Risk Assessment Method for Spontaneous Combustion of Pyrophoric Iron Sulfides
Abstract
:1. Introduction
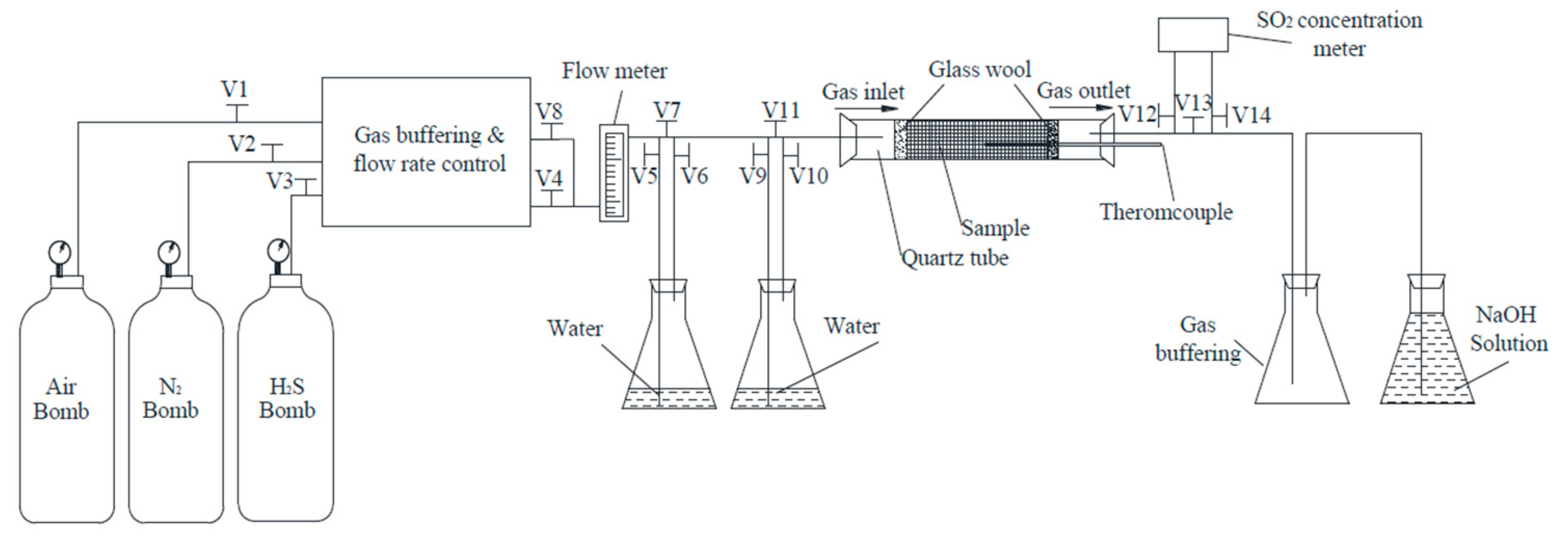
2. Experiments
2.1. Oxidation of PISs
2.1.1. Sample Preparation
2.1.2. Oxidation of Samples
