Ball Mill, Humic Acid, and Rock Phosphate-Modified Conocarpus Biochar for Efficient Removal of Heavy Metals from Contaminated Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production and Modification
2.2. Ball Milling
2.3. Biochar Characterization
2.4. Adsorption Experiment
2.4.1. Adsorption Isotherm
2.4.2. Kinetics Study
2.5. Statistics
3. Results and Discussion
3.1. Biochar Characterization
3.2. Adsorption Experiments
3.2.1. Equilibrium Adsorption
Cadmium (Cd)
Copper (Cu)
Zinc (Zn)
Lead (Pb)
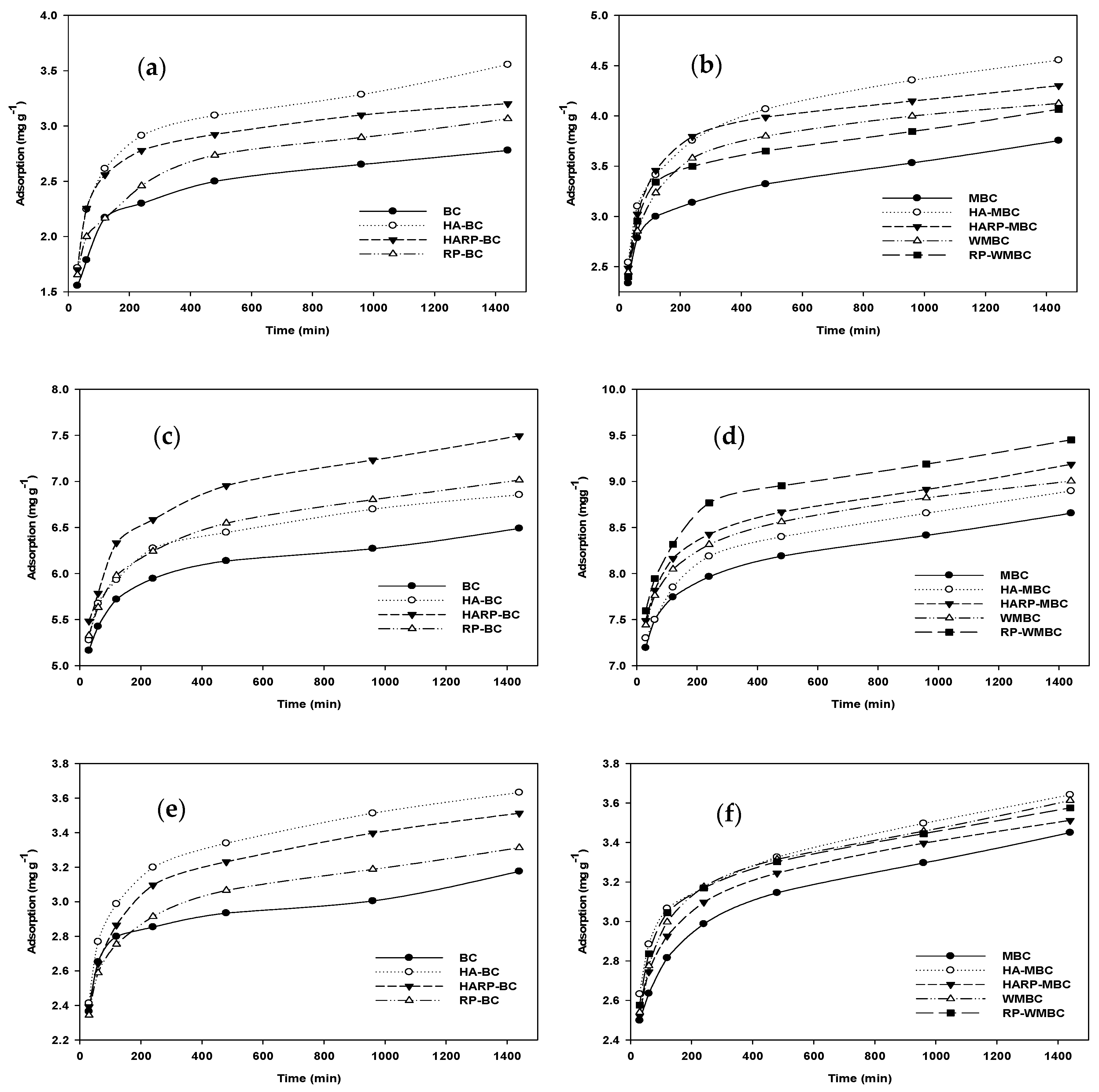
3.3. Kinetics Adsorption
3.3.1. Cadmium (Cd)
3.3.2. Copper (Cu)
3.3.3. Zinc (Zn)
3.3.4. Lead (Pb)
3.4. Mechanism for Metals Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| CEC | Cation exchange capacity |
| EC | Electrical conductivity |
| BC | Biochar |
| HA | Humic acid |
| HA-BC | Humic acid-modified biochar |
| RP | Rock phosphate |
| RP-BC | Rock phosphate-modified biochar |
| HARP-BC | Humic acid and rock phosphate-modified biochar |
| MBC | Ball-milled biochar |
| WMBC | Ball-milled biochar with water |
| HA-MBC | Humic acid-modified ball-milled biochar |
| RP-WMBC | Rock phosphate-modified ball-milled biochar with water |
| HARP-MBC | Humic acid and rock phosphate-modified ball-milled biochar |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier transformation infrared spectroscopy |
| BET | Brunauer–emmett–teller |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| LSD | Least significance difference |
References
- Swartjes, F.A. (Ed.) Dealing with Contaminated Sites: From Theory towards Practical Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry of the U.S. Department of Health and Human Services (ATSDR). Toxicological Profile for Thallium; Prepared by Clement International Corp., under Contract No. 205-88-0608; ATSDR: Atlanta, GA, USA, 2003.
