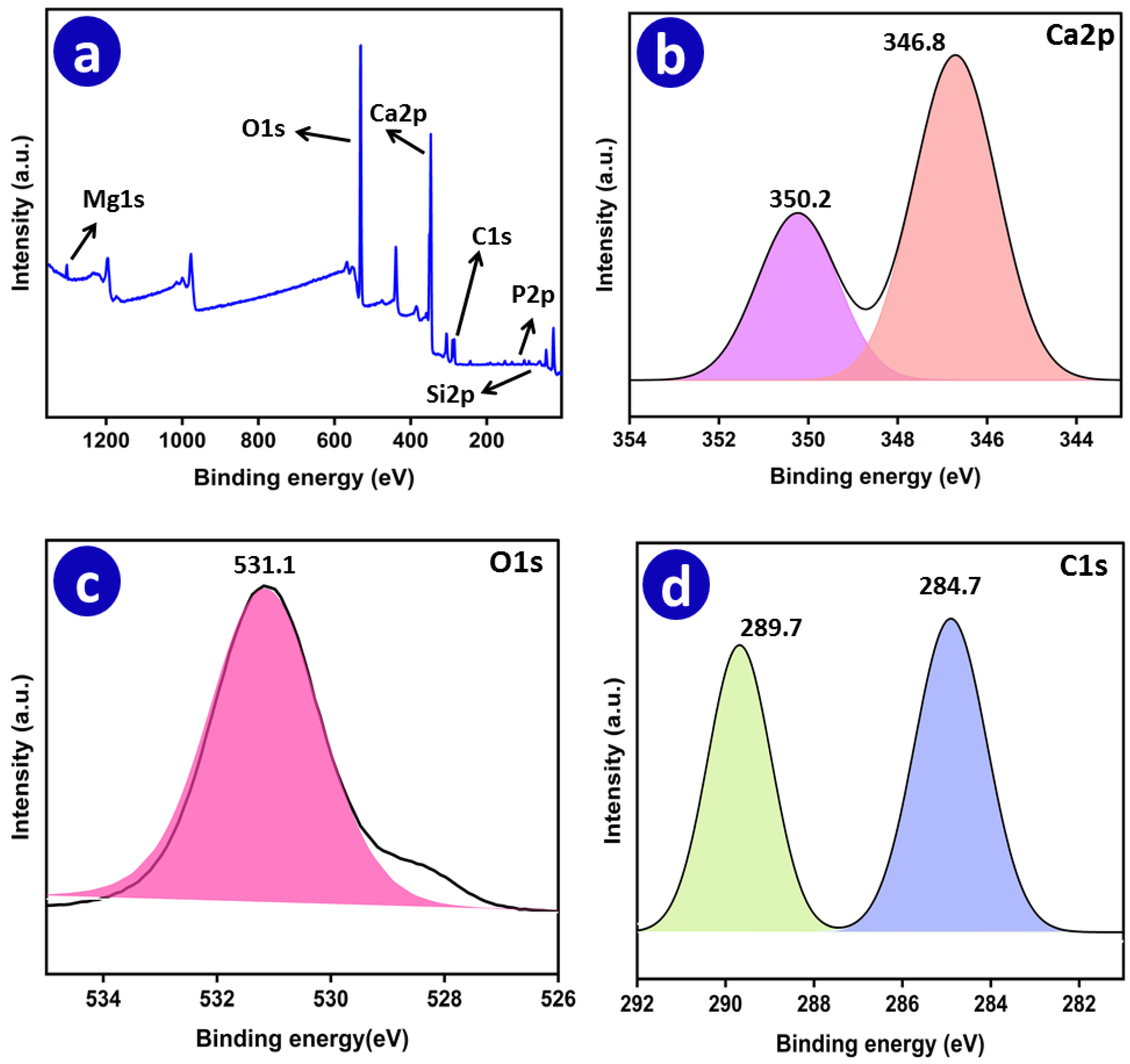
3.1.6. X-ray Photoelectron Spectroscopy
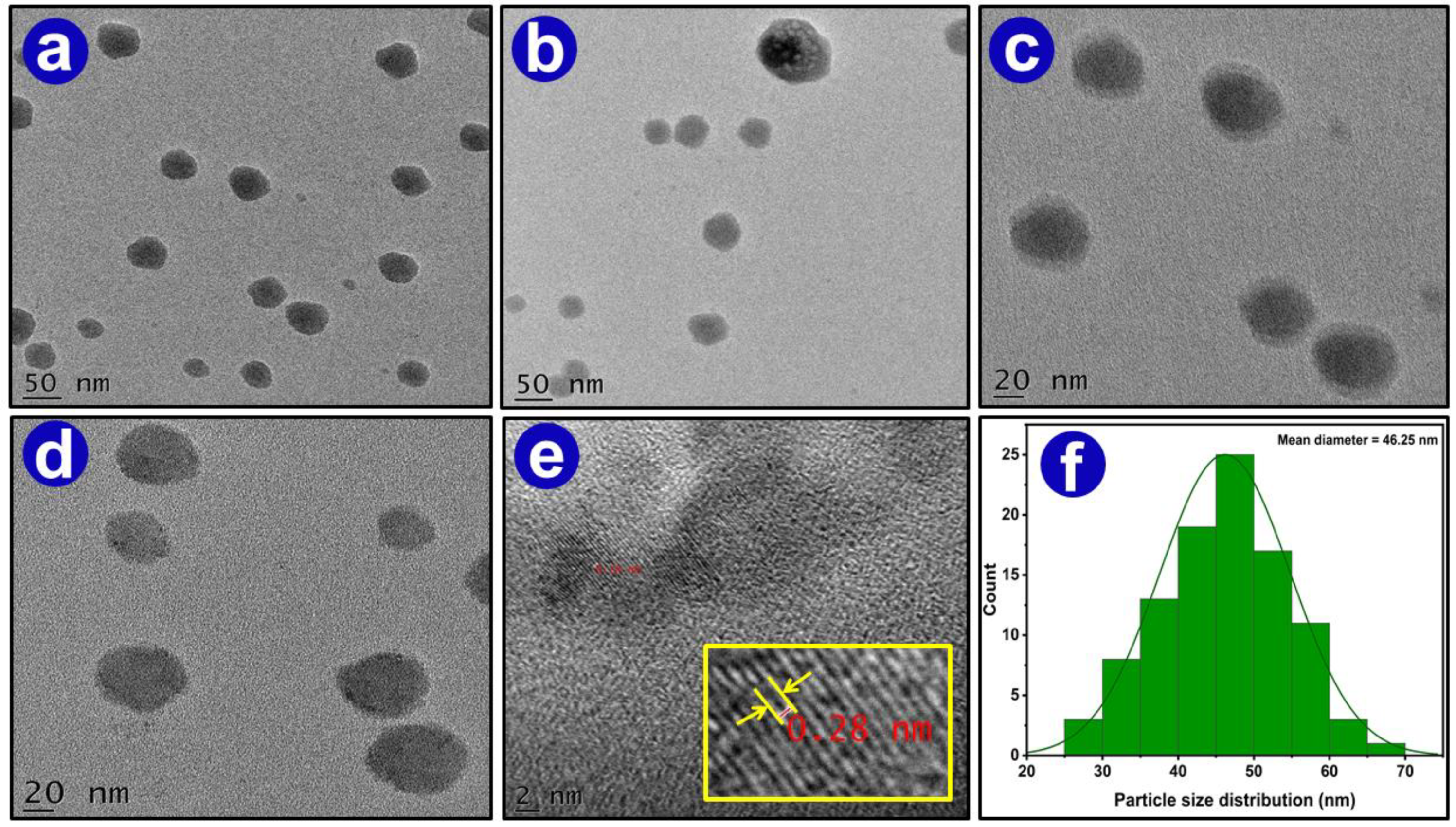
3.1.7. Transmission Electron Microscopy
3.2. Product Characterization
3.2.1. Fourier Transform Infrared Spectroscopy Analysis
3.2.2. Nuclear Magnetic Resonance Analysis
3.2.3. Gas Chromatography
3.2.4. Physicochemical and Fuel Properties
3.3. Optimization of the Transesterification Process
3.3.1. Catalyst Loading
3.3.2. Reaction Temperature
3.3.3. Methanol to Oil Molar Ratio
3.3.4. Reaction Time
3.4. Kinetics of Soybean Oil Esterification
3.5. Catalyst Heterogeneity Test
3.6. Catalyst Reusability Test
3.7. Comparison of CaO Nanocatalyst with Other Reported Heterogeneous Catalysts
4. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| NPs | Nanoparticles |
| CaO | Calcium oxide |
| Ca(OH)2 | Calcium hydroxide |
| Ca(NO3)2 | Calcium nitrate |
| 1H-NMR | Nuclear magnetic resonance |
| FT-IR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive X-ray spectroscopy |
| TEM | Transmission electron microscope |
| XPS | X-ray photoelectron spectroscopy |
| BET | Brunauer–Emmett–Teller |
| NaKTNT | Sodium titanate nanotubes doped with potassium |
| AIL-HPMo-MIL-100(Fe) | Acidic ionic liquid–phosphomolybdic acid–metal organic framework MIL–100(Fe) |
| TGA | Thermogravimetric analysis |
References
- Cong, W.J.; Nanda, S.; Li, H.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Metal-Organic Framework-Based Functional Catalytic Materials for Biodiesel Production: A Review. Green Chem. 2021, 23, 2595–2618. [Google Scholar] [CrossRef]
- Sahaym, U.; Norton, M.G. Advances in the Application of Nanotechnology in Enabling a “Hydrogen Economy”. J. Mater. Sci. 2008, 43, 5395–5429. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Li, H.; Kataki, R.; Agrawal, P.S.; Moyon, N.S.; Gurunathan, B.; Rokhum, S.L. Terminalia Arjuna Bark—A Highly Efficient Renewable Heterogeneous Base Catalyst for Biodiesel Production. Renew. Energy 2023, 212, 185–196. [Google Scholar] [CrossRef]
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Norhasyima, R.S. Comparison of Palm Oil, Jatropha Curcas and Calophyllum Inophyllum for Biodiesel: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G. Assessment of Sustainability Indicators for Biodiesel Production. Appl. Sci. 2017, 7, 869. [Google Scholar] [CrossRef] [Green Version]
- Borges, M.E.; Díaz, L. Recent Developments on Heterogeneous Catalysts for Biodiesel Production by Oil Esterification and Transesterification Reactions: A Review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste Snail Shell Derived Heterogeneous Catalyst for Biodiesel Production by the Transesterification of Soybean Oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef]
