Aspartic Acid-Based Nano-Copper Induces Resilience in Zea mays to Applied Lead Stress Via Conserving Photosynthetic Pigments and Triggering the Antioxidant Biosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Asp-CuNPs
2.1.1. X-ray Diffraction (XRD) Analysis
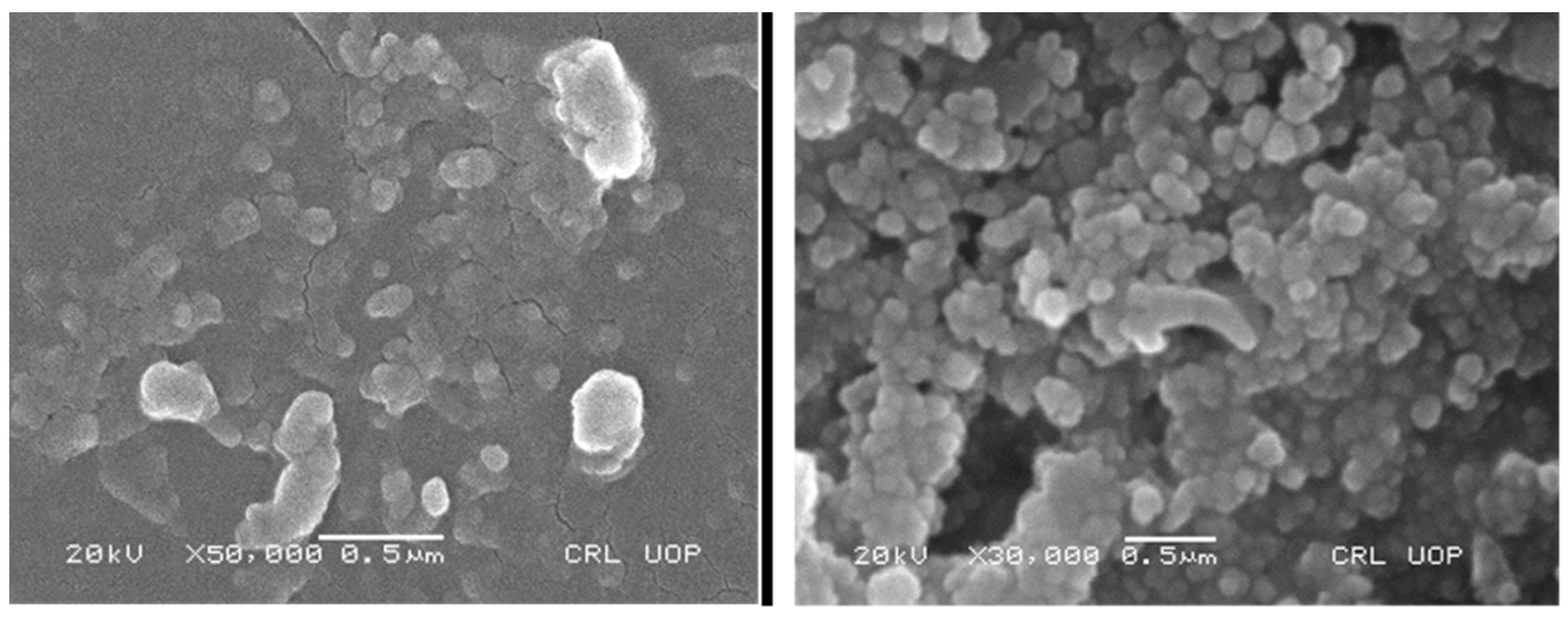
2.1.2. Scanning Electron Microscopy (SEM)
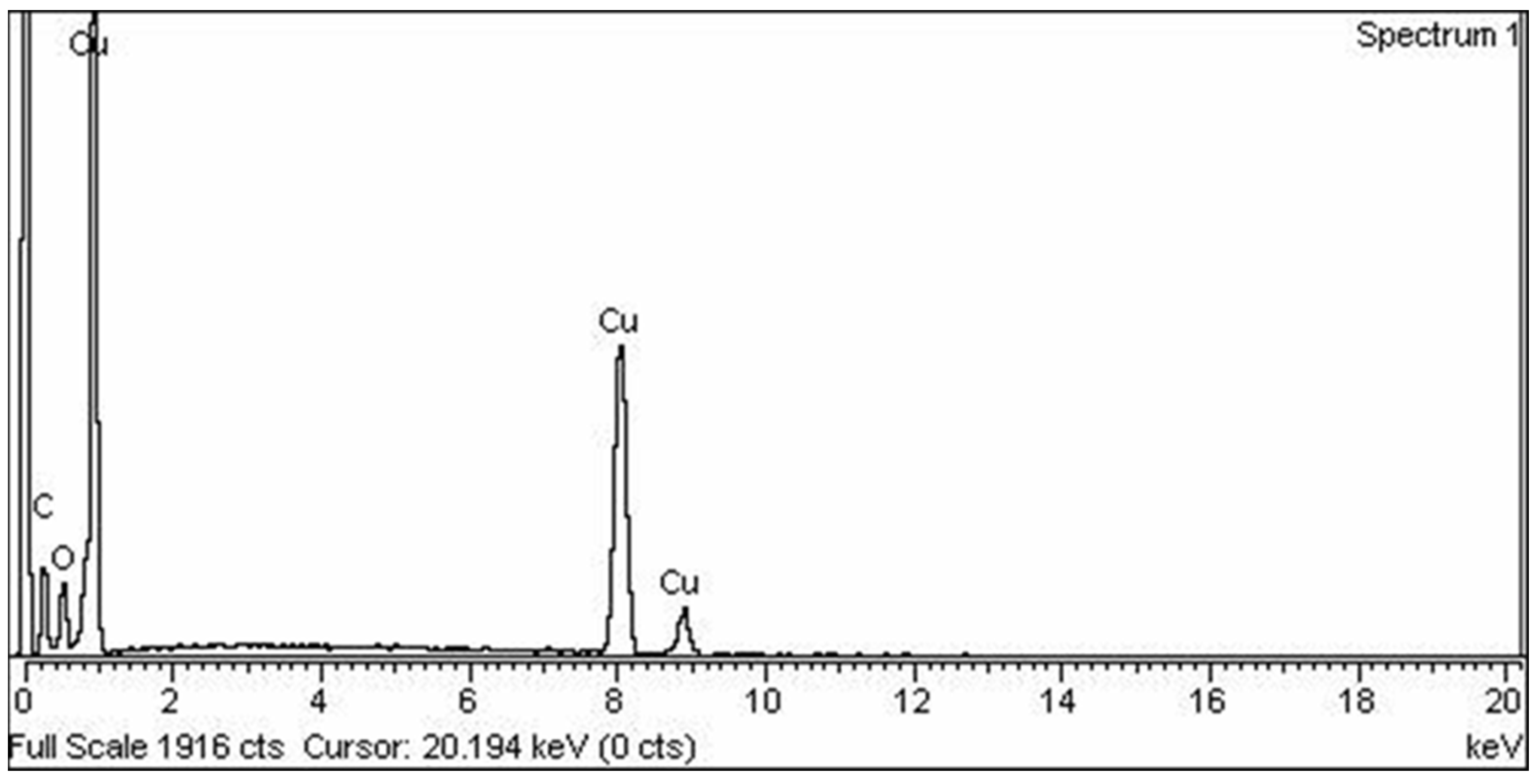
2.1.3. Energy Dispersive X-ray Spectroscopy (EDS)
2.1.4. Thermogravimetric Analysis (TGA)
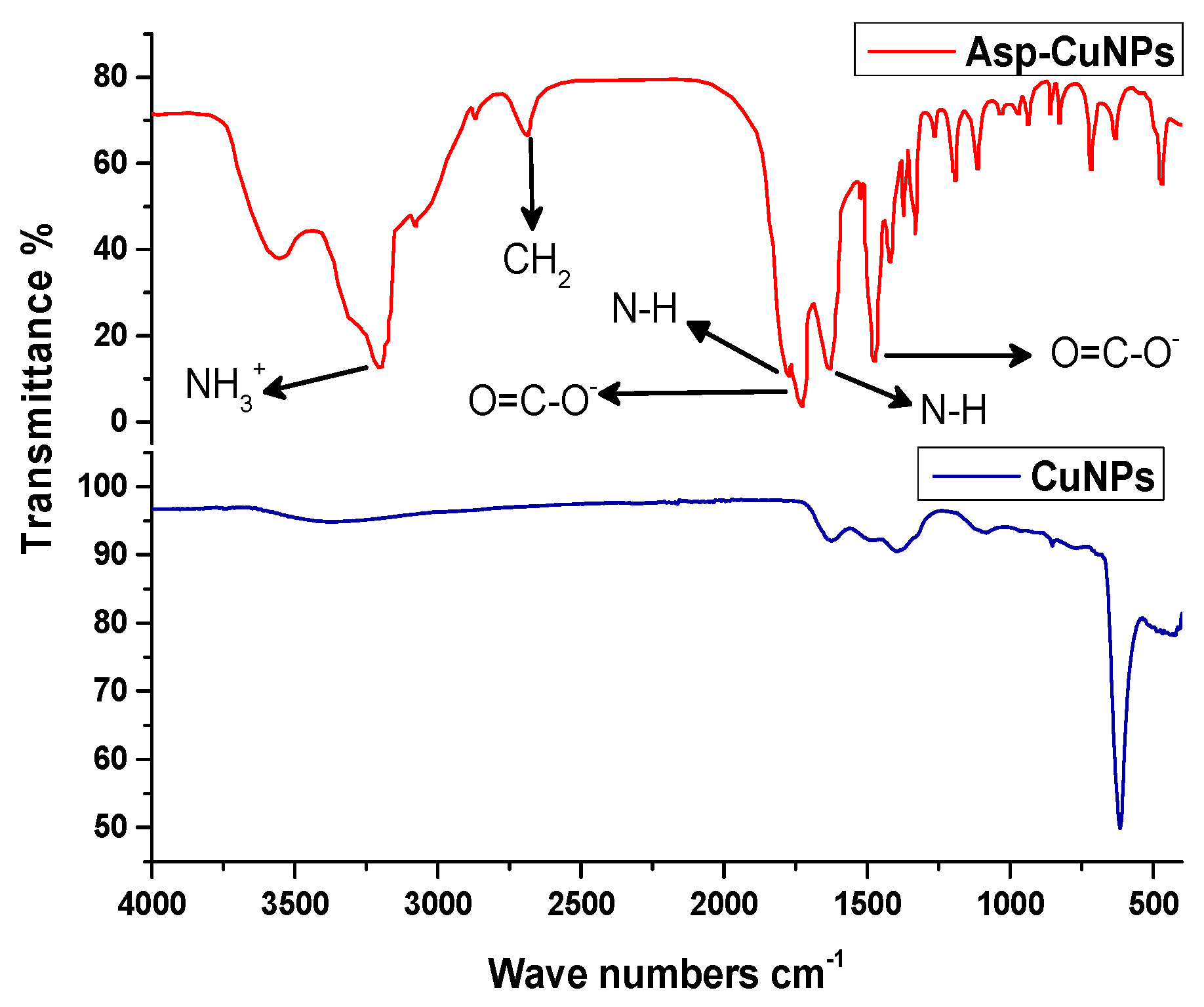
2.1.5. Infrared Spectroscopy
2.2. Plant Material and Growth Conditions
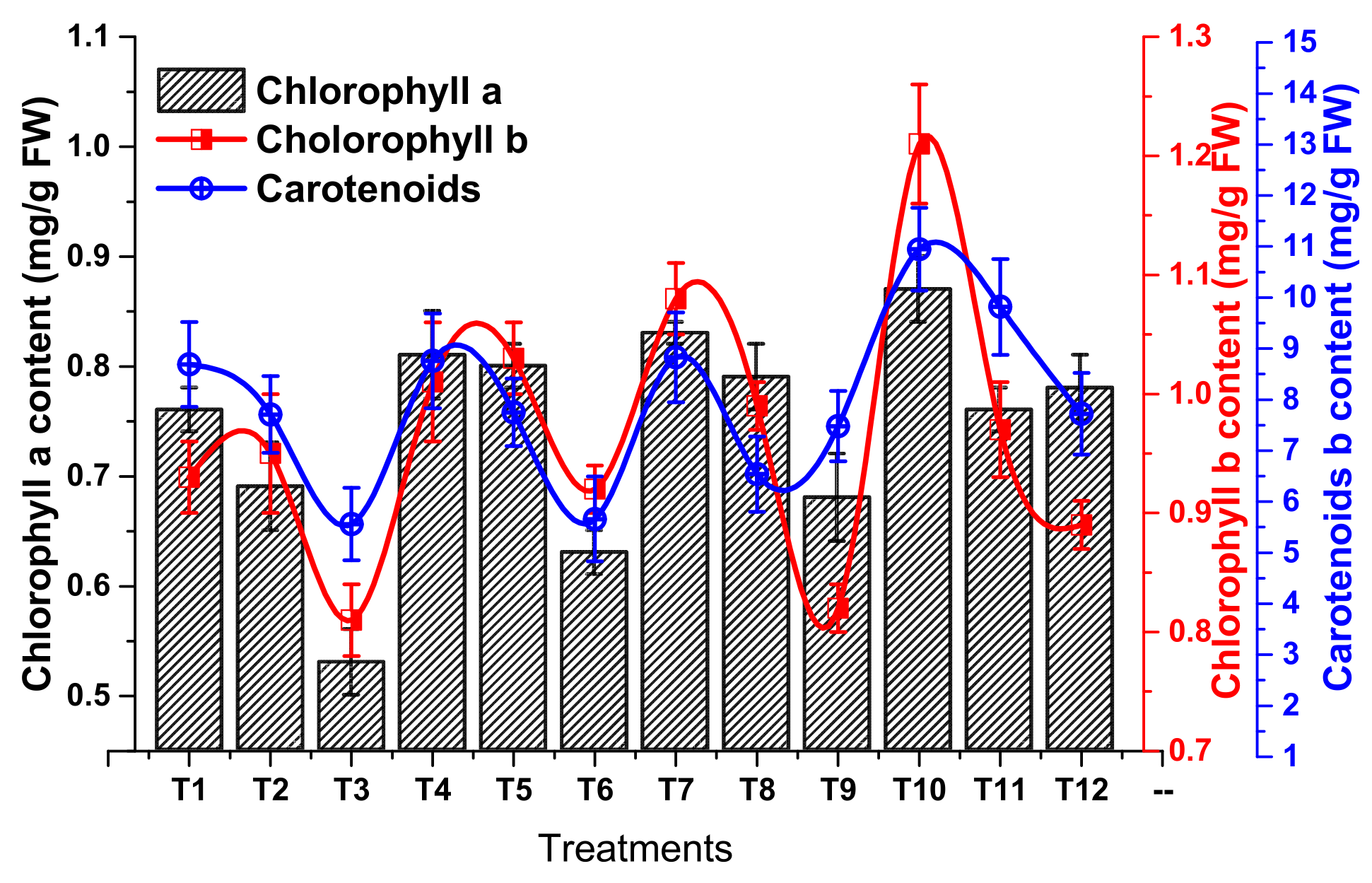
2.2.1. Determination of Photosynthetic Pigment Contents
2.2.2. Leaf Protein Contents
2.2.3. Estimation of Total Soluble Sugar
2.2.4. Proline Contents of Leaves
2.2.5. Extraction of Antioxidant Enzymes
2.2.6. Determination of Phenol Content
2.2.7. Determination of Pb Concentration
2.3. Statistics
3. Results
3.1. Characterization of Asp-CuNPs
3.1.1. Germination Indices and Seedling Growth
3.1.2. Response of Zea mays under Field Conditions
3.1.3. Biochemical Response of Zea mays
3.1.4. Response of the Oxidation/Antioxidation System of Zea mays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Ullah, Z.; Gul, F.; Iqbal, J.; Abbasi, B.A.; Kanwal, S.; Chalgham, W.; El-Sheikh, M.A.; Diltemiz, S.E.; Mahmood, T. Biogenic Synthesis of Multifunctional Silver Oxide Nanoparticles (Ag2ONPs) Using Parieteria alsinaefolia Delile Aqueous Extract and Assessment of Their Diverse Biological Applications. Microorganisms 2023, 11, 1069. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Zielony, T.; Gajewski, M. Effect of Aminoplant and Asahi on yield and quality of lettuce grown on rockwool. Conf. Biostimulators Mod. Agric. 2008, 2, 7–8. [Google Scholar]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Qyyum, A.; Kanwal, S. Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgata: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl. Organomet. Chem. 2019, 33, e4947. [Google Scholar] [CrossRef]
