Effect of Solvent Pretreatment on the Flash Pyrolysis Performance of Yinggema Lignite
Abstract
:1. Introduction
2. Experimental
2.1. Material
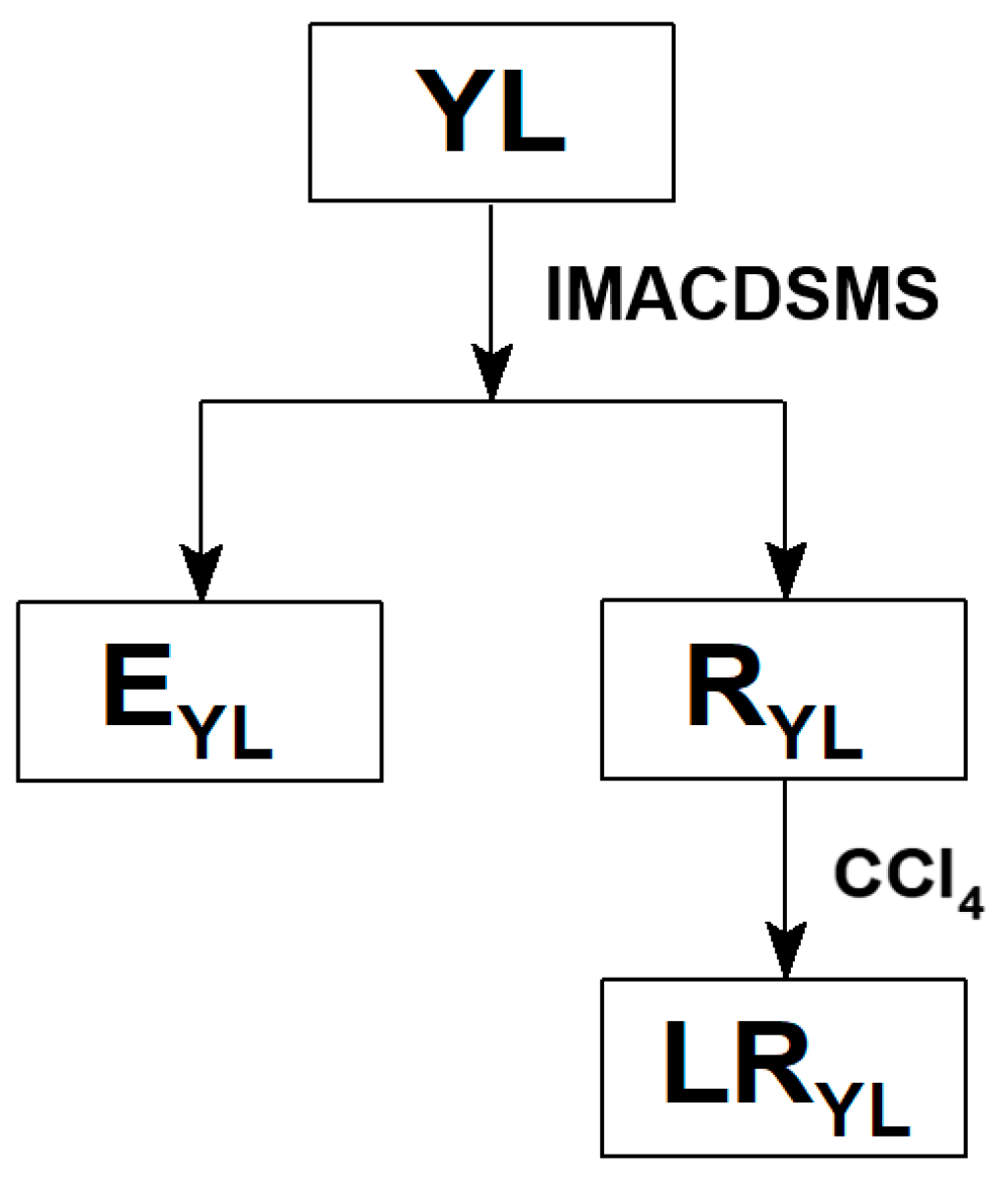
2.2. Preparation of LRYL
2.3. Analysis Methods
2.3.1. Proximate and Ultimate Analyses
2.3.2. FTIR Analysis
2.3.3. TG-DTG Analysis
2.3.4. TG-FTIR-GC/MS Analysis
- (1)
- TG analysis in the experiment. The test temperature was controlled at 40–800 °C, with a heating rate of 20 °C/min, the purge gas flow rate was selected as 20 mL/min, and a set of data was recorded every 6 s;
- (2)
- FTIR analysis in the experiment (scanning range: 400–4000 cm−1, resolution: 4 cm−1). Spectrum Omnic 8.2 was used for the recording and analysis of FTIR data, with which the baseline can be automatically corrected and related information on volatile gases can be analyzed synchronously;
- (3)
- GC/MS analysis in the experiment (mass-to-charge ratio (m/z) range: ≥5, scan time: 1 s). Time-intensity data is recorded by mass spectrometry and TurboMass Ver6.1.2 software is used for processing and analysis. The results were affected by air in the initial detection stage, so only stable data were selected. The ion flow diagram of the required m/z signal was isolated from the total ion flow diagram and it was distributed to corresponding gas species to obtain evolution information of the gas intensity.