- Marx, S.K.; Rashid, S.; Stromsoe, N. Global-scale patterns in anthropogenic Pb contamination reconstructed from natural archives. Environ. Pollut. 2016, 213, 283–298. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal (loid) s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef]
- Dong, C.; Taylor, M.P.; Kristensen, L.J.; Zahran, S. Environmental contamination in an Australian mining community and potential influences on early childhood health and behavioural outcomes. Environ. Pollut. 2015, 207, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil. Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
- Li, Y.; Yue, Q.; Gao, B. Adsorption kinetics and desorption of Cu (II) and Zn (II) from aqueous solution onto humic acid. J. Hazard. Mater. 2010, 178, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, Z.; Alessi, D.S. Stabilization-based soil remediation should consider long-term challenges. Front. Environ. Sci. Eng. 2018, 12, 16. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Zhang, X.; Cai, X.; Li, R.; Xie, X.; Wang, Z. Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water. Sci. Total Environ. 2019, 688, 1205–1215. [Google Scholar] [CrossRef]
- Kong, X.R.; Liu, Y.X.; Pi, J.C.; Li, W.H.; Liao, Q.H.G.; Shang, J.G. Low-cost magnetic herbal biochar: Characterization and application for antibiotic removal. Environ. Sci. Pollut. R. 2017, 24, 6679–6687. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Ahmad, M.; Rafique, M.I.; Akanji, M.A.; Al-Wabel, M.I.; Al-Swadi, H.A.; Al-Farraj, A.S. Silica modified biochar mitigates the adverse effects of salt and drought stress and improves safflower (Carthamus tinctorius L.) growth. J. Soils Sediments 2022, 23, 1–21. [Google Scholar] [CrossRef]
- Huang, P.; Ge, C.; Feng, D.; Yu, H.; Luo, J.; Li, J.; Strong, P.J.; Sarmah, A.K.; Bolan, N.S.; Wang, H. Effects of metal ions and pH on ofloxacin sorption to cassava residue-derived biochar. Sci. Total Environ. 2018, 616, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.I.; Usman, A.R.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. In situ immobilization of Cr and its availability to maize plants in tannery waste–contaminated soil: Effects of biochar feedstock and pyrolysis temperature. J. Soils Sediments 2020, 20, 330–339. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Lead (II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J. Hazard. Mater. 2021, 412, 125255. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Shang, Q.; Han, J.; Liu, C. Enhanced oil adsorption and nano-emulsion separation of nanofibrous aerogels by coordination of pomelo peel-derived biochar. Ind. Eng. Chem. Res. 2020, 59, 8825–8835. [Google Scholar] [CrossRef]
- Madhavi, P.; Sailaja, V.; Prakash, T.R.; Hussain, S.A. Characterization of Biochar and Humic Acid and their Effect on Soil Properties in Maize. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 449–457. [Google Scholar]
- Zhang, Z.; Liu, B.; He, Z.; Pan, P.; Wu, L.; Lin, B.; Li, Q.; Zhang, X.; Wang, Z. The synergistic effect of biochar-combined activated phosphate rock treatments in typical vegetables in tropical sandy soil: Results from nutrition supply and the immobilization of toxic metals. Int. J. Environ. Res. Public Health 2022, 19, 6431. [Google Scholar] [CrossRef]
- Liu, W.J.; Chai, G.L.; Deng, W.B. A combination of finite mixture distribution model with geo-statistical models to study spatial patterns and hazardous areas of heavy metals in cropland soils of the Guanzhong Plain, Northwest China. Chemosphere 2021, 283, 131222. [Google Scholar] [CrossRef]
- Liu, H.; Dai, P.; Zhang, J.; Zhang, C.; Bao, N.; Cheng, C.; Ren, L. Preparation and evaluation of activated carbons from lotus stalk with trimethyl phosphate and tributyl phosphate activation for lead removal. Chem. Eng. J. 2013, 228, 425–434. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Fan, X.L.; Chang, D.W.; Chen, X.L.; Baek, J.B.; Dai, L.M. Functionalized graphene nanoplatelets from ball milling for energy applications. Curr. Opin. Chem. Eng. 2016, 11, 52–58. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd (II), Cu (II) and Pb (II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, W.; Shen, G.; Ji, G.; Zhang, Y.; Gao, C.; Han, L. Carbonization and ball milling on the enhancement of Pb (II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 2019, 273, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.S.; Ndungutse, J.M.; Li, Y.; Mabi, A.; Liu, X.; Vakili, M.; Memon, A.G.; Ai, L.; Chenfeng, Z.; Sheng, M. Modification of biochar with Fe3O4 and humic acid-salt for removal of mercury from aqueous solutions: A review. Environ. Pollut. Bioavail. 2022, 34, 352–364. [Google Scholar] [CrossRef]
- Richard, L.A. Diagnosis and Improvement of Saline and Alkali Soils; U. S. Department of Agriculture Handbook: Washington, DC, USA, 1954; Volume 60, p. 160.