- Sasidharan, D.; Namitha, T.; Johnson, S.P.; Jose, V.; Mathew, P. Synthesis of silver and copper oxide nanoparticles using Myristica fragrans fruit extract: Antimicrobial and catalytic applications. Sustain. Chem. Pharm. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Servin, A.D.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina [Velvet mesquite] treated with ZnO nanoparticles. Chem. Eng. J. 2011, 170, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Ajmal, M.; Ullah, R.; Muhammad, Z.; Khan, M.N.; Kakar, H.A.; Kaplan, A.; Okla, M.K.; Saleh, I.A.; Kamal, A.; Abdullah, A.; et al. Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung bean). Molecules 2023, 28, 5059. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- Ul Haq, T.; Ullah, R.; Khan, M.N.; Nazish, M.; Almutairi, S.M.; Rasheed, R.A. Seed Priming with Glutamic-Acid-Functionalized Iron Nanoparticles Modulating Response of Vigna radiata (L.) R. Wilczek (Mung bean) to Induce Osmotic Stress. Micromachines 2023, 14, 736. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green Synthesized Metal Oxide Nanoparticles Mediate Growth Regulation and Physiology of Crop Plants under Drought Stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Rasheed, A.; Li, H.; Tahir, M.M.; Mahmood, A.; Nawaz, M.; Shah, A.N.; Aslam, M.T.; Negm, S.; Moustafa, M.; Hassan, M.U.; et al. The Role of Nanoparticles in Plant Biochemical, Physiological, and Molecular Responses under Drought Stress: A Review. Front. Plant Sci. 2022, 13, 976179. [Google Scholar] [CrossRef]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; Hernández-Hernández, H.; López-Pérez, M.C.; González-Morales, S.