3. Results and Discussion
3.1. Coal Analysis
3.2. FTIR Analysis
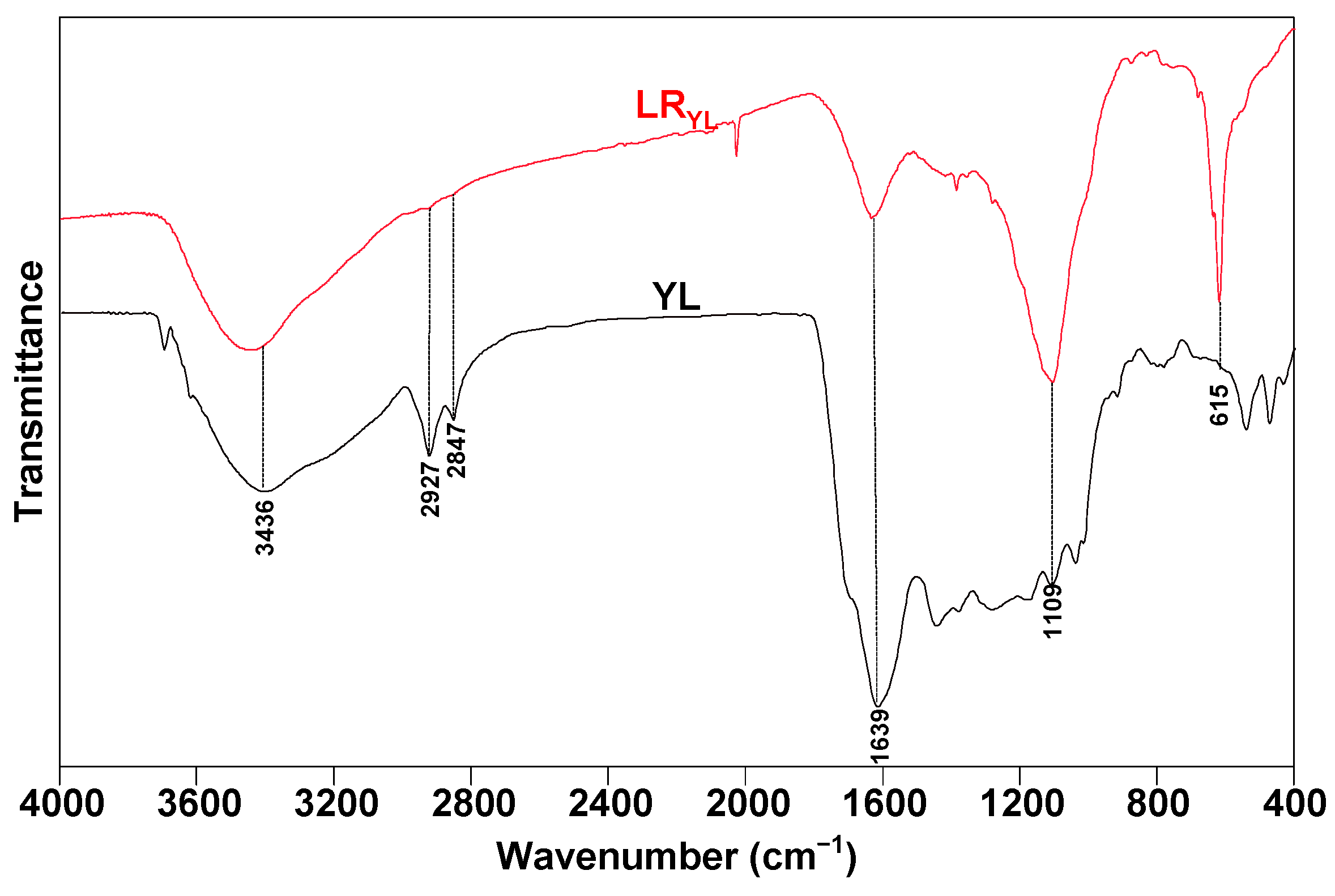
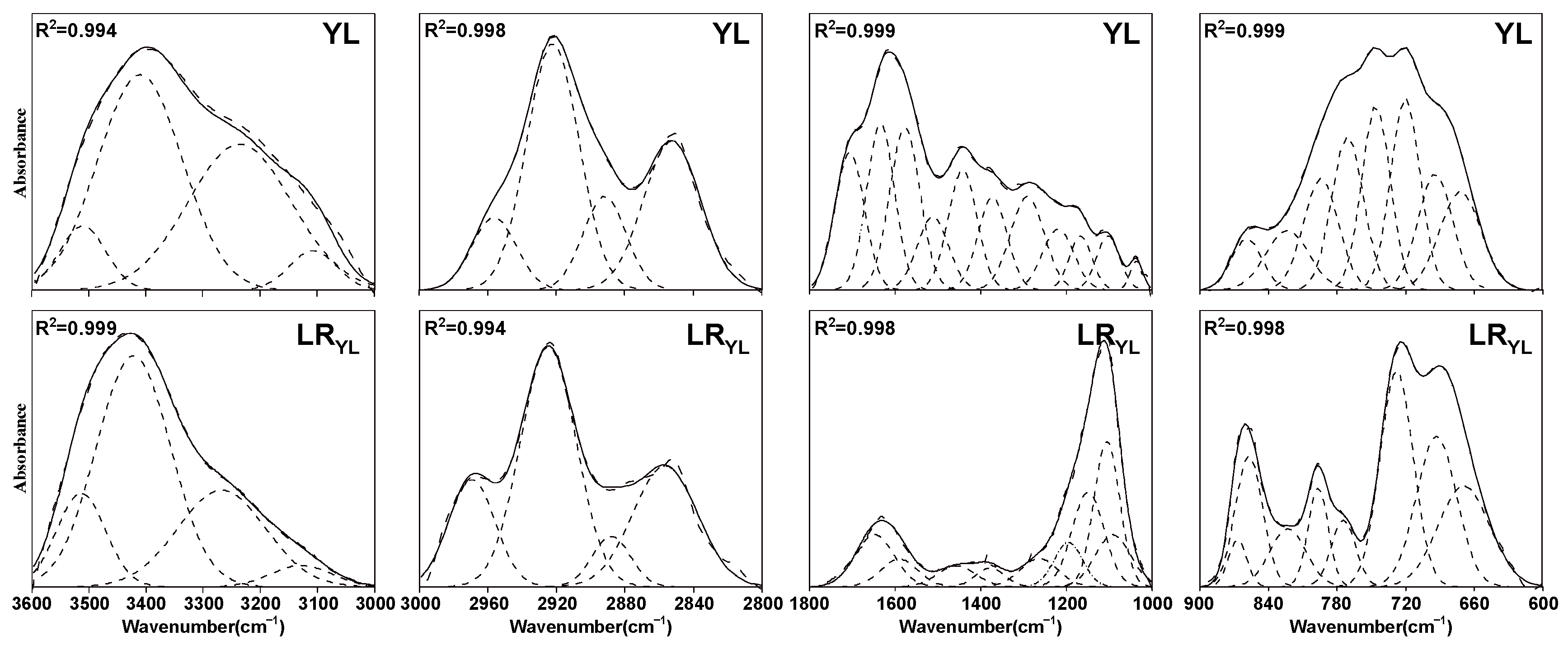
3.2.1. FTIR Profiles of the Samples
3.2.2. FTIR Semiquantitative Analysis
3.3. TG-DTG Analysis
3.4. TG-FTIR-GC/MS Test
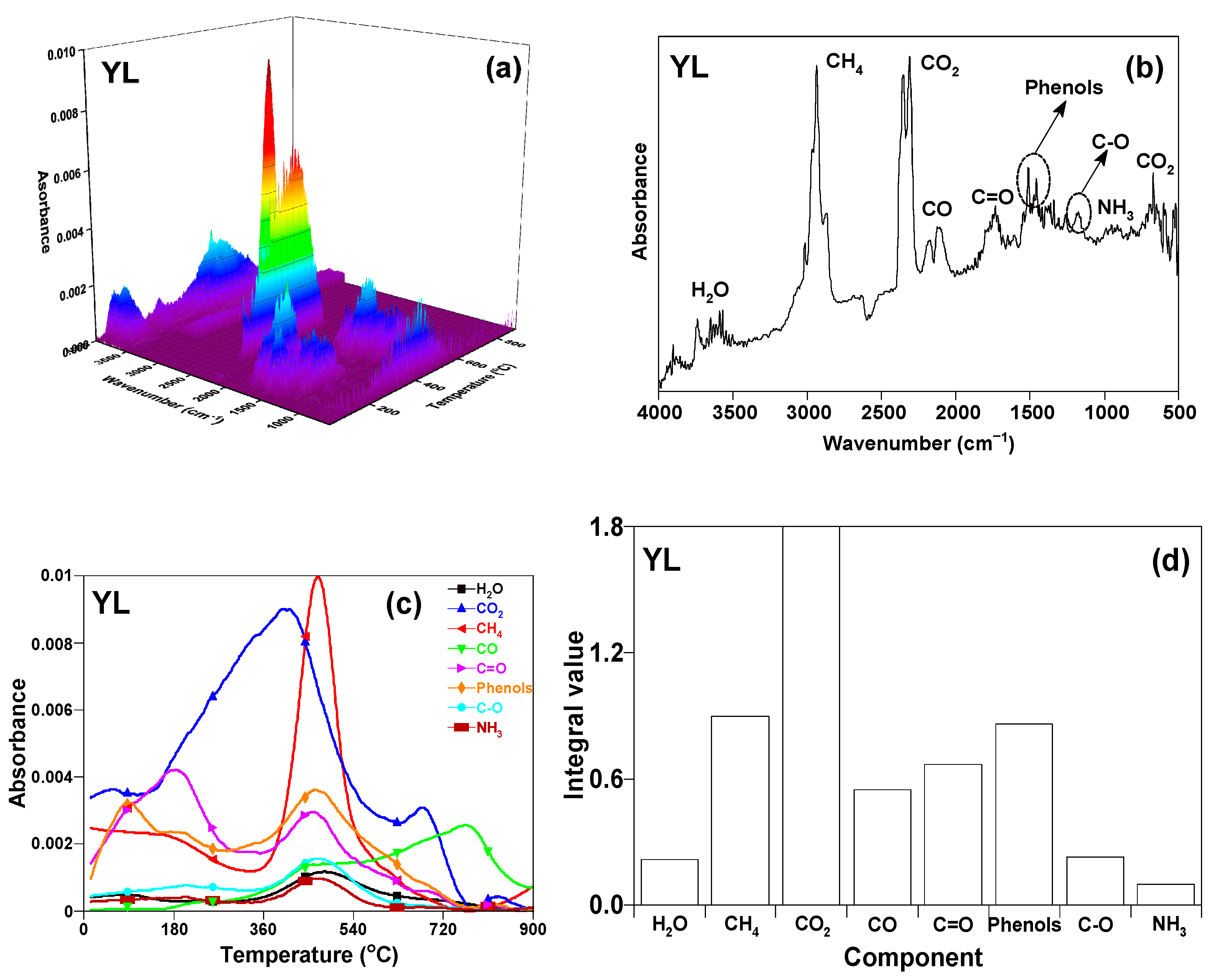
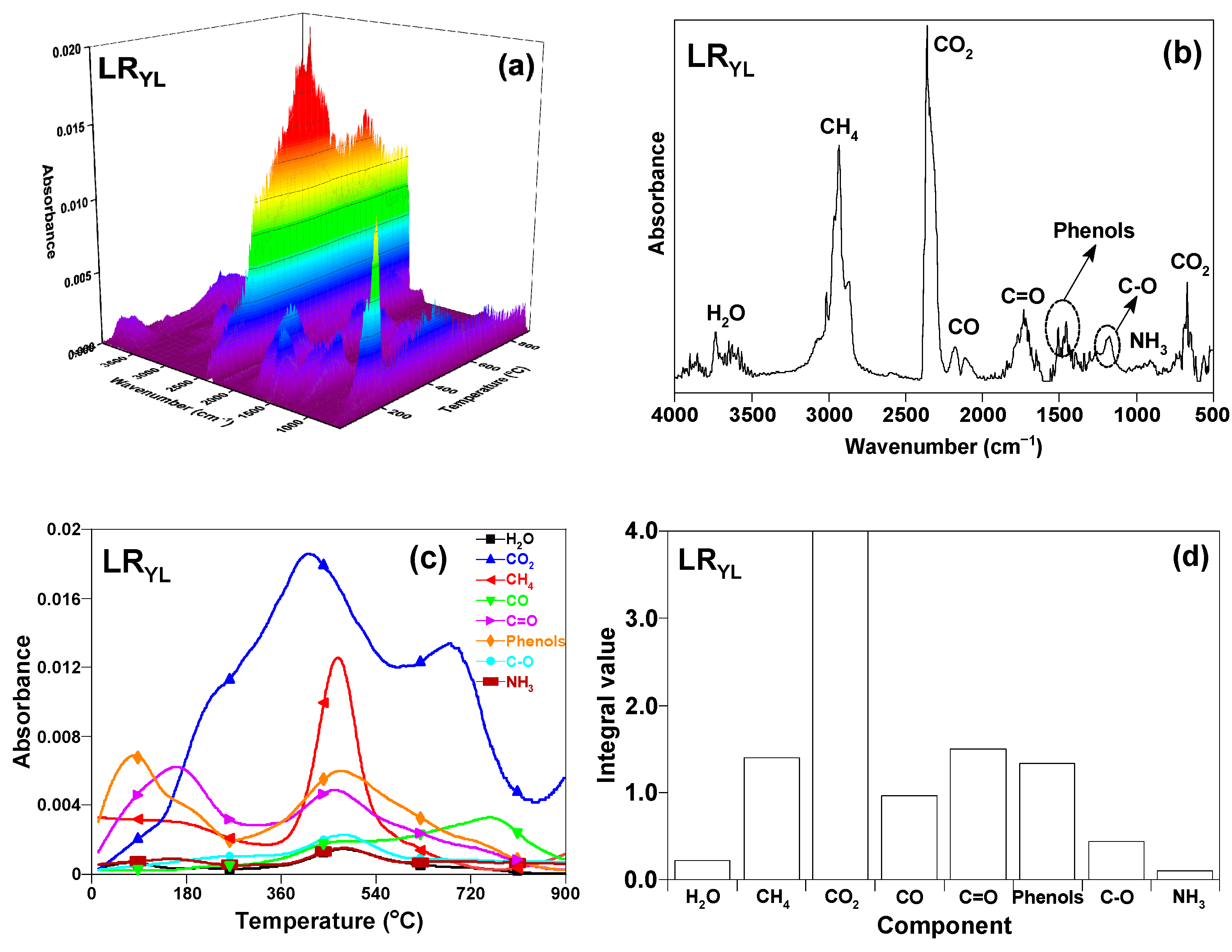
3.4.1. TG-FTIR Profile
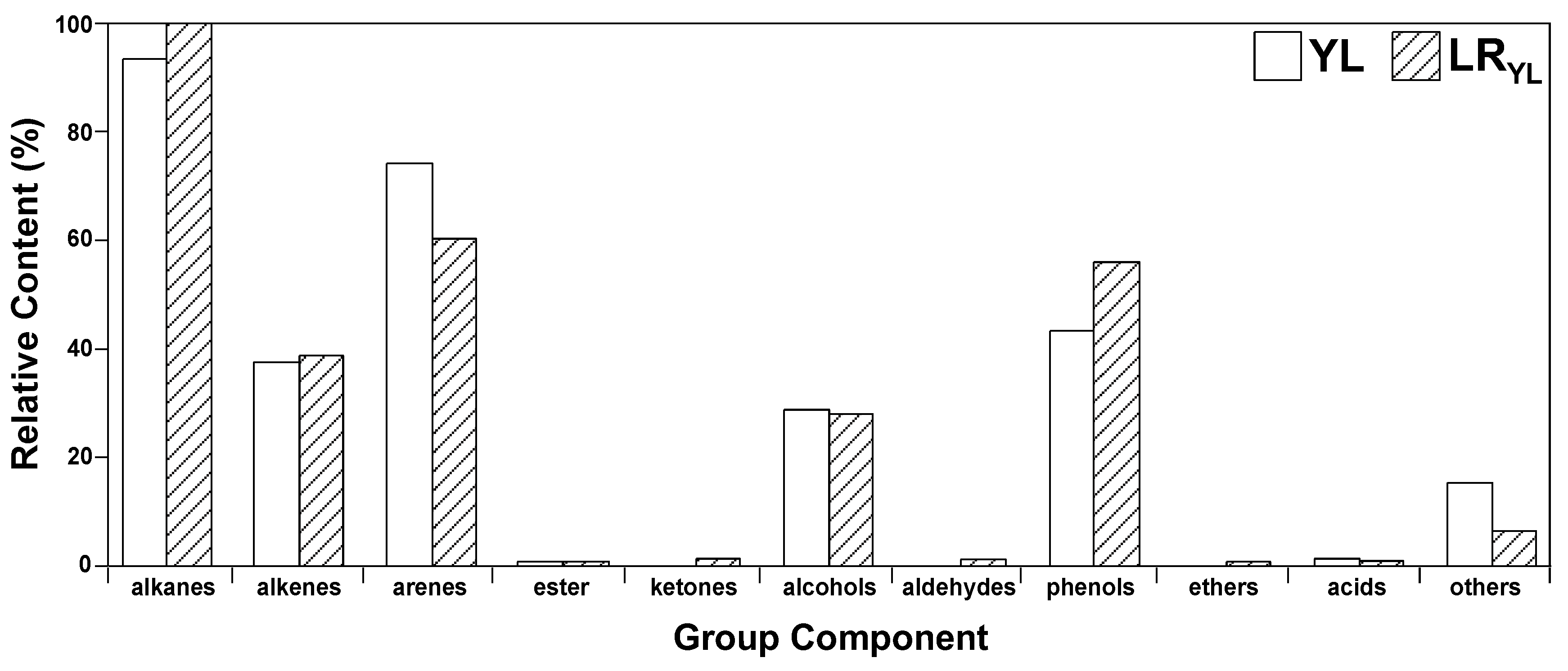
3.4.2. Flash Pyrolysis Products Distribution (GC/MS Results)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kan, H.; Wang, Y.; Mo, W.-L.; Wei, X.-Y.; Mi, H.-Y.; Ma, K.-J.; Zhu, M.-X.; Guo, W.-C.; Guo, J.; Niu, J.-M.; et al. Effect of solvent swelling with different enhancement method on the microstructure and pyrolysis performance of Hefeng subbituminous coal. Fuel 2023, 332, 126066. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Yu, J.-L.; Han, Y.-N.; Yin, F.-K.; Bhattacharya, S.; Stokie, D. Study of chemical structure changes of Chinese lignite upon drying in superheated steam, microwave, and hot air. Energy Fuels 2012, 26, 3651–3660. [Google Scholar] [CrossRef]
- Rathsack, P.; Kroll, M.-M.; Otto, M. Analysis of high molecular compounds in pyrolysis liquids from a german brown coal by FT-ICR-MS. Fuel 2014, 115, 461–468. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Tang, Y.-G.; Schobert, H.-H.; Guo, Y.-N.; Gao, W.-H.; Lu, X.-K. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. J. Anal. Appl. Pyrol. 