- Sparks, D.L. Methods of Soil Analysis; Soil Society of American: Madison, WI, USA, 1996. [Google Scholar]
- ASTM. Standard Method for Chemical Analysis of Wood Charcoal, 1762–1784; ASTM International Department: West Conshohocken, PA, USA, 1989. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 1940, 12, 327–356. [Google Scholar]
- Sparks, D.L. Kinetics of Soil Chemical Process; Academic Press: New York, NY, USA, 1989. [Google Scholar]
- Steael, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co., Inc.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr (VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. 2011 The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Song, B.; Chen, M.; Zhao, L.; Qiu, H.; Cao, X. Physicochemical property and colloidal stability of micron- and nano-particle biochar derived from a variety of feedstock sources. Sci. Total Environ. 2019, 661, 685–695. [Google Scholar] [CrossRef]
- Nasri, K.; Chtara, C.; Hassen, C.; Fiallo, M.; Sharrock, P.; Nzihou, A.; El Feki, H. Recrystallization of Industrial Triple Super Phosphate Powder. Ind. Eng. Chem. Res. 2014, 53, 14446–14450. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb (II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper (II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Charanworapan, C.; Suddhiprakarn, A.; Kheoruenromne, I.; Wiriyakitnateekul, W.; Gilkes, R.J. An evaluation of three Thai phosphate rocks for agronomic use based upon their chemical and mineralogical properties. Soil Sci. Plant Nutr. 2013, 59, 522–534. [Google Scholar] [CrossRef]
- Qian, T.T.; Li, D.C.; Jiang, H. Thermochemical behavior of tris (2-butoxyethyl) phosphate (TBEP) during co-pyrolysis with biomass. Environ. Sci. Technol. 2014, 48, 10734–10742. [Google Scholar] [CrossRef] [PubMed]
- Klupfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox properties of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Hiradate, S. Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J. Agric. Food Chem. 2014, 62, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, J.; Du, Q.; Cheng, K.; Yang, F. Analog synthesis of artificial humic substances for efficient removal of mercury. Chemosphere 2020, 250, 126606. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd (II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Ye, S.; Wu, H.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.; Ren, X.; Yi, H.; Cheng, M.; Zhang, C. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Ma, T.; Yang, C.; Jiang, X.; Dang, Z.; Li, X. Preparation of nanozero- valent iron modified amino biochar and its adsorption and desorption properties for Cd(II). Chin. J. Environ. Eng. 2016, 10, 5433–5439. [Google Scholar]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Chen, Y.; Wu, Y.; Li, H.; Wang, S.; Peng, Z.; Xu, R.; Zeng, Z. Novel magnetic pomelo peel biochar for enhancing Pb (II) and Cu (II) adsorption: Performance and mechanism. Water Air Soil. Pollut. 2020, 231, 404. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil. 2010, 337, 16. [Google Scholar] [CrossRef]
- Radnia, H.; Ghoreyshi, A.A.; Younesi, H. Isotherm and kinetics of Fe (II) adsorption onto chitosan in a batch process. Iran. J. Energy Environ. 2011, 2, 250–257. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Cho, J.-S.; Kang, S.-W.; Delaune, R.D.; Han, K.-J.; Seo, D.-C. Recycling of rice straw through pyrolysis and its adsorption behaviors for Cu and Zn ions in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 330–337. [Google Scholar] [CrossRef]
- Yang, S.; Hu, J.; Chen, C.; Shao, D.; Wang, X. Mutual effects of Pb (II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol. 2011, 45, 3621–3627. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Zhao, T.; Yao, Y.; Li, D.; Wu, F.; Zhang, C.; Gao, B. Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag (I) and Pb (II). Sci. Total Environ. 2018, 640, 73–79. [Google Scholar] [CrossRef]
- Ho, S.H.; Wang, D.; Wei, Z.S.; Chang, J.S.; Ren, N.Q. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar]
- Sugihara, G.; Shigematsu, D.S.; Nagadome, S.; Lee, S.; Sasaki, Y.; Igimi, H. Thermodynamic study on the Langmuir adsorption of various bile salts including taurine and glycine conjugates onto graphite in water. Langmuir 2000, 16, 1825–1833. [Google Scholar] [CrossRef]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, Z.; Liu, S.; Wu, Y. Potential of Punica granatum biochar to adsorb Cu (II) in soil. Sci. Rep. 2019, 9, 11116. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef]
- Kamari, A.; Yusoff, S.N.M.; Abdullah, F.; Putra, W.P. Biosorptive removal of Cu (II), Ni (II) and Pb (II) ions from aqueous solutions using coconut dregs residue: Adsorption and characterisation studies. J. Environ. Chem. Eng. 2014, 2, 1912–1919. [Google Scholar] [CrossRef]
- Mireles, S.; Ok, Y.; Kang, J.; Desk, S. Adsorptive and kinetic characterization of aqueous zinc removal by biochars. Environ. Earth Sci. 2016, 1, 80–86. [Google Scholar]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of Arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.M.; Rao, G.P.C.; Seshaiah, K.; Choudary, N.V.; Wang, M.C. Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag. 2008, 28, 849–858. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008, 223, 152–161. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Yin, G.; Song, X.; Tao, L.; Sarkar, B.; Sarmah, A.K.; Zhang, W.; Lin, Q.; Xiao, R.; Liu, Q.; Wang, H. Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd (II) from aqueous solutions. Chem. Eng. J. 2020, 389, 124465. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Kim, S.H.; Kang, S.W.; Jeong, C.Y.; Jeon, J.R.; Park, K.H.; Cho, J.S.; Delaune, R.D.; Seo, D.C. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Keskin, M.E.; Mazlum, S.; Mazlum, N. Hg(II) and Pb(II) adsorption on activated sludge biomass: Effective biosorption mechanism. Int. J. Miner. Process. 2008, 87, 1–8. [Google Scholar] [CrossRef]
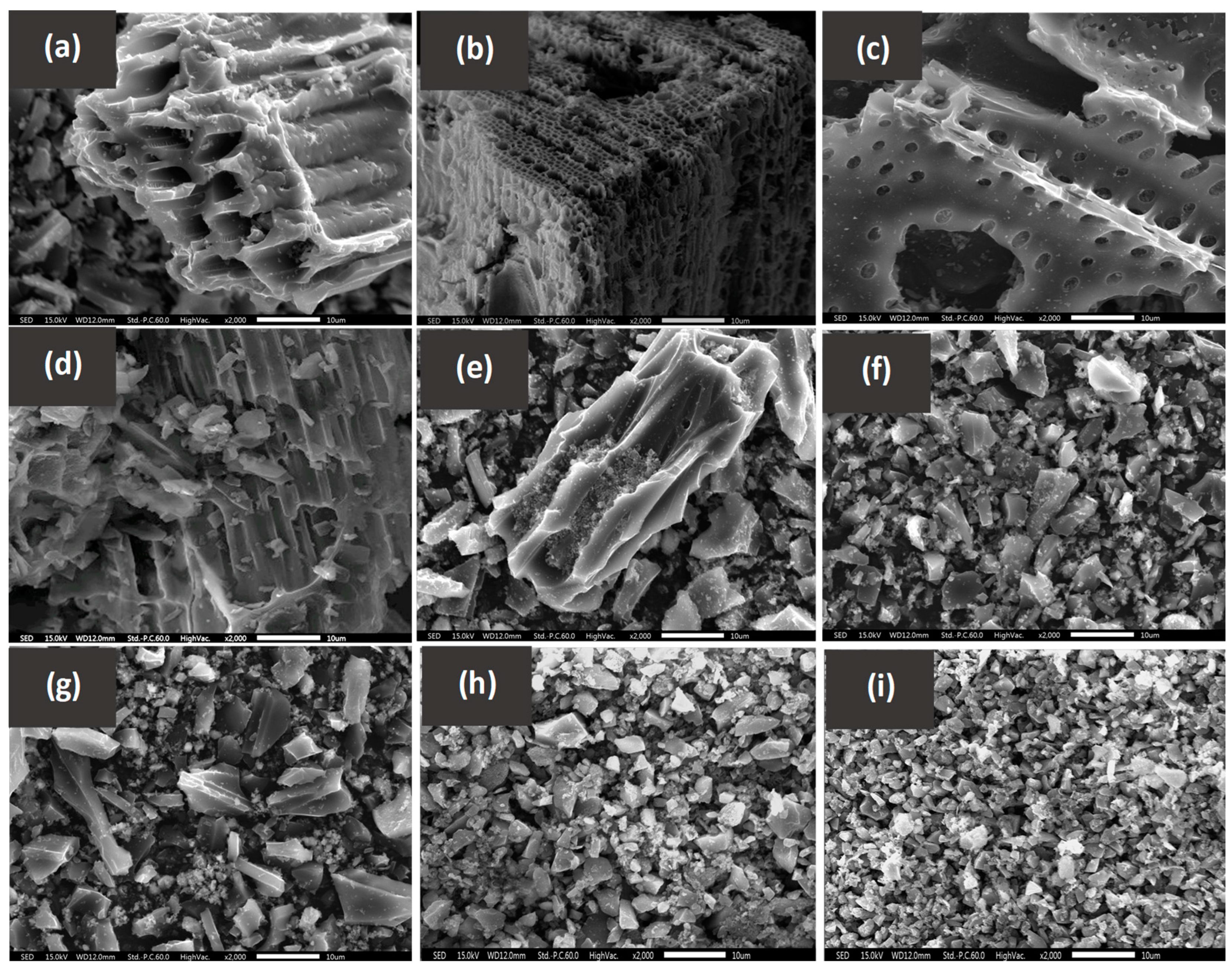

| Material | BC | HA-BC | HARP-BC | RP-BC | MBC | HA-MBC | HARP-MBC | WMBC | RP-WMBC |
|---|---|---|---|---|---|---|---|---|---|
| Moisture % | 2.30 ± 0.15 | 2.04 ± 0.02 | 2.35 ± 0.072 | 2.04 ± 0.50 | 1.96 ± 0.01 | 2.03 ± 0.09 | 2.30 ± 0.05 | 4.38 ± 0.21 | 2.43 ± 0.019 |
| Mobile matter % | 16.03 ± 1.17 | 11.49 ± 2.46 | 18.81 ± 1.93 | 13.55 ± 2.1 | 12.90 ± 3.21 | 15.81 ± 1.97 | 15.43 ± 1.35 | 17.36 ± 2.15 | 16.09 ± 3.70 |
| Ash % | 20.86 ± 3.14 | 24.56 ± 3.25 | 17.87 ± 5.48 | 19.64 ± 2.6 | 21.3 ± 3.99 | 20.57 ± 3.93 | 21.21 ± 4.64 | 20.97 ± 1.92 | 24.62 ± 3.64 |
| Resident matter % | 60.81 ± 4.88 | 61.91 ± 3.94 | 60.97 ± 3.40 | 64.77 ± 2.48 | 63.81 ± 1.81 | 61.60 ± 4.09 | 61.06 ± 5.50 | 57.29 ± 3.06 | 56.86 ± 4.37 |
| pH (1:10) | 10.14 ± 0.07 | 5.13 ± 0.18 | 7.96 ± 0.02 | 6.10 ± 0.02 | 9.37 ± 0.13 | 5.96 ± 0.34 | 7.95 ± 0.07 | 6.03 ± 0.04 | 6.41 ± 0.06 |
| EC † (dSm−1) | 0.56 ± 0.06 | 0.42 ± 0.04 | 0.36 ± 0.03 | 0.49 ± 0.03 | 0.62 ± 0.00 | 0.41 ± 0.064 | 0.49 ± 0.01 | 0.43 ± 0.015 | 0.59 ± 0.01 |
| Adsorbents | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (Å) |
|---|---|---|---|
| BC | 173.076 ± 8.793 | 0.1433 | 33.128 |
| HA-BC | 210.668 ± 6.786 | 0.2256 | 25.689 |
| HARP-BC | 207.993 ± 7.726 | 0.1337 | 25.717 |
| RP-BC | 188.850 ± 7.914 | 0.1462 | 36.908 |
| MBC | 234.99 ± 15.646 | 0.1724 | 29.350 |
| HA-MBC | 320.887 ± 7.902 | 0.1729 | 21.975 |
| HARP-MBC | 263.450 ± 8.315 | 0.1485 | 22.556 |
| WMBC | 241.671 ± 6.108 | 0.1380 | 22.851 |
| RP-WMBC | 272.645 ± 9.847 | 0.1760 | 25.826 |
| Adsorbent | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | KL (L g−1) | R2 | KF (mg g−1) | 1/n | R2 | B | A | R2 | |
| Cadmium (Cd) | |||||||||
| BC | 8.41 | 0.03 | 0.96 | 0.86 | 0.46 | 0.99 | 1484 | 0.69 | 0.94 |
| HA-BC | 9.28 | 0.07 | 0.99 | 1.68 | 0.39 | 0.97 | 1045 | 0.79 | 0.94 |