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Nanoparticles in Plants: Morphophysiological, Biochemical, and Molecular Responses. In Plant Life under Changing Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–322. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile Coating of Urea with Low-Dose ZnO Nanoparticles Promotes Wheat Performance and Enhances Zn Uptake Under Drought Stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, S.M.; Karimi, M.; Da Silva, J.A.T. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut 2012, 223, 2799–2806. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abda_Allaha, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signalling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, S.; Wani, O.A.; Lone, J.F.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Muhammad, L.; Khan, A.; Zhou, Y.; He, M.; Alrefaei, A.F.; Khan, M.; Ali, S. Physiological and ultrastructural changes in Dendranthema morifolium cultivars exposed to different cadmium stress conditions. Agriculture 2023, 13, 317. [Google Scholar] [CrossRef]
- Niyoifasha, C.J.; Borena, B.M.; Ukob, I.T.; Minh, P.N.; Al Azzawi, T.N.I.; Imran, M.; Ali, S.; Inthavong, A.; Mun, B.-G.; Lee, I.-J.; et al. Alleviation of Hg-, Cr-, Cu-, and Zn-Induced Heavy Metals Stress by Exogenous Sodium Nitroprusside in Rice Plants. Plants 2023, 12, 1299. [Google Scholar] [CrossRef]
- Mortezagholi, B.; Movahed, E.; Fathi, A.; Soleimani, M.; Mirhosseini, A.F.; Zeini, N.; Khatami, M.; Naderifar, M.; Kiasari, B.A.; Zareanshahraki, M. Plant-mediated synthesis of silver-doped zinc oxide nanoparticles and evaluation of their antimicrobial activity against bacteria cause tooth decay. Microsc. Res. Tech. 2022, 85, 3553–3564. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri [Linn.] Wettst. Plant growth metabolism. Proc. Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. A review. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative effects of ZnO nanoparticles, ZnO bulk particles, and Zn2+ on Brassica napus after long-term exposure: Changes in growth, biochemical compounds, antioxidant enzyme activities, and Zn bioaccumulation. Water Air Soil Pollut 2015, 226, 364. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J. Effect of copper salts and reducing agents on characteristics and antimicrobial activity of copper nanoparticles. Mater. Lett. 2014, 132, 307–311. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea [Pisum sativum L.]: Life cycle study. Front. Plant Sci. 2016, 6, 1242. [Google Scholar] [CrossRef] [Green Version]
- Rostami, F.; Ehsanpour, A.A. Application of silver thiosulfate (STS) on silver accumulation and protein pattern of potato (Solanum tubersum L.) under in vitro culture. Ann. Appl. Biol. 2009, 38, 46–54. [Google Scholar]
- Hasan, S.A.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium: Toxicity and tolerance in plants. J. Env. Biol. 2009, 30, 165–174. [Google Scholar]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef]
- Nejad, A.R.; Hatamian, M.; Kafi, M.; Souri, M.K.; Shahbazi, K. Interaction of Lead and Nitrate on Growth Characteristics of Ornamental Judas Tree [Cercis siliquastrum L.]. Open Agric. 2018, 3, 670–677. [Google Scholar] [CrossRef]
- Korkmaz, N.; Ceylan, Y.; Taslimi, P.; Karadağ, A.; Bülbül, A.S.; Şen, F. Biogenic nano silver: Synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Adv. Powder Technol. 2020, 31, 2942–2950. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sripriya, N.; Shanmugavel, M.; Manikandan, E.; Gnanamani, A.; Senthilkumar, P. Surface active gold nanoparticles biosynthesis by new approach for bionanocatalytic activity. J. Photochem. Photobiol. B Biol. 2018, 179, 119–125. [Google Scholar] [CrossRef]
