Effects of Climate-Change-Related Phenomena on Coastal Ecosystems in the Mexican Caribbean
Abstract
:1. Introduction
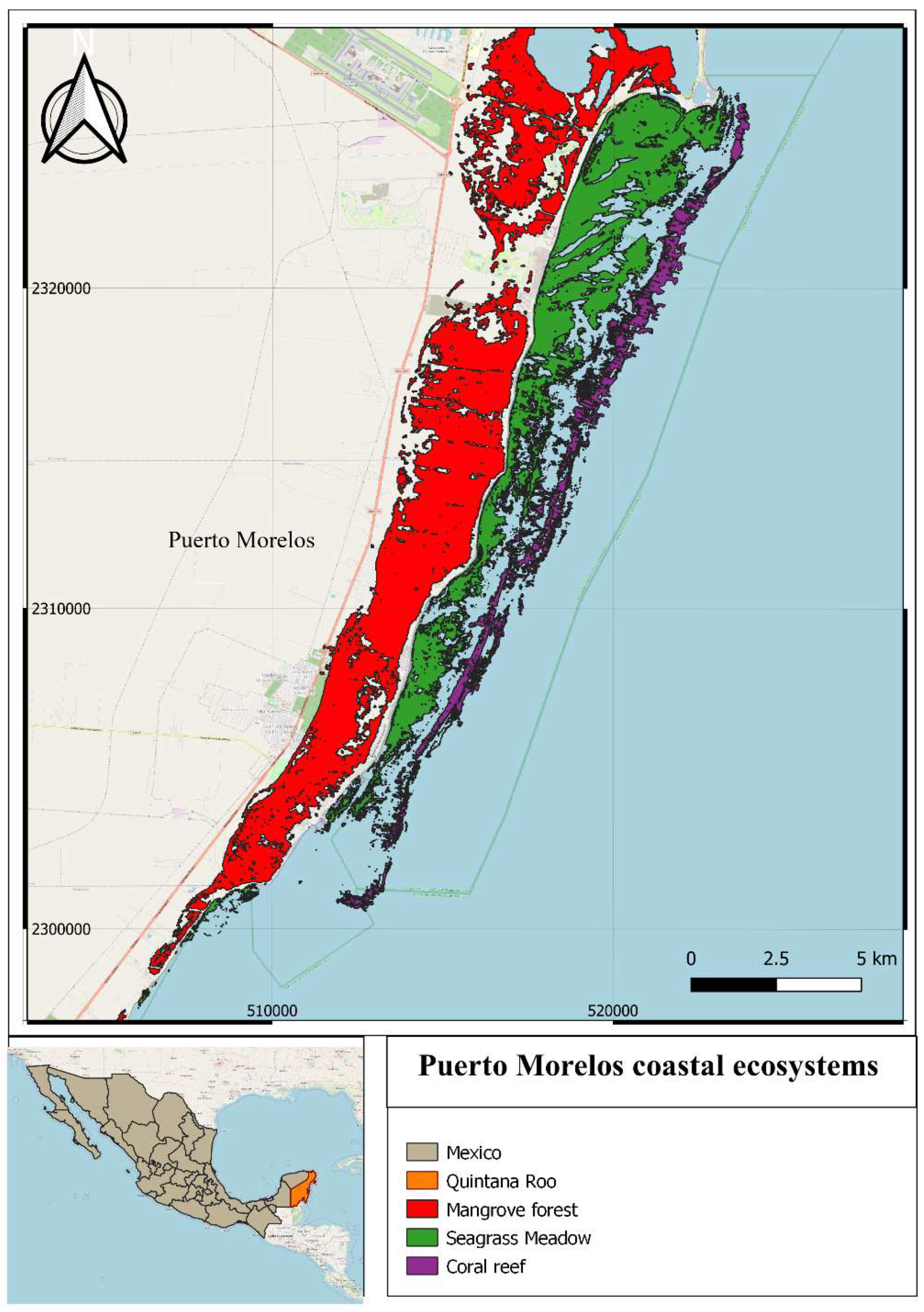
2. Study Area
3. Data and Methods
3.1. Data Sources
3.2. Data Analysis
4. Results
4.1. General Characterization of Climate Variables
4.2. Climate Change
4.2.1. Sea Level Anomalies (SLA)
4.2.2. Sea Surface Temperature (SST)
4.2.3. Atmospheric Pressure (AP)
4.2.4. Wind, Waves and Storms
4.2.5. Precipitation (P)
4.3. Yucatan Peninsula Tectonic Activity
4.4. The Present State of the Ecosystems
5. Discussion
| Variable | Global/Regional Trend (Per Year) | Local Trend (Per Year) | Observations |
|---|---|---|---|
| Sea Level Rise | +3.9 mm | +2.5 mm | Regional SLR for the north Atlantic and Caribbean [8] |
| Sea Surface Temperature | +1.5–4.0 °C * | +0.4–1.2 °C * | [8] gives scenarios for annual global sea SST |
| Atmospheric Pressure | no change | no change | Global trend from [52] |
| Wind | no change | no change | Similar results to regional data [52] |
| Waves | no change | no change | Similar results to regional data [52] |
| Precipitation | no change | no change | Similar results to regional data [53] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Name | Category | Date | Wind Speed(m/s) | Influence Radius (km) | Influence Time (Hours) | Persistence (Hours) |
|---|---|---|---|---|---|---|---|
| 1852 | Not named | H2 | 8-oct | 90 | 100 | 6 | 6 |
| 1857 | Not named | H2 | 28-sep | 90 | 100 | 6 | |
| 1857 | Not named | H1 | 26-sep | 75 | 80 | 6 | |
| 1857 | Not named | H1 | 26-sep | 75 | 80 | 12 | |
| 1857 | Not named | H1 | 19-aug | 65 | 80 | 12 | 36 |
| 1870 | Not named | TS | 02-nov | 50 | 40 | 2 | 2 |
| 1873 | Not named | H1 | 05-oct | 80 | 80 | 18 | |
| 1873 | Not named | H1 | 05-oct | 80 | 80 | 6 | 24 |
| 1877 | Not named | H1 | 29-sep | 70 | 80 | 8 | 8 |
| 1880 | Not named | H2 | 09-aug | 90 | 100 | 6 | |
| 1880 | Not named | H1 | 09-aug | 70 | 80 | 2 | |
| 1880 | Not named | TS | 06-oct | 50 | 40 | 4 | 12 |
| 1881 | Not named | TS | 16-aug | 40 | 40 | 2 | 2 |
| 1887 | Not named | H2 | 17-sep | 85 | 100 | 6 | |
| 1887 | Not named | H1 | 17-sep | 75 | 80 | 1 | 7 |
| 1888 | Not named | H1 | 06-sep | 70 | 80 | 3 | |
| 1888 | Not named | TS | 09-oct | 60 | 40 | 2 | 5 |
| 1891 | Not named | H4 | 10-oct | 110 | 150 | 18 | |