| HARP-BC | 9.14 | 0.07 | 0.99 | 1.49 | 0.39 | 0.99 | 1309 | 1.1 | 0.9 |
| RP-BC | 9.09 | 0.07 | 0.97 | 1.64 | 0.37 | 0.99 | 1445 | 1.58 | 0.91 |
| MBC | 26.62 | 0.01 | 0.96 | 1.6 | 0.6 | 0.96 | 1128 | 3.76 | 0.8 |
| HA-MBC | 30.89 | 0.09 | 0.96 | 1.75 | 0.56 | 0.99 | 569 | 0.82 | 0.91 |
| HARP-MBC | 27.53 | 0.02 | 0.98 | 1.7 | 0.55 | 0.99 | 853 | 2.68 | 0.9 |
| WMBC | 26.97 | 0.06 | 0.97 | 1.59 | 0.56 | 0.98 | 827 | 1.94 | 0.89 |
| RP-WMBC | 24.02 | 0.03 | 0.98 | 1.58 | 0.59 | 0.98 | 829 | 2.47 | 0.87 |
| Copper (Cu) | |||||||||
| BC | 17.17 | 0.11 | 0.93 | 4.15 | 0.29 | 0.99 | 1251 | 17.8 | 0.91 |
| HA-BC | 16.42 | 0.09 | 0.95 | 3.64 | 0.32 | 0.99 | 1202 | 13.1 | 0.88 |
| HARP-BC | 16.17 | 0.09 | 0.95 | 3.63 | 0.33 | 0.98 | 1290 | 18.72 | 0.84 |
| RP-BC | 16.4 | 0.12 | 0.96 | 4.11 | 0.31 | 0.99 | 1218 | 19.02 | 0.89 |
| MBC | 21.46 | 0.11 | 0.96 | 6.3 | 0.29 | 0.99 | 1018 | 36.4 | 0.91 |
| HA-MBC | 18.92 | 0.09 | 0.95 | 5.31 | 0.28 | 0.99 | 1186 | 44.17 | 0.9 |
| HARP-MBC | 17.34 | 0.22 | 0.95 | 5.91 | 0.24 | 0.99 | 1235 | 77.29 | 0.9 |
| WMBC | 17.55 | 0.2 | 0.95 | 6.22 | 0.54 | 0.99 | 827 | 71.94 | 0.89 |
| RP-WMBC | 24.02 | 0.03 | 0.96 | 6.41 | 0.23 | 0.99 | 1217 | 77.3 | 0.91 |
| Zinc (Zn) | |||||||||
| BC | 4.19 | 0.12 | 0.94 | 1.01 | 0.31 | 0.99 | 2916 | 0.78 | 0.91 |
| HA-BC | 7.98 | 0.04 | 0.97 | 0.8 | 0.47 | 0.99 | 1499 | 0.6 | 0.89 |
| HARP-BC | 8.22 | 0.04 | 0.97 | 0.87 | 0.45 | 0.99 | 1497 | 0.69 | 0.91 |
| RP-BC | 7.19 | 0.03 | 0.94 | 0.78 | 0.44 | 0.97 | 2028 | 1.02 | 0.88 |
| MBC | 11.78 | 0.02 | 0.91 | 1.1 | 0.45 | 0.94 | 1690 | 2.01 | 0.88 |
| HA-MBC | 15.34 | 0.01 | 0.94 | 0.71 | 0.58 | 0.97 | 1261 | 2.8 | 0.81 |
| HARP-MBC | 10.94 | 0.05 | 0.91 | 1.69 | 0.38 | 0.97 | 1527 | 3.12 | 0.83 |
| WMBC | 11.3 | 0.03 | 0.96 | 1.1 | 0.45 | 0.98 | 1500 | 2.47 | 0.83 |
| RP-WMBC | 18.14 | 0.01 | 0.95 | 0.76 | 0.57 | 0.96 | 1317 | 3.18 | 0.8 |
| Lead (Pb) | |||||||||
| BC | 11.13 | 0.06 | 0.86 | 3.44 | 0.35 | 0.97 | 1093 | 9.36 | 0.95 |
| HA-BC | 12.67 | 0.28 | 0.91 | 4.02 | 0.29 | 0.99 | 1241 | 12.64 | 0.95 |
| HARP-BC | 14.83 | 0.48 | 0.92 | 4.48 | 0.26 | 0.99 | 1267 | 16.8 | 0.97 |
| RP-BC | 13.7 | 0.33 | 0.92 | 4.53 | 0.28 | 0.99 | 1126 | 13.16 | 0.97 |
| MBC | 15.61 | 0.28 | 0.9 | 6.3 | 0.28 | 0.99 | 942 | 25.22 | 0.96 |
| HA-MBC | 17.47 | 0.51 | 0.88 | 5.48 | 0.27 | 0.98 | 999 | 18.34 | 0.95 |
| HARP-MBC | 18.85 | 0.67 | 0.91 | 6.57 | 0.27 | 0.98 | 928 | 22.53 | 0.96 |
| WMBC | 16.56 | 0.47 | 0.91 | 6.38 | 0.27 | 0.98 | 919 | 22.22 | 0.96 |
| RP-WMBC | 18.41 | 0.58 | 0.93 | 6.42 | 0.25 | 0.98 | 894 | 33.01 | 0.95 |