- Guemari, F.; Laouini, S.E.; Rebiai, A.; Bouafia, A.; Meneceur, S.; Tliba, A.; Majrashi, K.A.; Alshareef, S.A.; Menaa, F.; Barhoum, A. UV-Visible Spectroscopic Technique-Data Mining Tool as a Reliable, Fast, and Cost-Effective Method for the Prediction of Total Polyphenol Contents: Validation in a Bunch of Medicinal Plant Extracts. Appl. Sci. 2022, 12, 9430. [Google Scholar] [CrossRef]
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, F.; Shao, W.; Ge, C.; Li, W. Shape-controllable and versatile synthesis of copper nanocrystals with amino acids as capping agents. Nanoscale 2015, 7, 8811–8818. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, H.; Choi, D.; Hwang, I. Plant hormonal regulation of nitrogen-fixing nodule organogenesis. Mol. Cell 2012, 34, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and Foliar Application of Amino Acids Improve Variables of Nitrogen Metabolism and Productivity in Soybean Crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Forde, B.G. Glutamate signalling in roots. J. Exp. Bot. 2014, 65, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead Toxicity in Cereals: Mechanistic Insight into Toxicity, Mode of Action, and Management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Hakeem, K.R.; Alharby, H.F.; Rehman, R.U. Lead Toxicity Alters the Antioxidant Defense Machinery and Modulates the Biomarkers in Tartary Buckwheat Plants. Int. Biodeterior. Biodegrad. 2020, 151, 104992. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M. Lead Toxicity in Plants: Impacts and Remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Kanwal, A.; Farhan, M.; Sharif, F.; Hayyat, M.U.; Shahzad, L.; Ghafoor, G.Z. Effect of industrial wastewater on wheat germination, growth, yield, nutrients and bioaccumulation of lead. Sci. Rep. 2020, 10, 11361. [Google Scholar] [CrossRef]
- Gent, L.; Forde, B.G. How do plants sense their nitrogen status? J. Exp. Bot. 2017, 68, 2531–2539. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, transport, and regulation of amino acids: What is missing in rice? Crop. J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; Yamaya, T.; Yamaguchi, J. Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice. Plant Cell Physiol. 2003, 44, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.C.; Chung, T.Y.; Juo, Y.A.; Hsieh, M.H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, B.M.; Oh, G.G.K.; Lee, C.P.; Millar, A.H. Metabolite regulatory interactions control plant respiratory metabolism via Target of Rapamycin (TOR) kinase activation. Plant Cell 2019, 32, 666–682. [Google Scholar] [CrossRef]
- Forde, B.G.; Roberts, M.R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000prime Rep. 2014, 6, 37. [Google Scholar] [CrossRef]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2015, 43, 1–25. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids a_ects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Javed, M.R.; Bashir, A. Lead toxicity in cereals and its management strategies: A critical review. Water Air Soil Pollut. 2018, 229, 211. [Google Scholar] [CrossRef]