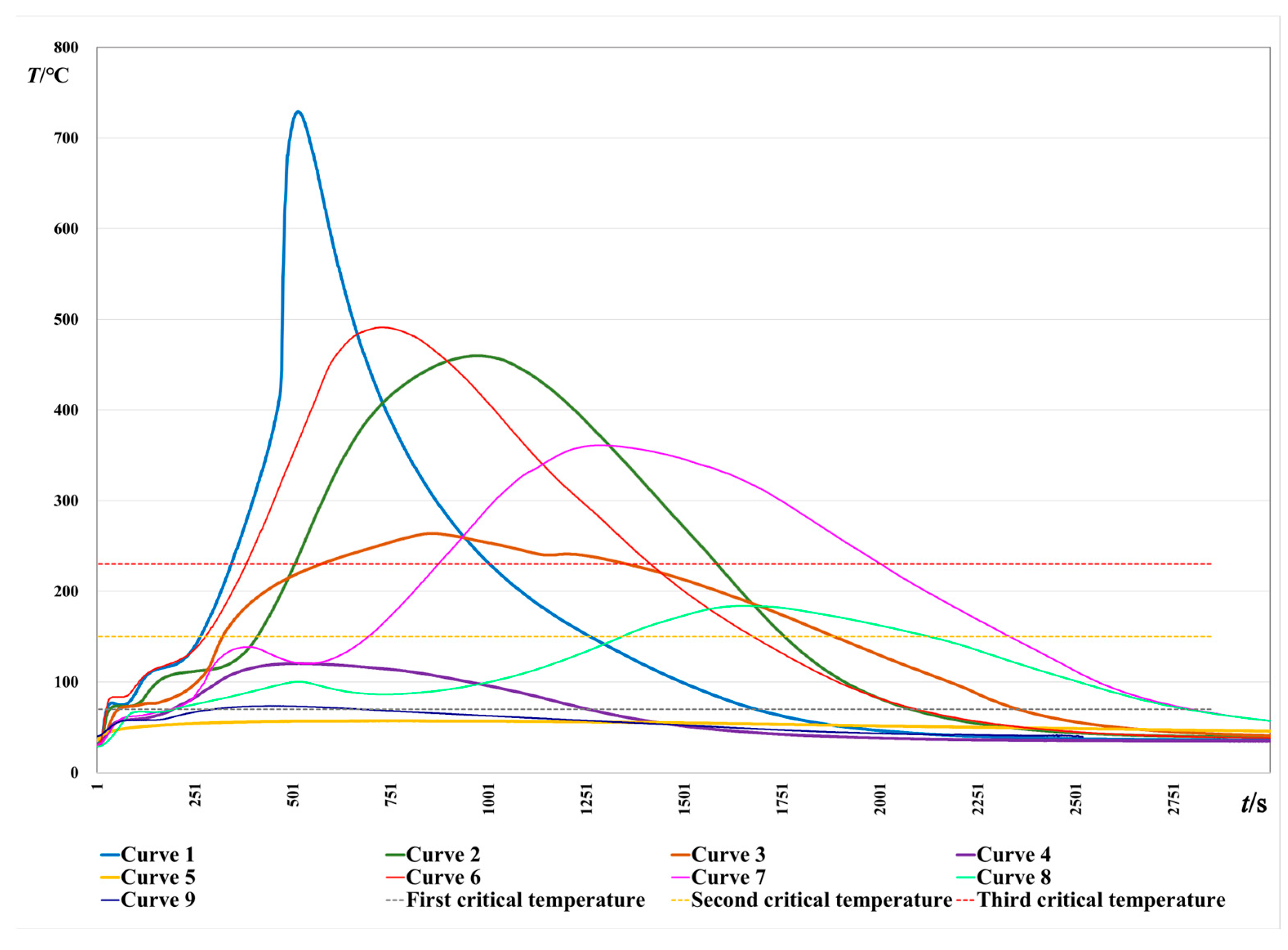
2.2. Results and Preliminary Analysis
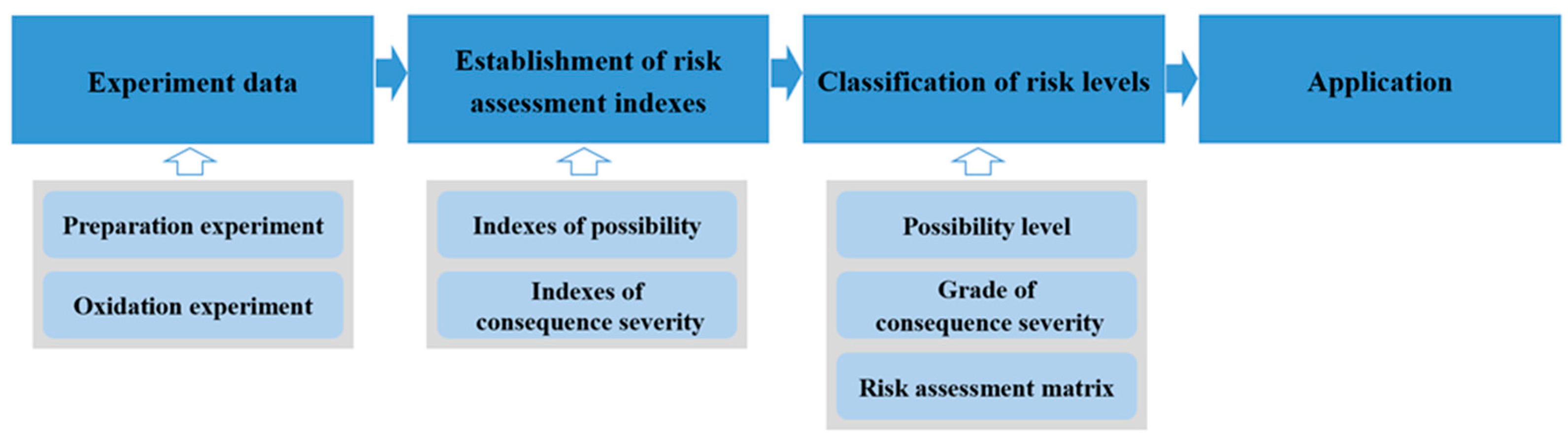
3. Establishment of Risk Assessment Indexes
3.1. Basic Principles
- Scientific feasibility
- 2.