| Cadmium (Cd) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First-Order | Pseudo-Second-Order | Intraparticle-Diffusion | Power Function | |||||||||
| Adsorbents | k1 | R2 | k2′ | qe | h | R2 | kid | C | R2 | kf | b | R2 |
| BC | 0.0003 | 0.72 | 0.008 | 2.82 | 0.07 | 0.99 | 0.034 | 1.612 | 0.87 | 0.145 | 0.004 | 0.95 |
| HA-BC | 0.0004 | 0.71 | 0.006 | 3.59 | 0.07 | 0.99 | 0.048 | 1.882 | 0.85 | 0.169 | 0.077 | 0.98 |
| HARP-BC | 0.0003 | 0.63 | 0.011 | 3.25 | 0.09 | 0.99 | 0.038 | 1.934 | 0.78 | 0.145 | 0.163 | 0.90 |
| RP-BC | 0.0003 | 0.78 | 0.052 | 2.51 | 0.06 | 0.99 | 0.037 | 1.679 | 0.91 | 0.153 | 0.034 | 0.98 |
| MBC | 0.0002 | 0.77 | 0.093 | 3.05 | 0.10 | 0.99 | 0.040 | 2.441 | 0.89 | 0.108 | 0.541 | 0.95 |
| HA-MBC | 0.0003 | 0.75 | 0.005 | 4.63 | 0.11 | 0.99 | 0.055 | 2.653 | 0.89 | 0.141 | 0.619 | 0.93 |
| HARP-MBC | 0.0003 | 0.64 | 0.069 | 4.40 | 0.14 | 0.99 | 0.048 | 2.706 | 0.80 | 0.132 | 0.549 | 0.92 |
| WMBC | 0.0003 | 0.77 | 0.099 | 3.11 | 0.12 | 0.99 | 0.047 | 2.563 | 0.85 | 0.130 | 0.509 | 0.95 |
| RP-WMBC | 0.0002 | 0.67 | 0.160 | 3.87 | 0.12 | 0.99 | 0.042 | 2.619 | 0.81 | 0.119 | 0.562 | 0.91 |
| Copper (Cu) | ||||||||||||
| BC | 0.0001 | 0.77 | 0.009 | 6.50 | 0.37 | 0.99 | 0.037 | 5.197 | 0.87 | 0.056 | 1.462 | 0.95 |
| HA-BC | 0.0002 | 0.75 | 0.008 | 6.89 | 0.36 | 0.99 | 0.044 | 5.349 | 0.85 | 0.065 | 1.463 | 0.98 |
| HARP-BC | 0.0002 | 0.78 | 0.01 | 7.54 | 0.30 | 0.99 | 0.058 | 5.474 | 0.78 | 0.080 | 1.440 | 0.91 |
| RP-BC | 0.0002 | 0.80 | 0.050 | 7.50 | 0.31 | 0.99 | 0.049 | 5.316 | 0.91 | 0.070 | 1.444 | 0.98 |
| MBC | 0.0001 | 0.83 | 0.094 | 7.41 | 0.55 | 0.99 | 0.041 | 7.193 | 0.89 | 0.045 | 1.826 | 0.94 |
| HA-MBC | 0.0001 | 0.81 | 0.007 | 8.92 | 0.52 | 0.99 | 0.047 | 7.240 | 0.89 | 0.051 | 1.813 | 0.93 |
| HARP-MBC | 0.0001 | 0.80 | 0.062 | 9.95 | 0.54 | 0.99 | 0.047 | 7.499 | 0.80 | 0.050 | 1.850 | 0.92 |
| WMBC | 0.0001 | 0.80 | 0.102 | 8.40 | 0.58 | 0.99 | 0.045 | 7.449 | 0.85 | 0.048 | 1.851 | 0.95 |
| RP-WMBC | 0.0001 | 0.76 | 0.140 | 9.01 | 0.54 | 0.99 | 0.052 | 7.628 | 0.81 | 0.055 | 1.850 | 0.94 |
| Zinc (Zn) | ||||||||||||
| BC | 0.0002 | 0.71 | 0.015 | 2.17 | 0.15 | 0.99 | 0.019 | 2.464 | 0.82 | 0.064 | 0.689 | 0.95 |
| HA-BC | 0.0002 | 0.72 | 0.010 | 3.66 | 0.13 | 0.99 | 0.033 | 2.511 | 0.86 | 0.098 | 0.595 | 0.98 |
| HARP-BC | 0.0002 | 0.75 | 0.01 | 3.55 | 0.12 | 0.99 | 0.031 | 2.434 | 0.89 | 0.096 | 0.574 | 0.90 |
| RP-BC | 0.0002 | 0.77 | 0.091 | 3.18 | 0.13 | 0.99 | 0.027 | 2.385 | 0.90 | 0.084 | 0.593 | 0.98 |
| MBC | 0.0002 | 0.84 | 0.127 | 3.97 | 0.12 | 0.99 | 0.028 | 2.459 | 0.95 | 0.082 | 0.637 | 0.99 |
| HA-MBC | 0.0002 | 0.81 | 0.010 | 4.66 | 0.14 | 0.99 | 0.027 | 2.661 | 0.92 | 0.078 | 0.727 | 0.93 |