| 1891 | Not named | H2 | 10-oct | 85 | 100 | 12 | 30 |
| 1893 | Not named | H2 | 29-sep | 85 | 100 | 6 | |
| 1893 | Not named | H1 | 29-sep | 80 | 80 | 6 | 12 |
| 1895 | Not named | H2 | 27-aug | 85 | 100 | 6 | 6 |
| 1903 | Not named | H2 | 13-aug | 85 | 100 | 6 | 6 |
| 1909 | Not named | H3 | 25-aug | 90 | 100 | 6 | |
| 1909 | Not named | TS | 08-aug | 40 | 40 | 4 | 10 |
| 1912 | Not named | TS | 13-oct | 55 | 40 | 3 | 3 |
| 1913 | Not named | TS | 25-jun | 50 | 40 | 3 | 3 |
| 1916 | Not named | H1 | 03-jul | 65 | 80 | 4 | 4 |
| 1922 | Not named | H2 | 18-oct | 95 | 100 | 12 | 12 |
| 1931 | Not named | TS | 25-jun | 45 | 40 | 1 | 1 |
| 1933 | Not named | H4 | 22-sep | 125 | 150 | 12 | 12 |
| 1935 | Not named | TD | 30-aug | 25 | 40 | 0.5 | 0.5 |
| 1936 | Not named | TS | 15-aug | 35 | 40 | 4 | |
| 1936 | Not named | TS | 13-jul | 40 | 40 | 3 | 7 |
| 1938 | Not named | H3 | 26-aug | 105 | 100 | 6 | 6 |
| 1942 | Not named | H2 | 28-aug | 90 | 100 | 6 | 6 |
| 1944 | Not named | H1 | 20-sep | 70 | 80 | 6 | 6 |
| 1951 | Charlie | H4 | 20-aug | 115 | 150 | 6 | 6 |
| 1964 | Not named | TD | 04-jun | 30 | 40 | 6 | 6 |
| 1967 | Beulah | H2 | 17-sep | 90 | 100 | 6 | 6 |
| 1969 | Not named | TD | 12–13jun | 25 | 40 | 18 | 18 |
| 1973 | Delia | TD | 02-sep | 30 | 40 | 6 | 6 |
| 1974 | Not named | TD | 24-sep | 30 | 40 | 4 | 4 |
| 1975 | Eloise | TS | 21-sep | 45 | 40 | 5 | |
| 1975 | Not named | TD | 10-nov | 30 | 40 | 4 | 9 |
| 1979 | Henri | TD | 15-sep | 25 | 40 | 3 | 3 |
| 1980 | Allen | H5 | 07-aug | 165 | 200 | 6 | 6 |
| 1988 | Gilbert | H5 | 09-sep | 160 | 200 | 12 | |
| 1988 | Keith | TS | 21-nov | 60 | 40 | 2 | 14 |
| 1992 | Isidore | TD | 24sep | 38 | 60 | 12 | 36 |
| 1995 | Roxane | H3 | 10–11 oct | 100 | 100 | 3 | 3 |
| 2003 | Claudette | TS | 11-jul | 50 | 40 | 6 | 6 |
| 2004 | Ivan | H5 | 14-sep | 145 | 200 | 1 | 1 |
| 2005 | Emily | H4 | 18-jul | 115 | 150 | 1 | |
| 2005 | Wilma | H4 | 22-oct | 115 | 150 | 48 | 49 |
| 2007 | Olga | TD | 15-dec | 30 | 40 | 3 | 3 |
| 2008 | Dolly | TS | 21-jul | 45 | 40 | 1 | 1 |
| 2010 | Paula | H2 | 13-oct | 85 | 100 | 6 | 6 |
| 2011 | Rina | TS | 28-oct | 50 | 40 | 4 | 4 |
| 2020 | Delta | H2 | 07-oct | 90 | 100 | 2 | |
| 2020 | Zeta | H1 | 27-oct | 75 | 80 | 1 | 3 |
Appendix B
The State and Changes in the Coastal Ecosystems of Puerto Morelos
- Mangroves



- 2.