- Simplicity and independence
3.2. Possibility of Spontaneous Combustion
3.2.1. Maximum Temperature Rise Rate v1
3.2.2. Maximum Oxidation Temperature Tmax
3.3. Severity of Oxidation to Spontaneous Combustion
3.3.1. Characteristic Duration τ
3.3.2. Third Instantaneous Heating Rate v2
4. Classification of Risk Levels
4.1. Risk Classification
4.1.1. Basic Principles
4.1.2. Possibility Levels of Spontaneous Combustion
4.1.3. Severity Grades of Oxidation to Spontaneous Combustion
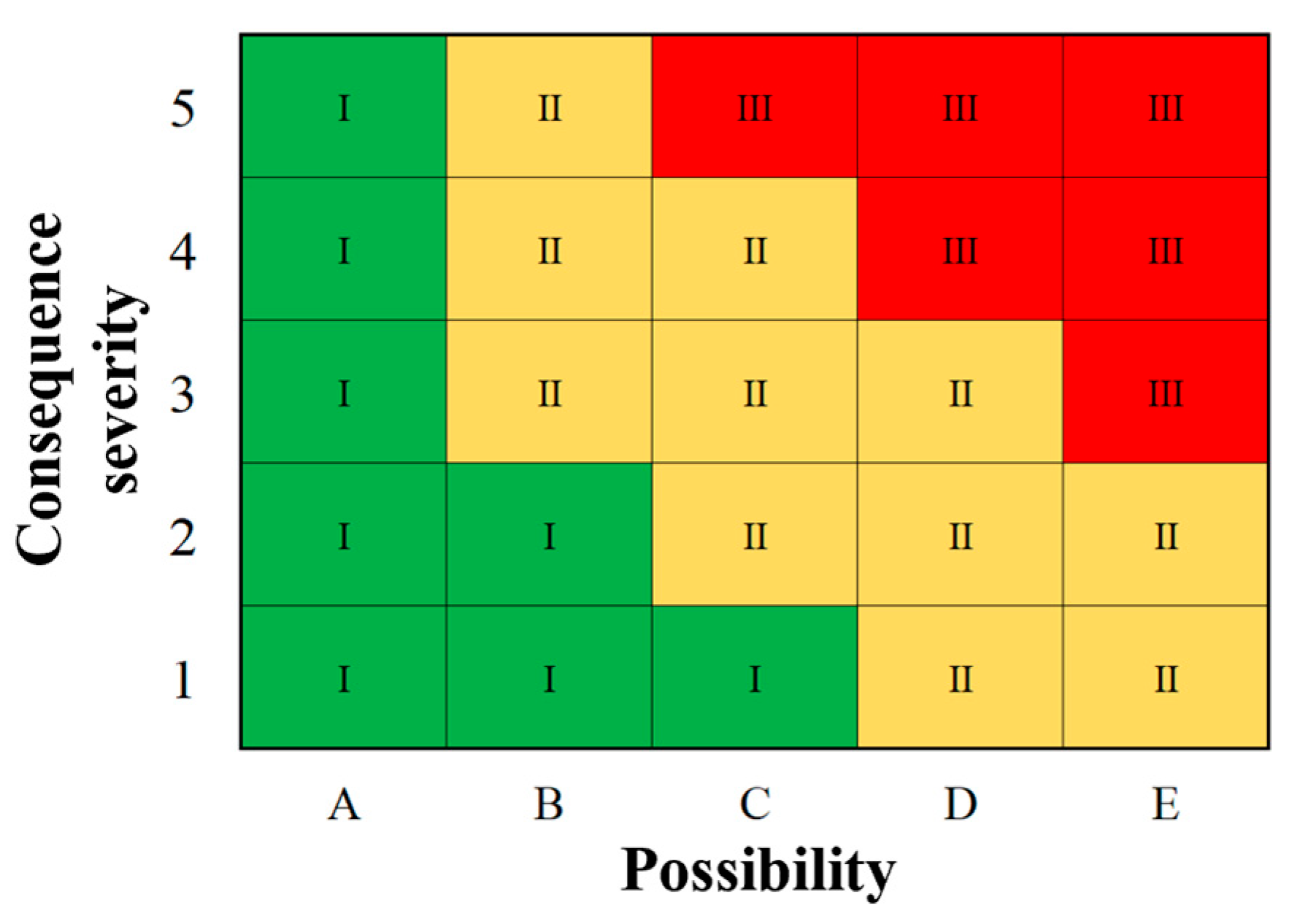
4.2. Risk Evaluation Matrix
4.3. Risk Assessment Procedures
- The possibility evaluation indexes, Tmax and v1, are obtained using the experimental data, and its product is calculated to determine the possibility of the spontaneous combustion of PISs;
- The consequence severity assessment indexes, τ and v2, are obtained, and the product is calculated to determine the consequence severity C of the spontaneous combustion of PISs;
- According to the possibility grade and the severity grade, the risk assessment matrix is used to determine the risk level of the spontaneous combustion of PISs.
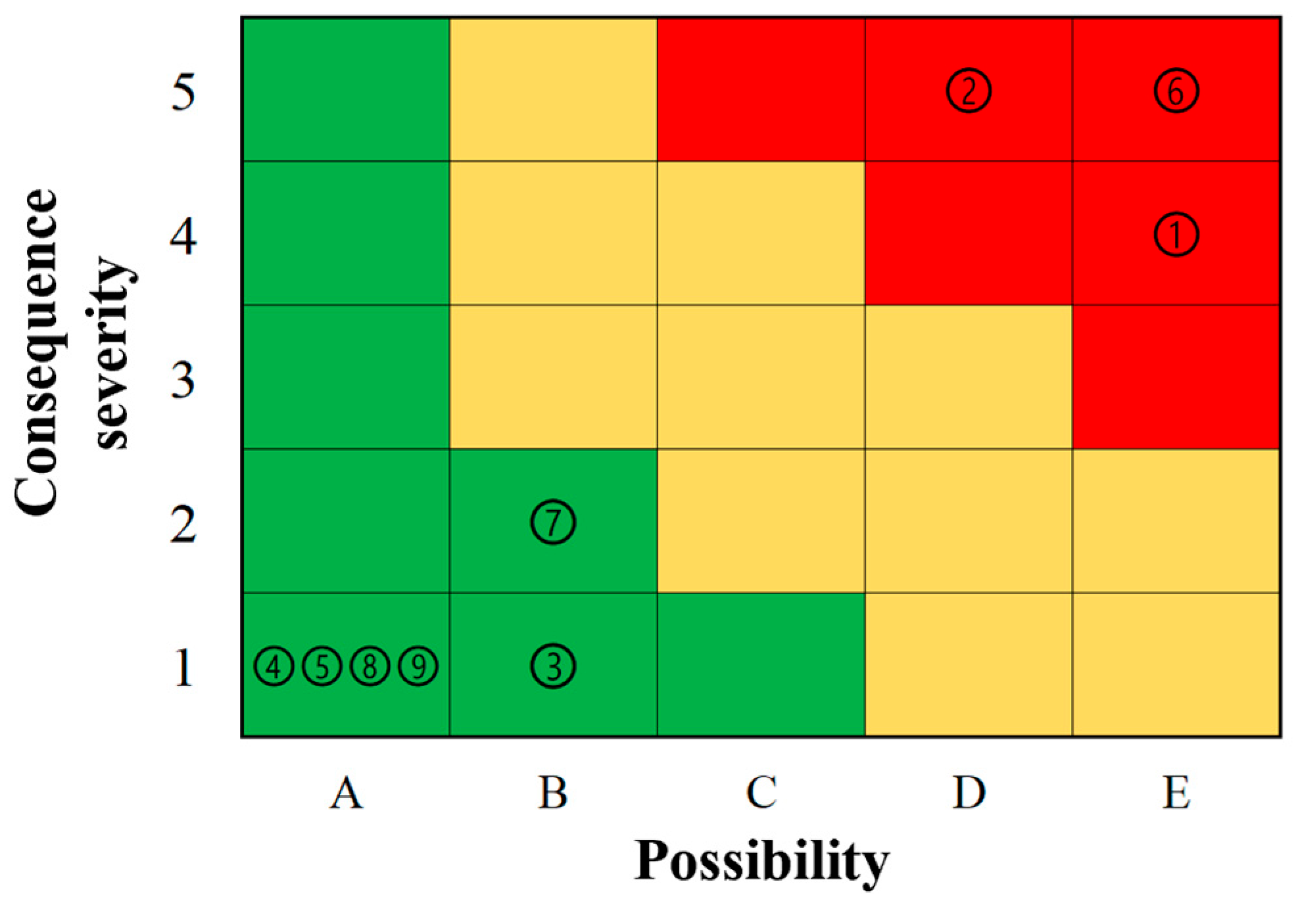
5. Application
5.1. Possibility Calculation
5.2. Severity Estimation
5.3. Risk Level Determination
6. Conclusions and Future Works
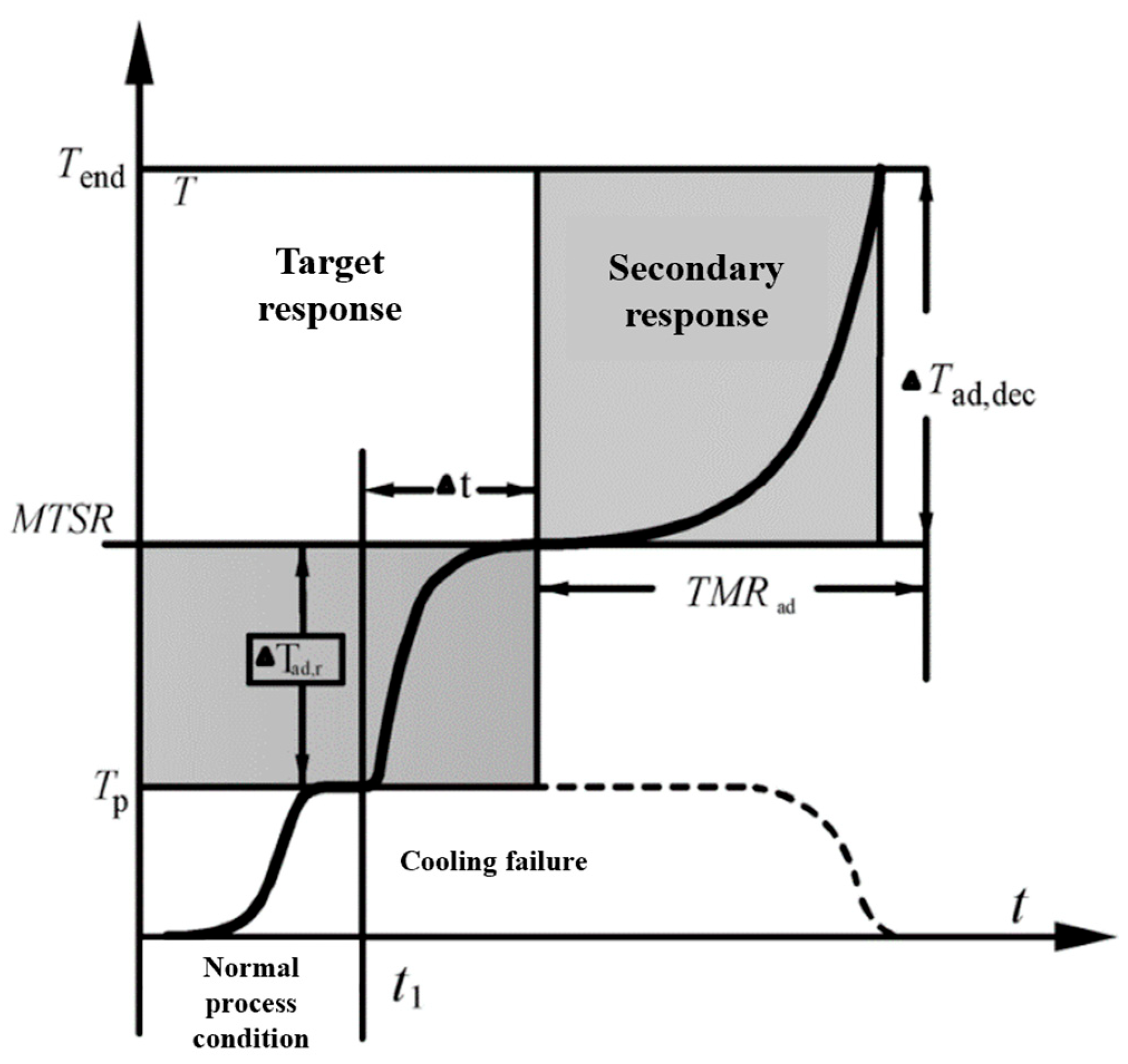
- Through the analysis of experimental data, it was found that the oxidation temperature of PISs was different under different conditions, and the number of heating stages was also different. In addition, the oxidation and spontaneous combustion process of PISs experienced three heating stages, which is similar to the thermal runaway process caused by cooling failure in chemical processes;
- Risk assessment indexes suitable for the oxidation and spontaneous combustion of PISs was proposed, including four risk assessment parameters: v1, Tmax, τ, and v2. Among them, v1 and Tmax characterize the possibility of spontaneous combustion, and τ and v2 characterize the severity of spontaneous combustion;
- The product value of Tmax and v1 is used to represent the possibility of spontaneous combustion, and it is divided into five grades. The product of τ and v2 is used to represent the severity of the consequences of oxidation to spontaneous combustion, and it is divided into five grades. A risk matrix is established to evaluate the semi-quantitative risk of PIS oxidation to spontaneous combustion. A complete risk assessment method for PIS oxidation to spontaneous combustion is established;
- Risk assessment methods are used to assess the risk of nine oxidation processes of PISs. The results show that curves 1, 2, and 6 are high risks, while curves 3, 4, 5, 7, 8, and 9 are low risks.