- Yacob, M.R.; Kabir, I.; Radam, A.; Abed, K.A.; El Morsi, A.K.; Sayed, M.M.; Shaib, A.A.E.; Gad, M.S.; Carlo, A.; Carolina, L.; et al. Catalysis in Biodiesel Production by Tranesterification Process-An Insight. Egypt. J. Pet. 1998, 9, 332–337. [Google Scholar]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Barekati-Goudarzi, M.; Boldor, D.; Nde, D.B. In-Situ Transesterification of Seeds of Invasive Chinese Tallow Trees (Triadica sebifera L.) in a Microwave Batch System (GREEN3) Using Hexane as Co-Solvent: Biodiesel Production and Process Optimization. Bioresour. Technol. 2016, 201, 97–104. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G.; Prasertdham, P. A Green Sulfonated Carbon-Based Catalyst Derived from Coffee Residue for Esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Hernández-Hipólito, P.; Juárez-Flores, N.; Martínez-Klimova, E.; Gómez-Cortés, A.; Bokhimi, X.; Escobar-Alarcón, L.; Klimova, T.E. Novel Heterogeneous Basic Catalysts for Biodiesel Production: Sodium Titanate Nanotubes Doped with Potassium. Catal. Today 2015, 250, 187–196. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, L. Exploiting Waste: Towards a Sustainable Production of Biodiesel Using: Musa Acuminata Peel Ash as a Heterogeneous Catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of Nano CaO–Based Catalysts in Biodiesel Synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, E. Catalytic Performance and Characterization of Cs–Ca/SiO2–TiO2 Nanocatalysts for Biodiesel Production. J. Mol. Catal. A Chem. 2015, 404–405, 131–138. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel Fuel Production with Solid Superacid Catalysis in Fixed Bed Reactor under Atmospheric Pressure. Catal. Commun. 2004, 5, 721–723. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of Soybean Oil to Biodiesel Using SrO as a Solid Base Catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, M.; Liu, Y.; Fan, M.; Jiang, P.; Dong, Y. Sr Doping Magnetic CaO Parcel Ferrite Improving Catalytic Activity on the Synthesis of Biodiesel by Transesterification. Fuel 2016, 186, 787–791. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of Heterogeneous Base Catalysts for Biodiesel Production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef]
- Shafiee, A.; Baneshi, M.; Varma, R.S.; Mostafavi, E.; Iravani, S. Biosynthesized Metallic Nanocatalysts in the Removal and Degradation of Pollutants. Mater. Lett. 2022, 326, 132911. [Google Scholar] [CrossRef]
- Al-mohaimeed, A.M.; Al-onazi, W.A.; El-tohamy, M.F. Multifunctional Eco-Friendly Synthesis of ZnO Nanoparticles. Molecules 2022, 27, 579. [Google Scholar] [CrossRef]
- Anand, K.V.; Mahalakshmi, D.; Selvan, S.M.; Ravi, M.; Kannan, M.; Govindaraju, K.; Shalan, A.E. Biomass Extract of Green Macroalga Halimeda Opuntia Assisted ZnO Nanoparticles: Preparation, Physico-Chemical Characterization, and Antibacterial Activity against Vibrio Harveyi. Biomass Convers. Biorefinery 2022, 1, 1–9. [Google Scholar] [CrossRef]