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Hu, X.-B.; Xu, H.; Mo, W.-L.; Fan, X.; Guo, W.-C.; Guo, J.; Niu, J.-M.; Mi, H.-Y.; Ma, Y.-Y.; Wei, X.-Y. Effect of sequential thermal dissolution on structure and pyrolysis characteristics of Naomaohu lignite. Fuel 2023, 331, 125930. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zong, Z.-M.; Cao, J.-P.; Ma, Y.-M.; Han, L.; Liu, G.-F.; Zhao, W.; Li, W.-Y.; Xie, K.-C.; Bai, K.-F.; et al. Difference in chemical composition of carbon disulfide extractable fraction between vitrinite and inertinite from Shenfu-Dongsheng and Pingshuo coal. Fuel 2008, 87, 565–567. [Google Scholar] [CrossRef]
- Zhou, G.-L.; Wu, J.-J.; Miao, Z.-Y.; Hu, X.-L.; Li, X.; Shi, X.; Cai, Z.-D.; Shang, Y.-K. Effects of process paramenters on pore structure of semi-coke prepared by solid heat carrier with dry distination. Int. J. Min. Sci. Technol. 2013, 23, 423–428. [Google Scholar] [CrossRef]
- Li, X.; Priyanto, D.-E.; Ashida, R.; Miura, K. Two-stage conversion of low-rank coal or biomass into liquid fuel under mild conditions. Energy Fuels 2015, 29, 3127–3133. [Google Scholar] [CrossRef]
- Kong, J.; Wei, X.-Y.; Zhao, M.-X.; Li, Z.-K.; Yan, H.-L.; Zheng, Q.-X.; Zong, Z.-M. Effects of sequential extraction and thermal dissolution on the structure and composition of Buliangou subbituminous coal. Fuel Process. Technol. 2016, 148, 324–331. [Google Scholar] [CrossRef]