- From the results of the risk analysis of cases, it is found that all the risk levels of the spontaneous combustion of PISs are level I and III, and there is no level II, which may be due to the small number of risk levels. In future studies, we plan to classify the risk level into five levels to assess the risk of spontaneous combustion of PISs in more detail;
- In order to apply the risk assessment method practically, the prediction models of Tmax and τ are established based on the experimental data using machine learning algorithms. In this way, it is possible to achieve early access to assessment index data for early warning and significantly reduce the risk of spontaneous combustion of PISs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrington, S. Pipeline debris removal requires extensive planning. Pipeline Gas J. 2006, 233, 61–62. [Google Scholar]
- Kim, C.Y.; Escuadro, A.A.; Bedzyk, M.J. Interaction of H2S with α-Fe2O3(0001) surface. Surf. Sci. 2007, 601, 4966–4970. [Google Scholar] [CrossRef] [Green Version]
- Wolthers, M.; Gaast, S.J.V.D.; Rickard, D. The structure of disordered mackinawite. Am. Mineral. 2003, 88, 2007–2015. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Zhao, S.P.; Mao, G.B.; Zhang, M.G.; Wang, L.; Wang, Z.R. Experimental investigation on oxidation of sulfurized rust in oil tank. J. Loss Prev. Process Ind. 2015, 38, 156–162. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.; Saad, M. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Wang, Z.R.; Zhao, S.P.; Yang, H.Q.; Mao, G.B. Kinetic analysis for spontaneous combustion of sulfurized rust in oil tanks. J. Loss Prev. Process Ind. 2014, 32, 387–392. [Google Scholar] [CrossRef]
- Gao, J.C.; Ma, X.M.; Shen, J.; Meng, Q.; Zhou, S.Y. Synthesis of pyrophoric active ferrous sulfide with oxidation behavior under hypoxic conditions. Vacuum 2017, 143, 386–394. [Google Scholar] [CrossRef]
- Beilin, Y.A.; Nisel’son, L.A.; Begishev, I.R.; Filimonov, L.I.; Shishkanov, B.A.; Ashcheulova, I.I.; Podobaev, A.N.; Reformatskaya, I.I. Corrosion pyrophoric deposits as promoters of self-ignition of storage tanks with sour crude oil. Prot. Met. 2007, 43, 269–274. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Zhao, S.P.; Zhang, W.X.; Ni, L.; Zhang, M.G.; Wang, Z.R. Analysis on oxidation process of sulphurized rust in oil tank. J. Therm. Anal. Calorim. 2017, 128, 125–134. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemical of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef]