- El-Desouky, N.; Shoueir, K.; El-Mehasseb, I.; El-Kemary, M. Synthesis of Silver Nanoparticles Using Bio Valorization Coffee Waste Extract: Photocatalytic Flow-Rate Performance, Antibacterial Activity, and Electrochemical Investigation. Biomass Convers. Biorefinery 2022, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gemishev, O.; Panayotova, M.; Gicheva, G.; Mintcheva, N. Green Synthesis of Stable Spherical Monodisperse Silver Nanoparticles Using a Cell-Free Extract of Trichoderma Reesei. Materials 2022, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Ulaeto, S.B.; Mathew, G.M.; Pancrecious, J.K.; Nair, J.B.; Rajan, T.P.D.; Maiti, K.K.; Pai, B.C. Biogenic Ag Nanoparticles of Neem Extract; Its Structural Evaluation and Antimicrobial Effects against Pseudomonas Nitroreducens and Aspergillus Unguis. ACS Biomater. Sci. Eng. 2020, 6, 235–245. [Google Scholar] [CrossRef]
- Kattimani, V.R.; Yatish, K.V.; Pramoda, K.; Sakar, M.; Balakrishna, R.G. Acacia Furnesiana Plant as a Novel Green Source for the Synthesis of NiFe2O4 Magnetic Nanocatalyst and as Feedstock for Sustainable High Quality Biofuel Production. Fuel 2023, 348, 128549. [Google Scholar] [CrossRef]
- Maleki, B.; Esmaeili, H. Application of Fe3O4/SiO2@ZnO Magnetic Composites as a Recyclable Heterogeneous Nanocatalyst for Biodiesel Production from Waste Cooking Oil: Response Surface Methodology. Ceram. Int. 2022, 49, 11452–11463. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Li, C.; Chen, X.; Peng, W.; Aghbashlo, M.; Lam, S.S.; Tabatabaei, M. Managing the Hazardous Waste Cooking Oil by Conversion into Bioenergy through the Application of Waste-Derived Green Catalysts: A Review. J. Hazard. Mater. 2022, 424, 127636. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Ahmad, M.; Munir, M.; Sultana, S.; Zafar, M.; Dawood, S.; Rozina; Alghamdi, A.M.; Asif, S.; Bokhari, A.; et al. Assessing the Potential of Green CdO2 Nano-Catalyst for the Synthesis of Biodiesel Using Non-Edible Seed Oil of Malabar Ebony. Fuel 2023, 333, 126492. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Sivashanmugam, P. Biodiesel Production from a Novel Renewable Source through the Ultrasound-Assisted Transesterification Process, Using Energy Efficient Nanocatalyst Developed from Waste Material. Fuel 2023, 346, 128397. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, D.; Almojil, S.F.; Almohana, A.I.; Alali, A.F.; Zhou, Y.; Raise, A. CaO-MgFe2O4@K2CO3 as a Novel and Retrievable Nanocatalyst for Two-Step Transesterification of Used Frying Oils to Biodiesel. Process Saf. Environ. Prot. 2023, 172, 195–210. [Google Scholar] [CrossRef]
- Hanif, M.; Bhatti, I.A.; Shahzad, K.; Hanif, M.A. Biodiesel Production from Waste Plant Oil over a Novel Nano-Catalyst of Li-TiO2/Feldspar. Catalysts 2023, 13, 310. [Google Scholar] [CrossRef]
- Munir, M.; Saeed, M.; Ahmad, M.; Waseem, A.; Alsaady, M.; Asif, S.; Ahmed, A.; Shariq Khan, M.; Bokhari, A.; Mubashir, M.; et al. Cleaner Production of Biodiesel from Novel Non-Edible Seed Oil (Carthamus Lanatus L.) via Highly Reactive and Recyclable Green Nano CoWO3@rGO Composite in Context of Green Energy Adaptation. Fuel 2023, 332, 126265. [Google Scholar] [CrossRef]