- Iino, M.; Takanohashi, T.; Ohsuga, H.; Toda, K. Extraction of coals with CS2/N–methyl-2-pyro lidinone mixed solvent at room temperature. Fuel 1988, 67, 1639–1647. [Google Scholar] [CrossRef]
- Cooke, N.-E.; Fuller, O.-M.; Gaikwad, R.-P. Ultrasonic extraction of coal. Fuel 1989, 68, 1227–1233. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Qiu, P.-H.; Lin, D.; Qian, J.; Hou, H.-M.; Pei, J.-T. Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks. Fuel 2018, 216, 521–530. [Google Scholar] [CrossRef]
- Jiang, Y.; Zong, P.-J.; Tian, B.; Xu, F.-F.; Tian, Y.-Y.; Qiao, Y.-Y.; Zhang, J.-H. Pyrolysis behaviors and product distribution of Shenmu coal at high heating rate: A study using TG-FTIR and Py-GC/MS. Fuel 2019, 179, 72–80. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Wei, X.-Y.; Mo, W.-L.; Kang, Y.-H.; Zhang, X.-Q.; Shan, X.-K.; Liu, G.-H.; Fan, X. Catalytic hydroconversion of the light residue from Yinggemajianfeng lignite over a solid superacid. Fuel 2022, 310, 122470. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Wei, X.-Y.; Li, Z.-K.; Zhang, D.-D.; Zong, Z.-M. Two-step depolymerization of Zhaotong lignite in ethanol. Fuel 2017, 196, 391–397. [Google Scholar] [CrossRef]