- Asaki, Z.; Atsumi, T.; Kondo, Y. Oxidation of Fe1−xS pellet. Trans. Jpn. Inst. Met. 1986, 27, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.B.; Jiang, J.C.; Zhao, S.P.; Dou, Z.; Zhao, D. On the monitoring technology for the symbolic gas in the spontaneous oxidation of sulfur-corrosive products in the oil tanks. J. Saf. Environ. 2015, 15, 155–158. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhang, Z.H.; Li, P.; Zhao, S.L.; Li, F.; Tang, M. Monitoring of the tail exhaust of the sulfur dioxide from the oxidation gas in the iron sulfides. J. Saf. Environ. 2017, 17, 1716–1719. [Google Scholar] [CrossRef]
- Zhao, X.E.; Jiang, J.C. Forecasting techniques whether containing sulfur oil-tank would combust spontaneously. Chem. Eng. Oil Gas 2008, 37, 357–360. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Zhao, S.P. Experimental study on oxidization and spontaneous combustion of lower temperature sulfur corrosion products in oil tank. J. Nanjing Univ. Technol. Nat. Sci. Ed. 2014, 36, 118–122. [Google Scholar] [CrossRef]
- Zhao, X.F. Experimental Study on Oxidation Kinetics of Thermal Spontaneous Combustion of Ferrous Sulfide. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2013. [Google Scholar]
- Cai, X. Study on Low Temperature Oxidation Process and Kinetics of Iron Sulfides; Zhengzhou University: Zhengzhou, China, 2015. [Google Scholar]
- Man, X.M. Study on Spontaneous Combustion Reaction Characteristics and Apparent Kinetics of Active Iron Sulfide Compounds. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar] [CrossRef]
- Li, Z.J.; Jiang, W.J.; Chen, T.F. Kinetic analysis on oxidation and spontaneous combustion of iron sulfides. J. Saf. Sci. Technol. 2018, 14, 24–29. [Google Scholar] [CrossRef]
- Liu, H.; Hong, R.; Lang, Z.H.; Yao, J.; Ye, D.; Shan, J.; Liu, X. Evaluation of the spontaneous combustion tendency of corrosion products in oil tanks based on TOPSIS methodologies. J. Loss Prev. Process Ind. 2021, 71, 104475. [Google Scholar] [CrossRef]
- Zhang, S.J.; Meng, T.W.; Ma, X. Progress of ferrous sulfide compound spontaneous combustion and prevention technology. J. Health Saf. Environ. 2013, 13, 33–36. [Google Scholar] [CrossRef]
- Dou, Z.; Mebarki, A.; Ni, L.; Jiang, J.C.; Cai, Z.L.; Zhang, M.G.; Zhao, S.P.; Zhang, W.X.; Pensée, V. SVM application in hazard assessment: Self-heating for sulfurized rust. J. Loss Prev. Process Ind. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, C.; Wang, Z.G.; Shi, N. Analysis and assessment of iron sulfur compounds naphtha tanks spontaneous combustion. J. Health Saf. Environ. 2015, 15, 33–37. [Google Scholar] [CrossRef]
- Deng, C.H. Prevention of spontaneous combustion of iron sulfide in LD32-2 oilfield development and production. Petro Chem. Equip. 2015, 18, 92–94. [Google Scholar] [CrossRef]