- Coastal Dunes


- 3.
- Shoreline dynamics

- 4.
- Seagrass meadows


- 5.
- Coral Reefs


References
- Coastal Engineering Research Center [CERC]. Coastal Engineering Manual; Coastal Engineering Research Center: Ft. Belvoir, VA, USA, 2000. [Google Scholar]
- Silva-Casarín, R.; Villatoro Lacouture, M.M.; Ramos Durón, F.J.; Pedroza Paez, D.; Ortiz Perez, M.A.; Mendoza Baldwin, E.G.; Delgadillo Calzadilla, M.A.; Escudero Castillo, M.C.; Féliz Delgado, A.; Cid Salinas, A. Caracterización de la Zona Costera y Plantemaineto de Elementos Técnicos para la Elaboración de Criterios de Regulación y Manejo Sustentable; UNAM—Instituto de Ingeniería: Ciudad de México, México, 2014; pp. 11–62. [Google Scholar]
- Burkett, V.; Codignotto, J.O.; Forbes, D.L.; Mimura, N.; Beamish, R.J.; Ittekkot, V. IPCC. In Climate Change Impacts, Adaptation and Vulnerability; McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; pp. 23–101. [Google Scholar]
- Cuellar-Martínez, T.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.; López-Mendoza, P.G.; Carnero-Bravo, V.; Agraz-Hernández, C.M.; van Tussenbroek, B.I.; Sandoval-Gil, J.; Cardoso-Mohedano, J.G.; et al. Temporal records of organic carbon stocks and burial rates in Mexican blue carbon coastal ecosystems throughout the Anthropocene. Glob. Planet. Chang. 2020, 192, 103215. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Martínez, M.L.; Silva, R.; López-Portillo, J.; Feagin, R.A.; Martínez, E. Coastal ecosystems as an ecological membrane. J. Coast Res. 2020, 95, 97–101. [Google Scholar] [CrossRef]
- Natural Resources Defense Council. Available online: https://www.nrdc.org/ (accessed on 31 March 2022).
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 325–360. [Google Scholar]
- Silva, R.; Martínez, M.L.; Van Tussenbroek, B.I.; Guzmán-rodríguez, L.O.; Mendoza, E.; López-portillo, J. A framework to manage coastal squeeze. Sustainability 2020, 12, 10610. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar] [CrossRef] [Green Version]
- Pedrozo-Acuña, A.; Damania, R.; Laverde-Barajas, M.A.; Mira-Salama, D. Assessing the consequences of sea-level rise in the coastal zone of Quintana Roo, México: The costs of inaction. J. Coast. Conserv. 2015, 19, 227–240. [Google Scholar] [CrossRef]
- Pfeffer, W.T.; Harper, J.T.; O’Neel, S. Kinematic constraints on glacier contributions to 21st-century sea level rise. Science 2008, 321, 1340–1343. [Google Scholar] [CrossRef]
- Ruíz-Ramírez, J.D.; Euán-Ávila, J.I.; Rivera-Monroy, V.H. Vulnerability of Coastal Resort Cities to Mean Sea Level Rise in the Mexican Caribbean. Coast. Manag. 2019, 47, 23–43. [Google Scholar] [CrossRef]
- Boretti, A. A realistic expectation of sea level rise in the Mexican Caribbean. J. Ocean. Eng. Sci. 2019, 4, 379–386. [Google Scholar] [CrossRef]
- Gómez, I.; Silva, R.; Lithgow, D.; Rodríguez, J.; Banaszak, A.T.; van Tussenbroek, B. A Review of Disturbances to the Ecosystems of the Mexican Caribbean, Their Causes and Consequences. J. Mar. Sci. Eng. 2022, 10, 644. [Google Scholar] [CrossRef]
- White, P.S.; Pickett, S. Natural disturbance and patch dynamics: An introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: New York, NY, USA, 1985; pp. 3–13. [Google Scholar]
- Cazenave, A.; Nerem, R.S. Present-day sea level change: Observations and causes. Rev. Geoph. 2004, 42, 1–20. [Google Scholar] [CrossRef]
- McLean, R.F.; Tsyban, A.; Burkett, V.; Codignott, J.O.; Forbes, D.L.; Mimura, N.; Beamish, R.J.; Ittekkot, V. Coastal Zones and Marine Ecosystems. In Climate Change 2001: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 343–379. [Google Scholar]
- Pacchiano Alemán, R. Programa de Manejo Parque Nacional Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc; SEMARNAT-Secretaría de Medio Ambiente y Recursos Naturales: Diario Oficial de la Federación. México. 2016; Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5446471&fecha=02/08/2016#gsc.tab=0 (accessed on 30 May 2020).