- He, X.-Q.; Liu, X.-F.; Nie, B.-S.; Song, D.-Z. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Xie, X.; Liu, L.; Lin, D.; Zhao, Y.; Qiu, P.-H. Influence of different state alkali and alkaline earth metal on chemical structure of Zhundong coal char pyrolyzed at elevated pressures. Fuel 2019, 254, 115691. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Qiu, P.-H.; Xie, X.; Chen, X.-Y.; Lin, D.; Sun, S.-Z. Impacts of chemical fractionation on Zhundong coal’s chemical structure and pyrolysis reactivity. Fuel Process. Technol. 2017, 155, 144–152. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhao, Y.; Liu, L.; Zhang, L.-Y.; Zhang, Z.; Qiu, P.-H. Evaluation of chemical structure, pyrolysis reactivity and gaseous products of Shenmu coal of different particle sizes. J. Anal. Appl. Pyrol. 2018, 130, 294–304. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Q.-Y.; Guo, X.-J.; Wu, W.-Z.; Liu, Z.-Y. Pyrolysis behavior and bonding information of coal—A TGA study. Fuel Process. Technol. 2013, 108, 125–132. [Google Scholar] [CrossRef]
- Cai, H.-M.; Liu, J.-Y.; Xie, W.-M.; Kuo, J.-H.; Buyukadaba, M.; Evrendilek, F. Pyrolytic kinetics, reaction mechanisms and products of waste tea via TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 184, 436–447. [Google Scholar] [CrossRef]
- Lei, Z.; Cheng, Z.-W.; Ling, Q.; Liu, X.-C.; Cui, P.; Zhao, Z.-G. Investigating the trigger mechanism of Shenfu bituminous coal pyrolysis. Fuel 2022, 313, 122995. [Google Scholar] [CrossRef]