- Huang, J.X. Study on Spontaneous Combustion Mechanism and Risk Prediction Technology of Sulfur-Containing Ore. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2010. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhao, S.L.; Li, P.; Han, Y. Spontaneous combustion process of hydrogen sulfide corrosion products formed at room temperature. Acta Pet. Sin. 2012, 28, 122–126. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, S.D. Discussion on explosion causes of large gas pipeline. J. Wuhan Iron Steel Univ. 1979, 1–5. [Google Scholar]
- Chu, C.W.; Zhu, Z.C.; Bian, H.T.; Jiang, J.C. Design of self-heating test platform for sulfide corrosion and oxidation based on Fuzzy PID temperature control system. Meas. Control 2021, 54, 1082–1096. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Garg, A.; Subasinghe, N.D. In situ high-temperature phase transformation studies on pyrite. Fuel 2009, 88, 988–993. [Google Scholar] [CrossRef]
- Debouza, M.; Al-Durra, A.; Al-Wahedi, K.; Abou-Khousa, M. Assessment of black powder concentrations in natural gas pipeline networks. IEEE Access 2020, 8, 71395–71404. [Google Scholar] [CrossRef]
- Al-Sulaiman, S.; Sandip, K.; Shady, H. Control of black powder in gas and condensate pipelines. Mater. Performance 2019, 58, 24–29. [Google Scholar]
- Hany, G.; Khaled, Z.A.; Salaheldin, E. New environmentally friendly acid system for iron sulfide scale removal. Sustainability 2019, 11, 6727. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Zhao, S. Influence of relative humidity on pyrophoricity of iron sulfides. J. Qingdao Univ. Sci. Technol. 2009, 30, 304–306. [Google Scholar] [CrossRef]
- Jiang, L. Study on Thermal Runaway and Discharge Characteristics of Peracetic Acid Production Process. Master’s Thesis, Dalian University of Technology, Dalian, China, 2017. [Google Scholar]
- Wang, W.; Shi, J.; Geng, L.Y.; Tang, J.Q.; Gong, J.M. Research status of damage evaluation method of fire equipment and damage evaluation of material mechanics and corrosion resistance. Mater. Mech. Eng. 2020, 44, 1–5. [Google Scholar] [CrossRef]
- Cao, L.Y.; Shao, S.S.; Wang, Y.A.; Li, Z.F.; Liu, J.J. Fitness for service assessment of fire vacuum tower in oil refinery. J. Press. Vessel Technol. 2015, 32, 66–72. [Google Scholar]
- Hao, J.B. Combustion and Explosion Science; China Petrochemical Press: Beijing, China, 2012. [Google Scholar]
- Wei, H.Y.; Bai, W.S.; Hao, L. Chemical Process Safety Assessment; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Kong, D.; Peng, R.; Sun, X.; Zhang, J.; Ping, P.; Du, J. Study of the influence of crude oil on the spontaneous combustion risk of sulfurized rust in crude oil tanks. Fuel 2019, 255, 1125816. [Google Scholar] [CrossRef]
- Q/SH 0560-2013; HSE risk matrix Standard. China National Petroleum Corporation Limited: Beijing, China, 2013; pp. 37–39.