- Sánchez-Quinto, A.; Costa, J.C.; da Zamboni, N.S.; Sanches, F.H.C.; Principe, S.C.; Viotto, E.V.; Faroni-Perez, L. Development of a conceptual framework for the management of biodiversity and ecosystem services in the Mexican Caribbean. Biota Neotrop. 2020, 20, 1–18. [Google Scholar] [CrossRef]
- SIMAR-CONABIO. Available online: https://simar.conabio.gob.mx/ (accessed on 28 June 2022).
- Carabias Lillo, J.; Provencio, E.; De la Maza Elvira, J.; Gutiérrez Carbonell, D.; Gómez Cruz, M. Programa de Manejo Parque Nacional Arrecife de Puerto Morelos; CONANP: Ciudad de México, México, 2000; p. 224. [Google Scholar]
- INEGI. Censos Económicos 2014. Available online: http://www.inegi.org.mx/ (accessed on 4 May 2021).
- Copernicus Climate Change Service. Available online: https://cds.climate.copernicus.eu/ (accessed on 10 April 2020).
- Servicio Académico de Monitoreo Meteorológico Oceanográfico. Available online: https://sammo.icmyl.unam.mx/ (accessed on 10 November 2020).
- Historical Hurricane Tracks. Available online: https://coast.noaa.gov/ (accessed on 15 March 2022).
- CONABIO Geoinformation Portal. Available online: http://geoportal.conabio.gob.mx (accessed on 23 June 2022).
- Olauson, J. ERA5: The new champion of wind power modelling? Renew. Energy 2018, 126, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Odériz, I.; Silva, R.; Mortlock, T.R.; Mendoza, E. Climate Drivers of Directional Wave Power on the Mexican Coast. Ocean Dyn. 2020, 70, 1253–1265. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Kopp, R.E.; Hay, C.C.; Little, C.M.; Mitrovica, J.X. Geographic Variability of Sea-Level Change. Curr. Clim. Chang. Rep. 2015, 1, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Olivera Gómez, A.Y. Reconstrucción paleo ecológica del sur de Quintana Roo, México, durante el Holoceno. In Contribuciones De La Ciencia Al Manejo Costero Integrado De La BAHÍA De Chetumal Y Su Área De Influencia; Rosado-May, F.J., Romero Mayo y, A., R. Navarrete, D.J., Eds.; Universidad de Quintana Roo, Q. Roo: Chetumal, México, 2002; pp. 5–15. [Google Scholar]
- Lugo-Hubp, J.; Aceves-Quesada, J.F.; Espinasa-Pereña, R. Rasgos geomorfológicos mayores de la península de Yucatán. Rev. Del Inst. De Geol. 1992, 10, 143–150. [Google Scholar]
- Sapper, K. Geología de la Península de Yucatán. In Enciclopedia Yucatanense; Enciclopedia Yucatanense: Mérida, México, 1945; Volume 1, pp. 207–213. [Google Scholar]
- Robles-Ramos, R. Geología y Geohidrología. In Los Recursos Del Sureste Y Su Aprovechamiento; Instituto Mexicano de Recursos Naturales Renovables: Ciudad de México, México, 1958; pp. 55–92. [Google Scholar]
- Espejel, I. A Phytogeographical Analysis of Coastal Vegetation in the Yucatan Peninsula. J. Biogeogr. 1987, 14, 499. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Silveira, J.A.; Teutli-Hernández, C.; Zaldívar-Jiménez, A.; Pérez- Ceballos, R.; Cortés-Balán, O.; Osorio-Moreno, I.; Ramírez-Ramírez, J.; Caamal-Sosa, J.; Andueza-Briceño, M.T.; Torres, R.y.H. Hernández-Aranda. Programa Regional para la Caracterización y el Monitoreo de Ecosistemas de Manglar del Golfo de México y Caribe Mexicano: Península de Yucatán. Centro de Investigación y de Estudios Avanzados-Mérida. SNIB-CONABIO, proyecto No. FN009. México city. 2014. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfFN009.pdf (accessed on 30 May 2020).