- Alsaiari, M.; Ahmad, M.; Munir, M.; Zafar, M.; Sultana, S.; Dawood, S.; Almohana, A.I.; Hassan, M.H.A.M.; Alharbi, A.F.; Ahmad, Z. Efficient Application of Newly Synthesized Green Bi2O3 Nanoparticles for Sustainable Biodiesel Production via Membrane Reactor. Chemosphere 2023, 310, 136838. [Google Scholar] [CrossRef]
- Maleki, B.; Esmaeili, H.; Mansouri, M.; Kumar, D.; Singh, B. Enhanced Conversion of Dairy Waste Oil to Biodiesel via Novel and Highly Reactive UiO-66-NH2/ZnO/TiO2 Nano-Catalyst: Optimization, Kinetic, Thermodynamic and Diesel Engine Studies. Fuel 2023, 339, 126901. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Enhanced Low-Temperature Production of Biodiesel from Waste Cooking Oil: Aluminum Industrial Waste as a Precursor of Efficient CaO/Al2O3 Nano-Catalyst. Fuel 2023, 351, 128897. [Google Scholar] [CrossRef]
- Wang, J.; Xia, A.; Deng, Z.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Intensifying Biofuel Production Using a Novel Bionic Flow-Induced Peristaltic Reactor: Biodiesel Production as a Case Study. Biofuel Res. J. 2022, 9, 1721–1735. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of Calcium Oxide as a Heterogeneous Catalyst in Biodiesel Production: A Review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological Agents for Synthesis of Nanoparticles and Their Applications. J. King Saud Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, B.; Das, B.; Kalita, P.; Basumatary, B. Utilization of Renewable and Sustainable Basic Heterogeneous Catalyst from Heteropanax Fragrans (Kesseru) for Effective Synthesis of Biodiesel from Jatropha Curcas Oil. Fuel 2021, 286, 119357. [Google Scholar] [CrossRef]
- Bharti, P.; Singh, B.; Dey, R.K. Process Optimization of Biodiesel Production Catalyzed by CaO Nanocatalyst Using Response Surface Methodology. J. Nanostructure Chem. 2019, 9, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Bhattacharya, J. Microwave-Assisted Synthesis and Characterization of CaO Nanoparticles. Int. J. Nanosci. 2011, 10, 413–418. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg Shell Waste as Heterogeneous Nanocatalyst for Biodiesel Production: Optimized by Response Surface Methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Saikia, K.; Rajkumari, K.; Moyon, N.S.; Basumatary, S.; Halder, G.; Rashid, U.; Lalthazuala, S. Sulphonated Biomass-Based Catalyst for Solketal Synthesis by Acetalization of Glycerol—A Byproduct of Biodiesel Production. Fuel Process. Technol. 2022, 238, 107482. [Google Scholar] [CrossRef]
- Liu, D.J.; Garcia, A.; Wang, J.; Ackerman, D.M.; Wang, C.J.; Evans, J.W. Kinetic Monte Carlo Simulation of Statistical Mechanical Models and Coarse-Grained Mesoscale Descriptions of Catalytic Reaction-Diffusion Processes: 1D Nanoporous and 2D Surface Systems. Chem. Rev. 2015, 115, 5979–6050. [Google Scholar] [CrossRef] [Green Version]