- Shandong Provincial Administration of Work Safety. Implementation Guide for Safety Risk Classification Control of Chemical Enterprises (Trial Version); Administration of Work Safety Supervision: Jinan, China, 2016.
- Wan, G.J. Quantitative classification control of Sinopec safety risk based on risk value. J. Health Saf. Environ. 2018, 18, 40–43. [Google Scholar] [CrossRef]
- Onawole, A.T.; Hussein, I.A.; Saad, M.A.; Mahmoud, M.; Ahmed, M.E.M.; Nimir, H.I. Effect of pH on acidic and basic chelating agents used in the removal of iron sulphide scales: A computational study. J. Pet. Sci. Eng. 2019, 178, 649–654. [Google Scholar] [CrossRef]
- Payant, R.A.; Finch, J.A. The effect of sulfide mixtures on self-heating. Can. Metall. Q. 2010, 49, 429–434. [Google Scholar] [CrossRef]
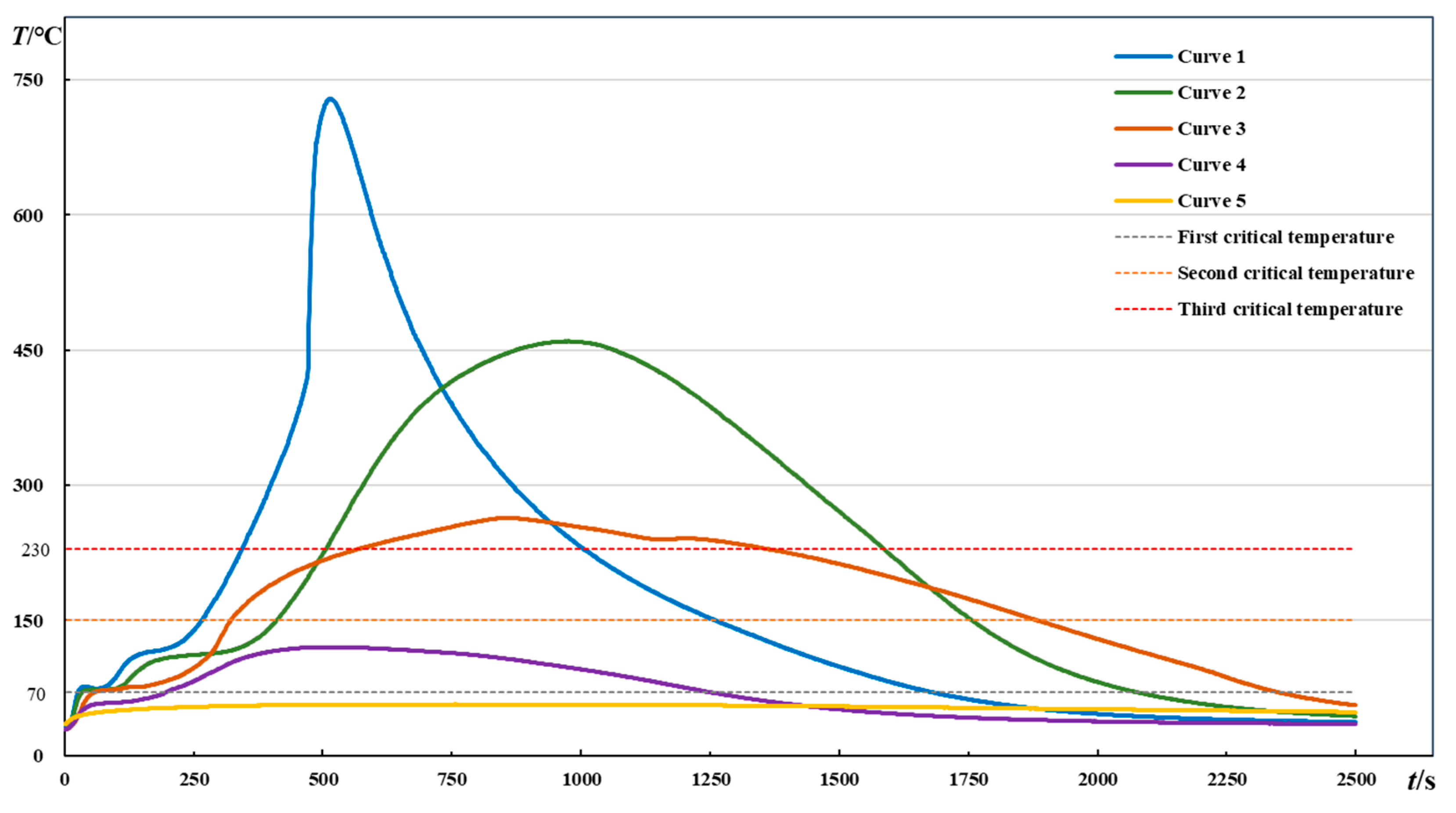


| Indexes | Curve 1 | Curve 2 | Curve 3 | Curve 4 | Curve 5 |
|---|---|---|---|---|---|
| Maximum temperature rise rate before reaching the third critical temperature (°C/s) | 4.50 | 3.56 | 2.00 | 1.44 | 0.81 |
| Temperature (°C) | 58.65 | 52.38 | 52.56 | 40.06 | 37.19 |
| Time (s) | 20 | 20 | 32 | 21 | 5 |
| Maximum temperature (°C) | 728.94 | 459.75 | 263.81 | 120.38 | 57.00 |
| Curves | Instantaneous Heating Rate Corresponding to the Third Critical Temperature (°C/s) | Temperature (°C) | Time (s) | Maximum Heating Rate between the Third Critical Temperature and the Maximum Temperature (°C/s) | Temperature (°C) | Time (s) |
|---|---|---|---|---|---|---|
| Curve 1 | 1.88 | 230 | 346 | 34 | 546.88 | 478 |
| Curve 2 | 1.56 | 230 | 509 | 1.63 | 238.75 | 517 |
| Curve 3 | 0.25 | 230 | 575 | 0.31 | 232.88 | 590 |
| Curve 4 | — | — | — | — | — | — |
| Curve 5 | — | — | — | — | — | — |
| L | Spontaneous Combustion Possibility Level | Explanation |
|---|---|---|
| (0, 230) | A | Lower |
| [230, 500) | B | Low |
| [500, 1000) | C | Medium |
| [1000, 2000) | D | High |
| [2000, 3500) | E | Higher |
| C | Consequence Severity Level | Explanation |
|---|---|---|
| (0, 300) | 1 | Mild |
| [300, 800) | 2 | General |
| [800, 1200) | 3 | Large |
| [1200, 1600) | 4 | Significant |
| [1600, 2000) | 5 | Particularly significant |
| Color | Risk Level | Risk Acceptability | Explanation | |
|---|---|---|---|---|
| Green | Ⅰ | Low risk | Acceptable | The risk level is within the acceptable range of the system without taking measures. |