- Martínez, M.; Moreno-Casasola, P.; Espejel, I.; Jiménez-Orocio, O.; Infante-Mata, D.; Rodríguez-Revelo, N. Diagnóstico General de las Dunas Costeras de México, Comisión Nacional Forestal; SEMARNAT: México City, México, 2014; p. 359. [Google Scholar]
- Escudero, M.; Reguero, B.G.; Mendoza, E.; Secaira, F.; Silva, R. Coral Reef Geometry and Hydrodynamics in Beach Erosion Control in North Quintana Roo, Mexico. Front. Mar. Sci. 2021, 8, 1–35. [Google Scholar] [CrossRef]
- Perera-Valderrama, S.; Cerdeira-Estrada, S.; Martell-Dubois, R.; Rosique-de la Cruz, L.O.; Caballero-Aragón, H.; Ressl, R.; Santamaría-del Ángel, E.; Álvarez-Filip, L.; Reyes-Bonilla, H.; Alva-Basurto, J.C.; et al. Protocolos De Monitoreo De La Biodiversidad Marina En Áreas Naturales Protegidas Del Caribe Mexicano; Ressl, H., Ed.; CONABIO: México City, México, 2020; p. 156. [Google Scholar] [CrossRef]
- van Tussenbroek Brigitta, I. Dynamics of seagrasses and associated algae in coral reef lagoons. Hidrobiológica 2011, 21, 293–310. [Google Scholar]
- de Almeida, L.R.; Ávila Mosqueda, S.V.; Silva, R.; Mendoza, E.; van Tussenbroek, B.I. Mapping the structure of mixed seagrasss meadows in the Mexican Caribbean. Front. Mar. Sci. 2022, 9, 1063007. [Google Scholar] [CrossRef]
- 43. Wilkinson, T.; Wiken, E.; Creel, J.B.; Hourigan, T.; Agardy, T.; Herrmann, H.; Janishevski, L.; Madden, C.; Morgan, L.; Padilla, M. Ecorregiones marinas de América del Norte; Comisión para la Cooperación Ambiental: Montreal, QC, Canada, 2009; p. 200. [Google Scholar]
- Álvarez-Filip, L.; Estrada-Saldívar, N.; Pérez-Cervantes, E.; Molina-Hernández, A.; González-Barrios, F.J. A rapid spread of the stony coral tissue loss disease out-break in the Mexican Caribbean. PeerJ 2019, 7, e8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, J.L. Dinámica de emersión del suelo y sucesión de la vegetación en el Arrecife Alacranes del Canal de Yucatán. Biótica 1984, 9, 41–64. [Google Scholar]
- Coke, J.G. Underwater Caves of the Yucatan Peninsula. In Encyclopedia of Caves, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Moseley, G.E.; Smart, P.L.; Richards, D.A.; Hoffmann, D.L. Speleothem constraints on marine isotope stage (MIS) 5 relative 616 sea levels, Yucatan Peninsula, Mexico. J. Quat. Sci 2013, 28, 293–300. [Google Scholar] [CrossRef]
- Sánchez, R.J.P. Evolución geológica del sureste mexicano, Golfo de México. Boletín De La Soc. Geológica Mex. 2007, 19–42. [Google Scholar] [CrossRef]
- Simms, A.R. Last interglacial sea levels within the Gulf of Mexico and northwestern Caribbean Sea. Earth Syst. Sci. Data 2020, 620, 1419–1439. [Google Scholar] [CrossRef]
- Otvos, E.G. Tectonic lineaments of Pliocene and Quaternary shorelines, northeast Gulf Coast. Geology 1981, 9, 398–404. [Google Scholar] [CrossRef]
- INAPESCA. Available online: https://www.gob.mx/inapesca (accessed on 2 February 2022).
- Rioja-Nieto, R.; Garza-Pérez, R.; Á lvarez-Filip, L.; Mariño-Tapia, I.; Enríquez, C. The Mexican caribbean: From Xcalak to holbox. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 637–653. [Google Scholar]
- Rodríguez-Martínez, R.E.; Ruíz-Rentería, F.; van Tussenbroek, B.; Barba-Santos, G.; Escalante-Mancera, E.; Jordán-Garza, G.E. Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Revista de Biología Tropical 2010, 58, 23–43. [Google Scholar]
- Kuffner, I.B. Sea-level rise could overwhelm coral reefs Frictionless when flat. Nature 2018, 558, 378–379. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.; Egnotovich, M.; Ford, M.; Platt, W. Regeneration in fringe mangrove forests damaged by hurricane Andrew. Plant Ecol. 2001, 157, 151–164. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Barba Santos, M.G.; Van Dijk, J.K.; Sanabria Alcaraz, S.M.; Téllez Calderón, M.L. Selective elimination of rooted plants from a tropical seagrass bed in a back-reef lagoon: A hypothesis tested by hurricane Wilma (2005). J. Coast Res. 2008, 24, 278–281. [Google Scholar] [CrossRef]
- van Tussenbroek Brigitta, I.; Cortés, J.; Collin, R.; Fonseca, A.C.; Gayle, P.M.H.; Guzmán, H.M.; Jácome, G.E.; Juman, R.; Koltes, K.H.; Oxenford, H.A. Caribbean-wide, long-term study of seagrass beds reveals local variations, shifts in community structure and occasional collapse. PLoS ONE 2014, 9, e90600. [Google Scholar] [CrossRef] [Green Version]
- van Tussenbroek, B.I.; Hernández-Arana, H.A.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; Gon-zález-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda Collado-Vides, A.L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Poll. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ávila Mosqueda, S.V. Variabilidad Espacial en Comunidades de Pastos Marinos Asociada a Afluencias Masivas de Sargassum spp. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2021. Available online: http://132.248.9.195/ptd2021/mayo/0811636/In-65dex.html (accessed on 17 December 2021).