- Daimary, N.; Boruah, P.; Eldiehy, K.S.H.; Pegu, T.; Bardhan, P.; Bora, U.; Mandal, M.; Deka, D. Musa Acuminata Peel: A Bioresource for Bio-Oil and by-Product Utilization as a Sustainable Source of Renewable Green Catalyst for Biodiesel Production. Renew. Energy 2022, 187, 450–462. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, H.; Hu, R. The Activity and Stability of CeO2@CaO Catalysts for the Production of Biodiesel. RSC Adv. 2018, 8, 32922–32929. [Google Scholar] [CrossRef] [PubMed]
- Dhankhar, S.; Bhalerao, G.; Ganesamoorthy, S.; Baskar, K.; Singh, S. Growth and Comparison of Single Crystals and Polycrystalline Brownmillerite Ca2Fe2O5. J. Cryst. Growth 2017, 468, 311–315. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Lv, L.P.; Kong, S.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal–Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef]
- Zeng, T.; Yu, M.; Zhang, H.; He, Z.; Chen, J.; Song, S. Fe/Fe3C@N-Doped Porous Carbon Hybrids Derived from Nano-Scale MOFs: Robust and Enhanced Heterogeneous Catalyst for Peroxymonosulfate Activation. Catal. Sci. Technol. 2017, 7, 396–404. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A novel α-Fe2O3@ g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Lu, D.; Hu, S.; Han, H. Preparation of KF/CaO Nanocatalyst and Its Application in Biodiesel Production from Chinese Tallow Seed Oil. Fuel 2010, 89, 2267–2271. [Google Scholar] [CrossRef]
- Shancita, I.; Masjuki, H.H.; Kalam, M.A.; Reham, S.S.; Shahir, S.A. Comparative Analysis on Property Improvement Using Fourier Transform Infrared Spectroscopy (FT-IR) and Nuclear Magnetic Resonance (NMR) (1H and 13C) Spectra of Various Biodiesel Blended Fuels. Energy Fuels 2016, 30, 4790–4805. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Ahmad, F.; Ahmad, M.; Zafar, M.; Khalid, N.; Khan, M.A. Identification, FT-IR, NMR (1H and 13C) and GC/MS Studies of Fatty Acid Methyl Esters in Biodiesel from Rocket Seed Oil. Fuel Process. Technol. 2011, 92, 336–341. [Google Scholar] [CrossRef]
- Doudin, K.I. Quantitative and Qualitative Analysis of Biodiesel by NMR Spectroscopic Methods. Fuel 2021, 284, 119114. [Google Scholar] [CrossRef]
- Mahmoud, H.R. Bismuth Silicate (Bi4Si3O12 and Bi2SiO5) Prepared by Ultrasonic-Assisted Hydrothermal Method as Novel Catalysts for Biodiesel Production via Oleic Acid Esterification with Methanol. Fuel 2019, 256, 115979. [Google Scholar] [CrossRef]
- Saikia, K.; Ngaosuwan, K.; Assabumrungrat, S.; Singh, B.; Okoye, P.U.; Rashid, U.; Rokhum, S.L. Sulphonated Cellulose-Based Carbon as a Green Heterogeneous Catalyst for Biodiesel Production: Process Optimization and Kinetic Studies. Biomass Bioenergy 2023, 173, 106799. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of Transesterification Methods for Production of Biodiesel from Vegetable Oils and Fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Fan, M.; Jiang, P. Catalytic Performance of a Novel Amphiphilic Alkaline Ionic Liquid for Biodiesel Production: Influence of Basicity and Conductivity. Renew. Energy 2016, 86, 99–105. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Transesterification of Low-Quality Triglycerides over a Zn/CaO Heterogeneous Catalyst: Kinetics and Reusability Studies. Energy Fuels 2013, 27, 3758–3768. [Google Scholar] [CrossRef]