- Qiang, L.-Y.; Bai, B.-Y.; Peng, Z.-W.; Zhang, S.-S.; Chang, H.; Su, M.; Xu, L.; Ma, X.-X. Research on the relationship between the structure and pyrolysis characteristics of pretreated Shendong coal. Fuel 2021, 305, 121515. [Google Scholar] [CrossRef]




| Sample | Proximate Analysis | Ultimate Analysis | H/C | O/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad # | C | H | N | S | O # | |||
| YL | 7.12 | 10.49 | 49.20 | 33.19 | 71.89 | 5.17 | 0.88 | 0.74 | 21.32 | 0.86 | 0.22 |
| LRYL | 6.79 | 6.53 | 43.91 | 42.77 | 57.63 | 4.26 | 2.7 | 0.54 | 34.87 | 1.03 | 0.53 |
| Wavenumber (cm−1) | Functional Group | Content (Area %) | |
|---|---|---|---|
| YL | LRYL | ||
| 3600–3500 | OH-π | 1.3545 | 2.4973 |
| 3500–3350 | Self-associated OH | 9.3762 | 12.0334 |
| 3350–3260 | OH-ether O | 7.0370 | 9.4466 |
| 3260–3170 | Cyclic OH | 0.8577 | 1.5695 |
| 2950–2930 | Asymmetric aliphatic –CH3 | 0.2727 | 0.3891 |
| 2930–2900 | Asymmetric aliphatic –CH2 | 1.4897 | 1.2416 |
| 2900–2870 | Aliphatic –CH | 0.4657 | 0.1348 |
| 2870–2850 | Symmetric aliphatic –CH2 | 0.9863 | 1.1413 |
| 1700 | Carboxylic acids C=O | 10.3221 | 1.6679 |
| 1650 | Conjugated C=O | 17.8629 | 3.9661 |
| 1600–1480 | C=C in ARs | 11.6004 | 9.9983 |
| 1480–1400 | Asymmetric –CH3, –CH2 | 8.9035 | 5.1319 |
| 1400–1240 | Symmetric –CH3 | 12.0257 | 9.2693 |
| 1240–1160 | Phenols C–OH | 8.0456 | 20.9652 |
| 1160–1090 | Grease C–O | 3.2081 | 14.5576 |
| 1090–1030 | Alkyl ethers | 3.9385 | 8.1450 |
| 900–860 | Five adjacent H deformation (1H) | 0.1110 | 0.1306 |