- Ladd, M.C.; Collado-Vides, L. Practical applications of monitoring results to improve managing for coral reef resilience: A case study in the Mexican Caribbean. Biodivers. Conserv. 2013, 22, 1591–1608. [Google Scholar] [CrossRef]
- Wang, C.; Enfield, D.B. The tropical Western Hemisphere warm pool. Geoph. Res. Lett. 2001, 28, 1635–1638. [Google Scholar] [CrossRef]
- Naomi, O. The Scientific Consensus on Climate Change: How do We Know We’re not Wrong? Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Dakos, V.; Matthews, B.; Hendry, A.P.; Levine, J.; Loeuille, N.; Norberg, J.; Nosil, P.; Scheffer, M.; De Meester, L. Ecosystem tipping points in an evolving world. Nat. Ecol. Evol. 2019, 3, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Tartaglione, C.A.; Smith, S.R.; O’Brien, J.J. ENSO impact on hurricane landfall probabilities for the Caribbean. J. Clim. 2003, 16, 2925–2931. [Google Scholar] [CrossRef]
- Islebe, G.; Sánchez, O. History of late holocene vegetation at quintana roo, caribbean coast of mexico. Plant Ecol. 2002, 670, 187–192. [Google Scholar] [CrossRef]
- Martínez, M.L.; Lithgow, D.; Vázquez, G.; García Franco, J.G. Diagnóstico de las necesidades y probabilidades de restauración en las dunas costeras de Quintana Roo. In Experiencias Mexicanas en la Restauración de los Ecosistemas; Chapter: 18. Eds Eliane Ceccon, Cristina Martínez; UNAM/CRIM/UAEM/CONABIO: México City, México, 2016; pp. 409–430. [Google Scholar]
- Ruíz-Rentería, F.; van Tussenbroek, B.I.; Jordan-Dahlgren, E. Puerto Morelos, Quintana Roo, Mexico. In Caricomp—Caribbean Coral Reef, Seagrass and Mangrove Sites; Kjerfve, B., Ed.; Unesco: Paris, France, 1998; pp. 57–66. [Google Scholar]
- 68. López-Mendoza, P.G.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; van Tussenbroek, B.I.; Cuellar-Martinez, T.; Pérez-Bernal, L.H. Temporal trends of organic carbon accumulation in seagrass meadows from the northern Mexican Caribbean. Catena 2020, 194, 104645. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Lira-Noriega, A.; González-Barrios, F.J.; Reyes-Bonilla, H.; Alvarez-Filip, L. Functional divergence from ecological baselines on Caribbean coral reefs. Ecography 2022, 3, e05811. [Google Scholar] [CrossRef]
- Coral reefs coverage map of the Mexican Caribbean: Cabo Catoche – Xcalak. Available online: https://simar.conabio.gob.mx/arrecifesam/ (accessed on 28 June 2022).
- Caballero-Aragón, H.; Perera-Valderrama, S.; Cerdeira-Estrada, S.; Martell-Dubois, R.; Rosique-de la Cruz, L.; Alvarez-Filip, L.; Ressl, R. Puerto Morelos coral reefs, their current state and classification by a scoring system. Diversity 2020, 12, 272. [Google Scholar] [CrossRef]
- Caballero-Aragón, H.; Perera-Valderrama, S.; Cerdeira-Estrada, S.; Martell-Dubois, R.; Rosique-de la Cruz, L.; Á lvarez-Filip, L.; Pérez-Cervantes, E.; Estrada-Saldivar, N.; Ressl, R. Dataset of coral reefs monitoring, Puerto Morelos, Mexico. Data Brief. 2022, 42, 108253. [Google Scholar] [CrossRef] [PubMed]
- McField, M.; Kramer, P.; Giró Petersen, A.; Soto, M.; Drysdale, I.; Craig, N.; Rueda-Flores, M. Mesoamerican Reef Report Card. 2020. Available online: https://www.researchgate.net/publication/322603385_2018_Mesoamerican_Reef_Report_Card?channel=doi&linkId=5a6254b20f7e9b6b8fd64421&showFulltext=true (accessed on 15 March 2023).
- Roff, G.; Joseph, J.; Mumby, P. Multi-decadal changes in structural complexity following mass coral mortality on a Caribbean 701 reef. Bio-geosciences 2020, 17, 5909–5918. [Google Scholar]
- Weil, E. Coral reef diseases in the Wider Caribbean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Antonius, A. The “band” diseases in coral reefs. In Proceedings of the 4th International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981; pp. 7–14. [Google Scholar]
- Antonius, A. Coral diseases in the Indo-Pacific: A first record. Mar. Ecol. 1985, 6, 197–218. [Google Scholar] [CrossRef]
- Rutzler, K.; Santavy, D.; Antonius, A. The black band disease of Atlantic reef corals. III. Distribution, ecology and development. Mar. Ecol. 1983, 4, 329–358. [Google Scholar] [CrossRef]
- Edmunds, P.J. Extent and effect of black band disease on a Caribbean reef. Coral Reefs. 1991, 10, 161–165. [Google Scholar] [CrossRef]
- Carlton, R.G.; Richardson, L.L. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: Black band disease of corals. FEMS Microbiol. Ecol. 1995, 18, 155–162. [Google Scholar] [CrossRef]
- Kuta, K.G.; Richardson, L.L. Abundance and distribution of black band disease on coral reefs in the northern Florida Keys. Coral Reefs 1996, 15, 219–223. [Google Scholar] [CrossRef]