| HARP-MBC | 0.0002 | 0.78 | 0.106 | 4.13 | 0.13 | 0.99 | 0.028 | 2.545 | 0.91 | 0.082 | 0.666 | 0.98 |
| WMBC | 0.0002 | 0.77 | 0.152 | 3.96 | 0.13 | 0.99 | 0.030 | 2.575 | 0.90 | 0.086 | 0.664 | 0.95 |
| RP-WMBC | 0.0002 | 0.76 | 0.243 | 3.76 | 0.15 | 0.99 | 0.027 | 2.636 | 0.87 | 0.079 | 0.710 | 0.90 |
| Lead (Pb) | ||||||||||||
| BC | 0.0001 | 0.65 | 0.010 | 7.20 | 0.54 | 0.99 | 0.036 | 5.961 | 0.77 | 0.051 | 1.610 | 0.93 |
| HA-BC | 0.0001 | 0.73 | 0.011 | 7.36 | 0.57 | 0.98 | 0.031 | 6.252 | 0.83 | 0.042 | 1.692 | 0.96 |
| HARP-BC | 0.0001 | 0.66 | 0.01 | 7.88 | 0.65 | 0.99 | 0.035 | 6.253 | 0.78 | 0.046 | 1.740 | 0.93 |
| RP-BC | 0.0002 | 0.50 | 0.010 | 7.67 | 0.56 | 0.99 | 0.038 | 6.351 | 0.85 | 0.050 | 1.682 | 0.96 |
| MBC | 0.0001 | 0.64 | 0.012 | 9.33 | 1.05 | 0.98 | 0.027 | 8.364 | 0.90 | 0.027 | 2.034 | 0.99 |
| HA-MBC | 0.0001 | 0.63 | 0.009 | 10.07 | 0.89 | 0.99 | 0.039 | 8.676 | 0.88 | 0.038 | 2.034 | 0.93 |
| HARP-MBC | 0.0001 | 0.71 | 0.011 | 10.58 | 1.27 | 0.99 | 0.042 | 9.181 | 0.65 | 0.042 | 2.068 | 0.96 |
| WMBC | 0.0001 | 0.80 | 0.012 | 9.68 | 1.13 | 0.99 | 0.035 | 8.501 | 0.69 | 0.036 | 2.018 | 0.99 |
| RP-WMBC | 0.0001 | 0.75 | 0.011 | 10.37 | 1.17 | 0.99 | 0.038 | 9.080 | 0.78 | 0.036 | 2.081 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhawas, M.S.; Rafique, M.I.; Ahmad, M.; Al-Wabel, M.I.; Usman, A.R.A.; Al-Swadi, H.A.; Al-Farraj, A.S. Ball Mill, Humic Acid, and Rock Phosphate-Modified Conocarpus Biochar for Efficient Removal of Heavy Metals from Contaminated Water. Sustainability 2023, 15, 11474. https://doi.org/10.3390/su151411474
Alhawas MS, Rafique MI, Ahmad M, Al-Wabel MI, Usman ARA, Al-Swadi HA, Al-Farraj AS. Ball Mill, Humic Acid, and Rock Phosphate-Modified Conocarpus Biochar for Efficient Removal of Heavy Metals from Contaminated Water. Sustainability. 2023; 15(14):11474. https://doi.org/10.3390/su151411474
Chicago/Turabian StyleAlhawas, Mansour S., Muhammad Imran Rafique, Munir Ahmad, Mohammad I. Al-Wabel, Adel R. A. Usman, Hamed Ahmed Al-Swadi, and Abdullah S. Al-Farraj. 2023. "Ball Mill, Humic Acid, and Rock Phosphate-Modified Conocarpus Biochar for Efficient Removal of Heavy Metals from Contaminated Water" Sustainability 15, no. 14: 11474. https://doi.org/10.3390/su151411474
APA StyleAlhawas, M. S., Rafique, M. I., Ahmad, M., Al-Wabel, M. I., Usman, A. R. A., Al-Swadi, H. A., & Al-Farraj, A. S. (2023). Ball Mill, Humic Acid, and Rock Phosphate-Modified Conocarpus Biochar for Efficient Removal of Heavy Metals from Contaminated Water. Sustainability, 15(14), 11474. https://doi.org/10.3390/su151411474