| Yellow | Ⅱ | Medium risk | Conditional acceptance | Reasonable and effective measures should be taken to reduce the risk level to grade I. |
| Red | Ⅲ | High risk | Unacceptable | The risk level exceeds the acceptable range of the system. Effective measures should be taken immediately to inhibit the oxidation reaction and reduce the risk of spontaneous combustion. |
| Indexes | Curve 1 | Curve 2 | Curve 3 | Curve 4 | Curve 5 | Curve 6 | Curve 7 | Curve 8 | Curve 9 |
|---|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | 728.94 | 459.75 | 263.81 | 120.38 | 57.00 | 491.19 | 360.94 | 183.88 | 73.44 |
| v1 (°C/s) | 4.50 | 3.56 | 2.00 | 1.44 | 0.81 | 4.81 | 1.19 | 1.13 | 0.69 |
| Tmax × v1 | 3280.2 | 1636.7 | 527.6 | 173.3 | 46.2 | 2363.8 | 428.6 | 206.9 | 50.5 |
| Probability level | E | D | B | A | A | E | B | A | A |
| Indexes | Curve 1 | Curve 2 | Curve 3 | Curve 4 | Curve 5 | Curve 6 | Curve 7 | Curve 8 | Curve 9 |
|---|---|---|---|---|---|---|---|---|---|
| τ (s) | 659 | 1078 | 781 | 0 | 0 | 1033 | 1133 | 0 | 0 |
| v2 (°C/s) | 1.88 | 1.56 | 0.25 | — | — | 1.5 | 0.31 | — | — |
| τ × v2 | 1238.9 | 1681.7 | 195.3 | 0 | 0 | 1549.5 | 351.2 | 0 | 0 |
| Severity | 4 | 5 | 1 | 1 | 1 | 5 | 2 | 1 | 1 |
| Curve 1 | Curve 2 | Curve 3 | Curve 4 | Curve 5 | Curve 6 | Curve 7 | Curve 8 | Curve 9 | |
|---|---|---|---|---|---|---|---|---|---|
| L | E | D | B | A | A | E | B | A | A |
| C | 4 | 5 | 1 | 1 | 1 | 5 | 2 | 1 | 1 |
| Risk level | Ⅲ | Ⅲ | Ⅰ | Ⅰ | Ⅰ | Ⅲ | Ⅰ | Ⅰ | Ⅰ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Z.; Li, L.-L.; Chen, L.-C. Risk Assessment Method for Spontaneous Combustion of Pyrophoric Iron Sulfides. Sustainability 2023, 15, 11605. https://doi.org/10.3390/su151511605
Dou Z, Li L-L, Chen L-C. Risk Assessment Method for Spontaneous Combustion of Pyrophoric Iron Sulfides. Sustainability. 2023; 15(15):11605. https://doi.org/10.3390/su151511605
Chicago/Turabian StyleDou, Zhan, Li-Li Li, and Liang-Chao Chen. 2023. "Risk Assessment Method for Spontaneous Combustion of Pyrophoric Iron Sulfides" Sustainability 15, no. 15: 11605. https://doi.org/10.3390/su151511605
APA StyleDou, Z., Li, L.-L., & Chen, L.-C. (2023). Risk Assessment Method for Spontaneous Combustion of Pyrophoric Iron Sulfides. Sustainability, 15(15), 11605. https://doi.org/10.3390/su151511605