- Kuta, K.G.; Richardson, L.L. Ecological aspects of black band disease of corals: Relationships between disease incidence and environmental factors. Coral Reefs 2002, 21, 393–398. [Google Scholar] [CrossRef]
- Richardson, L.L.; Kuta, K.G. Ecological physiology of the black band cyanobacterium Phormidium corallyticum. FEMS Microbiol. Ecol. 2003, 43, 287–298. [Google Scholar] [CrossRef]
- Myers, J.L.; Richardson, L.L.; Sekar, R. Molecular detection and ecological significance of the cyanobacteria Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 2007, 73, 5173–5182. [Google Scholar] [CrossRef] [Green Version]
- Rasoulouniriana, D.; Siboni, N.; Ben-Dov, E.; Kramarsky-Winter, E.; Loya, Y.; Kushmaro, A. Pseudoscillatoria coralii gen. nov., sp. nov., a cyanobacterium associated with coral black band disease (BBD). Dis. Aquat. Org. 2009, 87, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, K.; Jones, R.; Gibbs, D.; Richardson, L.L. The roles of temperature and light in black band disease (BBD) progression on corals of the genus Diploria in Bermuda. J. Inv. Path. 2011, 106, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Antonius, A. Kranke Korallenriff Zerstörung. Umsch. Wiss. Und Tech. 1976, 15, 493–494. [Google Scholar]
- Alker, A.P.; Smith, G.W.; Kim, K. Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean Sea fan corals. Hydrobiology 2001, 460, 105–111. [Google Scholar] [CrossRef]
- Ward, J.R.; Kim, K.; Harvell, C.D. Temperature affects coral disease resistance and pathogen growth. Mar. Ecol. Prog. Ser. 2007, 329, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Marfenina, O.E.; Fomicheva, G.M.; Vasilenko, O.V.; Naumova, E.M.; Kulko, A.B. Sporulation in saprotrophic and clinical strains of Aspergillus sydowii (Bain. and Sart.) Thom and Church under various environmental conditions. Microbiology 2010, 79, 753–758. [Google Scholar] [CrossRef]
- Patterson, K.L.; Porter, J.W.; Ritchie, K.B.; Polson, S.W.; Mueller, E.; Peters, E.C.; Santavy, D.L.; Smith, G.L. The etiology of white pox disease, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 2002, 13, 8725–8730. [Google Scholar] [CrossRef]
- Looney, E.E.; Sutherland, K.P.; Lipp, E.K. Effects of temperature, nutrients, organic matter, and coral mucus on the survival of the coral pathogen Serracia marcescens PDL 100. Environ. Microbiol. 2010, 12, 2479–2485. [Google Scholar] [CrossRef]
- Riegl, B. Effects of the 1996 and 1998 positive sea- surface temperature anomalies on corals, coral diseases, and fish in the Arabian Gulf (Dubai, UAE). Mar. Biol. 2002, 140, 29–40. [Google Scholar]
- Harvell, C.D.; Altizer, S.; Cattadori, I.M.; Harrington, L.; Weil, E. Climate change and wildlife diseases: When does the host matter most? Ecology 2009, 90, 912–920. [Google Scholar] [CrossRef]
- Cervino, J.M.; Hayes, R.L.; Goreau, T.J.; Smith, G.W. Zooxanthellae regulation in yellow blotch/band and other coral diseases contrasted with temperature related bleaching: In situ destruction vs. expulsion. Symbiosis 2004, 37, 63–85. [Google Scholar]
- Gil-Agudela, D. Garzón-Ferreira.: Spatial and seasonal variation of dark spots disease in coral communities of the Santa Marta area (Columbian Caribbean). Bull. Mar. Sci. 2002, 69, 619–629. [Google Scholar]
- Borger, J.L.; Steiner, C.C. The spatial and temporal dynamics of coral disease in Dominica, West Indies. Bull. Mar. Sci. 2005, 77, 137–154. [Google Scholar]
- Porter, J.W.; Torres, C.; Sutherland, K.P.; Meyers, M.K.; Callahan, M.K.; Ruzicka, R.; Colella, M. Prevalence, severity, lethality, and recovery of dark spots syndrome among three Floridian reef building corals. J. Exp. Mar. Biol. Ecol. 2011, 408, 79–87. [Google Scholar] [CrossRef]
- Kushmaro, A.; Rosenberg, E.; Fine, M.; Ben-Haim, Y.; Loya, Y. Effect of temperature on bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 1998, 171, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Toren, A.; Landau, L.; Loya, Y.; Rosenberg, E. Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl. Environ. Microbiol. 1998, 64, 1379–1384. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Banim, E.; Kushmaro, A.; Loya, Y.; Rosenberg, E. Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Environ. Microbiol. 1999, 1, 223–229. [Google Scholar] [CrossRef]
- Banin, E.; Israely, M.; Fine, Y.; Loya, Y.; Rosenberg, E. Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of thecoral-bleaching pathogen Vibrio shiloi to its host. Appl. Environ. Microbiol. 2001, 199, 33–37. [Google Scholar]