| 860–810 | Four adjacent H deformation (2H) | 0.7996 | 0.1693 |
| 810–750 | Three adjacent H deformation | 0.7713 | 0.2125 |
| 750–720 | Two adjacent H deformation | 0.5715 | 0.3327 |
| Index | Index Calculation | Assignment |
|---|---|---|
| Ia1 | Acaboxyl/Atotal | Carboxyl content |
| Ia2 | Aphenolic hydroxyl/Atotal | Phenolic hydroxyl content |
| Ia3 | Acarboxyl/Aphenolic hydroxyl | Relative change of carboxyl to phenolic hydroxyl |
| Ib1 | Aaliphatic hydrogen/Atotal | Aliphatic hydrogen content |
| Ib2 | Aaromatic hydrogen/Atotal | Aromatic hydrogen content |
| Ib3 | Aaliphatic hydrogen/Aaromatic hydrogen | Relative change of aliphatic to aromatic structure |
| Ic1 | ACH2/ACH3 | Length and branching degree of aliphatic side chain |
| Ic2 | Aaromatic hydrogen/Aaromatic ring c=c | Condensation degree of aromatic ring |
| Ic3 | A1H and 2H/Aaromatic hydrogen | Proportion of the aromatic rings with high substitution degree in the entire aromatic ring system |
| Sample | Ti (°C) | Tf (°C) | Tmax (°C) | ΔT1/2 (°C) | (dM/dT)max | (dM/dT)mean | D |
|---|---|---|---|---|---|---|---|
| YL | 200 | 700 | 450 | 67 | −0.1815 | −0.04812 | 1.44 × 10−9 |
| LRYL | 140 | 740 | 440 | 66 | −0.1716 | −0.04943 | 2.08 × 10−9 |
| Temperature Range (°C) | Possible Origin | Bond Energy (kJ·mol−1) |
|---|---|---|
| <300 | Release of bonded water and decomposition of carboxylic acid | <150 |