- Betiku, E.; Ajala, S.O. Modeling and Optimization of Thevetia Peruviana (Yellow Oleander) Oil Biodiesel Synthesis via Musa Paradisiacal (Plantain) Peels as Heterogeneous Base Catalyst: A Case of Artificial Neural Network vs. Response Surface Methodology. Ind. Crops Prod. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.H. Sustainable Biodiesel Production via Transesterification of Waste Cooking Oil by Using CaO Catalysts Prepared from Chicken Manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Girish, N.; Niju, S.P.; Begum, K.M.M.S.; Anantharaman, N. Utilization of a Cost Effective Solid Catalyst Derived from Natural White Bivalve Clam Shell for Transesterification of Waste Frying Oil. Fuel 2013, 111, 653–658. [Google Scholar] [CrossRef]
- Syazwani, O.N.; Teo, S.H.; Islam, A.; Taufiq-Yap, Y.H. Transesterification activity and characterization of natural CaO derived from waste venus clam (Tapes belcheri S.) material for enhancement of biodiesel production. Process Saf. Environ. Prot. 2017, 105, 303–315. [Google Scholar] [CrossRef]
- Yahya, N.Y.; Ngadi, N.; Wong, S.; Hassan, O. Transesterification of Used Cooking Oil (UCO) Catalyzed by Mesoporous Calcium Titanate: Kinetic and Thermodynamic Studies. Energy Convers. Manag. 2018, 164, 210–218. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to Value Addition: Utilization of Waste Brassica Nigra Plant Derived Novel Green Heterogeneous Base Catalyst for Effective Synthesis of Biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Churipard, S.R.; Manjunathan, P.; Chandra, P.; Shanbhag, G.V.; Ravishankar, R.; Rao, P.V.; Maradur, S.P. Remarkable Catalytic Activity of a Sulfonated Mesoporous Polymer (MP-SO3H) for the Synthesis of Solketal at Room Temperature. New J. Chem. 2017, 41, 5745–5751. [Google Scholar] [CrossRef]
- Ao, S.; Alghamdi, L.A.; Kress, T.; Selvaraj, M.; Halder, G.; Wheatley, A.E.H.; Lalthazuala, S. Microwave-Assisted Valorization of Glycerol to Solketal Using Biomass-Derived Heterogeneous Catalyst. Fuel 2023, 345, 128190. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-Efficient Conversion of Glycerol to Solketal over Heterogeneous Lewis Acid Catalysts. Green Chem. 2012, 14, 1611. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Quintinite as a Bifunctional Heterogeneous Catalyst for Biodiesel Synthesis. Appl. Catal. A Gen. 2011, 393, 36–43. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Synthesis of biodiesel from vegetable oil with methanol catalyzed by Li-doped magnesium oxide catalysts. Appl. Energy 2010, 87, 743–748. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Guanidine Post-Functionalized Crystalline ZIF-90 Frameworks as a Promising Recyclable Catalyst for the Production of Biodiesel via Soybean Oil Transesterification. Energy Convers. Manag. 2019, 198, 111922. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Yahya, N.Y.; Rahman, R.A. Synthesis, Characterization and Performance of Silica Impregnated Calcium Oxide as Heterogeneous Catalyst in Biodiesel Production. J. Clean. Prod. 2017, 146, 116–124. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Biodiesel Production from Acidic Oils Using Polyoxometalate-Based Sulfonated Ionic Liquids Functionalized Metal–Organic Frameworks. Catal. Lett. 2019, 149, 2916–2929. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Fihri, A.; Zahouily, M. Sodium Modified Fluorapatite as a Sustainable Solid Bi-Functional Catalyst for Biodiesel Production from Rapeseed Oil. Renew. Energy 2019, 133, 1295–1307. [Google Scholar] [CrossRef]


| Nanocatalyst | Size (nm) | Biodiesel Feedstocks | BD Yield (%) | Reaction Conditions f | Reusability Cycles | BD Yield with Reused Catalyst (%) | Activation Energy (kJ mol−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| NiFe2O4 (magnetic) | ~15.5 a | Acacia furnesiana | 93.1 | 14:1, 4.61, 65, 53 | 4 | 80 | ~44.5 | [26] |
| Fe3O4/SiO2@ZnO (magnetic) | 10.4 b | WCO | 97.2 | 9.90:1, 2.67, -, 30.1 | 7 | 88.4 | - | [27] |
| CaO/Al2O3 | - | WCO | 95 d | 7:1, 3, 45, 180 | 5 | 62.9d | 39 | [36] |
| CdO2 | 45 a | Diospyros malabarica | 94 | 9:1, 0.5, 90, 180 | 6 | ˂60 | - | [29] |
| CaO | 10 a | RM-SML | 98.3 d | 8:1 g, 3.5, 200W i, 90 | 7 | 66.3 d | [30] | |
| CaO-MgFe2O4 @K2CO3 | ˂100 c 12.0 b | UFO | 96.5 | 18:1, 4, 70, 300 | 4 | >90 | 64.6 | [31] |
| Li-TiO2/feldspar | 41.8 a | Karanja oil | 98.4 | 10:1, 2, 50, 120 | - | - | - | [32] |
| CoWO3@rGO | 23.4 a | CSO | 99.7 | 12:1, 0.8, 65, 120 | 9 | 93.5 | 178 | [33] |
| B2O3 | 43 b | Cannabis sativa | 92 | 12:1, 1.5, 92, 210 | 9 | 33.78 | 0.574 | [34] |
| UiO-66-NH2/ZnO/TiO2 | 28.3 c | DWSO | 98.7 d | 9.8:1, 2, 61, 30 | 7 | 90.14 d | 48.7 | [35] |
| Peak No | Retention Time (min) | Free Fatty Acid Composition | Composition (%) | Corresponding Acids |
|---|---|---|---|---|
| 1 | 12.605 | Hexadecanoic acid, methyl ester | 13.6 | C16:0 |
| 2 | 13.745 | 6,9-octadecadienoic acid, methyl ester | 36.2 | C18:2 |
| 3 | 13.775 | 9-octadecenoic acid, methyl ester, (E)- | 37.7 | C18:1 |
| 4 | 13.860 | 9,12,15-octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 1.01 | C18:3 |
| 5 | 13.905 | Stearic acid, methyl ester | 7.18 | C18:0 |
| 6 | 14.965 | Cis-11-eicosenoic acid, methyl ester | 1.26 | C20:1 |
| 7 | 15.090 | 18-methylnonadecanoic acid, methyl ester | 1.60 | C20:0 |
| 8 | 16.190 | Docosanoic acid, methyl ester | 1.37 | C22:0 |
| Property | ASTM Limits | Present Biodiesel | Testing Methods |
|---|---|---|---|
| Acid value (mg KOH/g) | <0.79 | 0.32 | D 664 |
| Density (g cm−3) | 0.86–0.90 | 0.885 | D 1448–1972 |