- Israely, T.; Banin, E.; Rosenberg, E. Growth, differentiation and death of Vibrio shiloi in coral tissue as a function of seawater temperature. Aquat. Microb. Ecol. 2001, 24, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ben-Haim, Y.; Thompson, F.L.; Thompson, C.C.; Cnockaert, M.C.; Hoste, B.; Swings, J.; Rosenberg, E. Vibrio corallyticus sp. nov a temperature-dependent pathogen of the coral Pocillopora damicronis. Int. J. Syst. Evol. Microbiol. 2003, 53, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Ben-Haim, Y.; Zicherman-Keren, M.; Rosenberg, E. Temperature- regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio corallyticus. Appl. Environ. Microbiol. 2003, 69, 4236–4242. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.; Shechtman, K.; Loya, Y.; Rosenberg, E. Bacteria appear to play important roles in both causing and preventing the bleaching of the coral Oculina patagonica. Mar. Ecol. Progress. 2013, 489, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Agostini, S.; Casareto, B.E.; Yoshinaga, K.; Suzuki, T.; Nakano, Y.; Fujimura, H.; Suzuki, Y. Bacterial enhancement of bleaching and physiological impacts on the coral Montipora digitata. J. Exp. Mar. Biol. Ecol. 2013, 440, 54–60. [Google Scholar] [CrossRef]
- Ilosvay Elías, X.E.; Contreras-Silva, A.I.; Alvarez-Filip, L.; Wild, C. Coral Reef Recovery in the Mexican Caribbean after 2005 Mass Coral Mortality—Potential Drivers. Diversity 2020, 12, 338. [Google Scholar] [CrossRef]
- Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C.; et al. Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS ONE 2010, 5, e13969. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Hernández, E.; Carrillo Salvador, L.; Meza Romero, I.; Ávalos Cueva, D. Variabilidad espacio temporal de la temperatura superficial del mar, en el mar caribe. Ra Ximhai 2017, 13, 243–265. [Google Scholar] [CrossRef]
- Lamb, J.B.; True, J.D.; Piromvaragorn, S.; Willis, B.L. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 2014, 178, 88–96. [Google Scholar] [CrossRef]











| Data Source | Instrument | Parameter | Initial Year | Final Year | Frequency |
|---|---|---|---|---|---|
| SAMMO | HOBO (1 m) | SST | 2002 | 2020 | 24 h |
| Pluviometer (mm) | Precipitation | 1992 | 2021 | Monthly | |
| ERA 5 | Reanalysis | SST, AP | 1980 | 2020 | 24 h |
| Wind and waves | 1979 | 2019 | 24 h | ||
| SLA | 1993 | 2020 | 24 h |
| SLA (mm) | SST (°C) | AP (Pa) | PP (mm) | Waves | |||
|---|---|---|---|---|---|---|---|
|
Tp (s) |
Hs (m) | ||||||
| Data Source | ERA5 | SAMMO | ERA5 | ERA5 | SAMMO | ERA5 | |
| Period | 1993–2020 | 2002–2020 | 1980–2020 | 1980–2020 | 1992–2021 | 2019–2020 | 2019–2020 |
| Min | −206.5 | 19.6 | 25.04 | 96,841 | 0.00 | 2.62 | 0.00 |
| 1st Q | −23.1 | 26.73 | 26.98 | 101,287 | 24.18 | 5.90 | 0.24 |
| Median | 31.9 | 28.13 | 28.05 | 101,471 | 58.65 | 6.79 | 0.47 |
| Mean | 31.82 | 28.05 | 28 | 101,474 | 87.62 | 6.87 | 0.58 |
| 3rd Q | 80.8 | 29.3 | 28.99 | 101,658 | 123.85 | 7.93 | 0.79 |
| Max | 282.2 | 34.03 | 30.85 | 103,040 | 462.70 | 16.59 | 11.23 |
| Identified Drivers/Disturbances | ||||
|---|---|---|---|---|
| Ecosystem (State) | Observed Changes | Local Level | Regional Level | Global Level |
Mangroves | Total 1996 ha; conservation state of 40% good, 36% fair, and 24% poor [37]. | Wastewater discharge, urbanization | Hurricanes | |
Dunes | Reduction from 92 ha in 2011 to 79 ha in 2017 [38]. | Urbanization | Hurricanes | |
Beach | More or less stable until 2004, severe erosion since 2015 [39]. | Urbanization | Hurricanes, Sargasso influx | |
Seagrass meadows | Total 1622 [40] ha Gradual decline in overall condition [41]. Since 2015, loss of near-coastal meadows [42]. | Wastewater discharge | Hurricanes, Sargasso influx | |
Coral reefs | Increasing algal cover [43], coral bleaching and diseases affecting stony corals [44]. | Aquatic activities, wastewater discharge | Hurricanes, Sargasso influx | Increasing SST, acidification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, O.; Mendoza, E.; van Tussenbroek, B.I.; Silva, R. Effects of Climate-Change-Related Phenomena on Coastal Ecosystems in the Mexican Caribbean. Sustainability 2023, 15, 12042. https://doi.org/10.3390/su151512042
Guzmán O, Mendoza E, van Tussenbroek BI, Silva R. Effects of Climate-Change-Related Phenomena on Coastal Ecosystems in the Mexican Caribbean. Sustainability. 2023; 15(15):12042. https://doi.org/10.3390/su151512042
Chicago/Turabian StyleGuzmán, Odette, Edgar Mendoza, Brigitta I. van Tussenbroek, and Rodolfo Silva. 2023. "Effects of Climate-Change-Related Phenomena on Coastal Ecosystems in the Mexican Caribbean" Sustainability 15, no. 15: 12042. https://doi.org/10.3390/su151512042
APA StyleGuzmán, O., Mendoza, E., van Tussenbroek, B. I., & Silva, R. (2023). Effects of Climate-Change-Related Phenomena on Coastal Ecosystems in the Mexican Caribbean. Sustainability, 15(15), 12042. https://doi.org/10.3390/su151512042