| 300–420 | Breakage of bonds between Cal and O, S and N, and S–S | 150–230 |
| 420–550 | Breakage of bonds between Cal and Cal, H, O, and Car–N | 210–320 |
| 550–650 | Breakage of bonds between Car and Cal, O, and S | 300–430 |
| 650–740 | Decomposition of carbonates in coals to generate CO2 | >400 |
| >700 | Polycondensation of Aromatic rings to generate H2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, W.-L.; Kan, H.; Wu, T.; Hu, X.-B.; Ma, Y.-Y.; Guo, J.; Guo, W.-C.; Wei, X.-Y.; Akram, N. Effect of Solvent Pretreatment on the Flash Pyrolysis Performance of Yinggema Lignite. Sustainability 2023, 15, 11760. https://doi.org/10.3390/su151511760
Mo W-L, Kan H, Wu T, Hu X-B, Ma Y-Y, Guo J, Guo W-C, Wei X-Y, Akram N. Effect of Solvent Pretreatment on the Flash Pyrolysis Performance of Yinggema Lignite. Sustainability. 2023; 15(15):11760. https://doi.org/10.3390/su151511760
Chicago/Turabian StyleMo, Wen-Long, Hui Kan, Ting Wu, Xiao-Bo Hu, Ya-Ya Ma, Jia Guo, Wen-Cang Guo, Xian-Yong Wei, and Naeem Akram. 2023. "Effect of Solvent Pretreatment on the Flash Pyrolysis Performance of Yinggema Lignite" Sustainability 15, no. 15: 11760. https://doi.org/10.3390/su151511760
APA StyleMo, W.-L., Kan, H., Wu, T., Hu, X.-B., Ma, Y.-Y., Guo, J., Guo, W.-C., Wei, X.-Y., & Akram, N. (2023). Effect of Solvent Pretreatment on the Flash Pyrolysis Performance of Yinggema Lignite. Sustainability, 15(15), 11760. https://doi.org/10.3390/su151511760