| Flash point (°C) | ≥130 | 147 | D 93 |
| Pour point (°C) | −15 to +6 | 3.9 | D 97 |
| Cloud point (°C) | −3 to + 12 | 3 | D 2500 |
| Water content (wt%) | - | 0.03 | - |
| Heat value (MJ L−1) | 30–40 | 34 | D 6571 |
| Cetane number | ≥47 | 77 | D 6890 |
| Kinematic viscosity (mm2 s−1)@ 40 °C | 1.9–6.0 | 2.87 | D 445 |
| Sulphur content (wt%) | 0.005 | 0.001 | D 6751 |
| Catalyst Loading (wt%) | Temperature (°C) | Methanol to Oil Ratio | Time (h) | Yield (%) |
|---|---|---|---|---|
| 4 | 75 | 18:1 | 2.0 | 59.4 |
| 6 | 75 | 18:1 | 2.0 | 70.5 |
| 8 | 75 | 18:1 | 2.0 | 92.1 |
| 10 | 75 | 18:1 | 2.0 | 91.6 |
| 12 | 75 | 18:1 | 2.0 | 90.2 |
| 8 | 45 | 18:1 | 2.0 | 54.3 |
| 8 | 55 | 18:1 | 2.0 | 68.5 |
| 8 | 65 | 18:1 | 2.0 | 94.9 |
| 8 | 75 | 18:1 | 2.0 | 94.1 |
| 8 | 85 | 18:1 | 2.0 | 93.2 |
| 8 | 65 | 6:1 | 2.0 | 55.2 |
| 8 | 65 | 9:1 | 2.0 | 78.4 |
| 8 | 65 | 12:1 | 2.0 | 84.3 |
| 8 | 65 | 15:1 | 2.0 | 95.4 |
| 8 | 65 | 18:1 | 2.0 | 94.4 |
| 8 | 65 | 15:1 | 0.5 | 51.6 |
| 8 | 65 | 15:1 | 1.0 | 64.6 |
| 8 | 65 | 15:1 | 1.5 | 79.9 |
| 8 | 65 | 15:1 | 2.0 | 96.1 |
| 8 | 65 | 15:1 | 2.5 | 95.1 |
| Sl No | Catalyst | Precursor | Conditions a | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1. | NaKTNT | Soybean | 20:1, 1, 80, 1 | 96.2 | [12] |
| 2. | Waste snail shell | Soybean | 6:1, 3, 900, 7 | 98.0 | [7] |
| 3. | K2O/NaX and Na2O/NaX | Safflower | 18:1, 12.5, 61, 7 | 98.0 | [7] |
| 4. | Quintite | Waste vegetable | 12:1, 10, 75, 2 | 96.0 | [72] |
| 5. | Li-doped MgO catalyst | Vegetable oil | 12:1, 9, 65, 2 | 93.9 | [73] |
| 6. | Metal-oxide catalysts (CaTiO3,CaMnO3, Ca2Fe2O5, CaZrO3) | Rapeseed | 6:1, - , 60, 10 | 90.0 | [19] |
| 7. | ZIF-90-Gua | Soybean | 15:1, 1, 65, 6 | 95.4 | [74] |
| 8. | Silica-impregnated Cao | Palm | 20;1, 3, 60, 2 | 90.0 | [75] |
| 9. | Na-modified fluorapatite (Na/FAP) | Rapeseed | 10:1, 6, 120, 8 | 98.0 | [77] |
| 10. | AIL-HPMo-MIL-100(Fe) | Soybean | 30:1, 9, 120, 8 | 92.3 | [76] |
| 11. | CaO | Soybean | 15:1, 8, 65, 2.0 | 96.1 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satapathy, A.; Saikia, K.; Rokhum, S.L. Biodiesel Production Using a Banana Peel Extract-Mediated Highly Basic Heterogeneous Nanocatalyst. Sustainability 2023, 15, 11332. https://doi.org/10.3390/su151411332
Satapathy A, Saikia K, Rokhum SL. Biodiesel Production Using a Banana Peel Extract-Mediated Highly Basic Heterogeneous Nanocatalyst. Sustainability. 2023; 15(14):11332. https://doi.org/10.3390/su151411332
Chicago/Turabian StyleSatapathy, Ananya, Kankana Saikia, and Samuel Lalthazuala Rokhum. 2023. "Biodiesel Production Using a Banana Peel Extract-Mediated Highly Basic Heterogeneous Nanocatalyst" Sustainability 15, no. 14: 11332. https://doi.org/10.3390/su151411332
APA StyleSatapathy, A., Saikia, K., & Rokhum, S. L. (2023). Biodiesel Production Using a Banana Peel Extract-Mediated Highly Basic Heterogeneous Nanocatalyst. Sustainability, 15(14), 11332. https://doi.org/10.3390/su151411332