- Akhtar, S.; Khan, Z.I.; Ahmad, K.; Nadeem, M.; Ejaz, A.; Hussain, M.I.; Ashraf, M.A. Assessment of lead toxicity in diverse irrigation regimes and potential health implications of agriculturally grown crops in Pakistan. Agric. Water Manag. 2022, 271, 107743. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Ullah, R.; Sher, S.; Muhammad, Z.; Jan, S.A.; Nafees, M. Modulating response of sunflower [helianthus annus L.] to induced salinity stress through application of engineered urea functionalized hydroxyapatite nanoparticles. Micro. Res. Tech. 2021, 85, 244–252. [Google Scholar] [CrossRef]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K. Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef]
- Hussain, F.; Hadi, F.; Rongliang, Q. Effects of zinc oxide nanoparticles on antioxidants, chlorophyll contents, and proline in Persicaria hydropiper L. and its potential for Pb phytoremediation. Environ. Sci. Pollut. Res. 2021, 28, 34697–34713. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Ashraf, U.; Tang, X. Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere 2017, 176, 141–155. [Google Scholar] [CrossRef]







| Controls (Hydro-Primed/ Hydro-Sprayed) | Asp-CuNP Treatments (Priming/Foliar Spray) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 µg/mL | 5.0 µg/mL | 10.0 µg/mL | |||||||||
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
| Pb level (mg/kg of soil)/mg/L | |||||||||||
| 0 | 500 | 1000 | 0 | 500 | 1000 | 0 | 500 | 1000 | 0 | 500 | 1000 |
| Treatments | T50 (Days) | MGT (Days) | FGP | GI | Radial Length (cm) | Plumule Length (cm) |
|---|---|---|---|---|---|---|
| T1 | 3.38 bc | 4.74 de | 80 cd | 21.38 ef | 4.97 | 6.43 a |
| T2 | 4.05 b | 6.33 b | 72 d | 24.35 de | 4.78 | 4.92 abc |
| T3 | 5.33 a | 7.28 a | 56 e | 19.29 f | 3.44 | 3.95 c |
| T4 | 3.14 bc | 4.11 de | 88 bc | 30.91 bc | 5.26 | 6.42 a |
| T5 | 3.62 bc | 5.92 bc | 80 cd | 25.63 de | 4.9 | 5.52 abc |
| T6 | 4.22 ab | 6.55 b | 76 d | 24.20 de | 4.09 | 5.77 abc |
| T7 | 2.83 cd | 3.97 ef | 96 ab | 36.74 a | 4.8 | 6.93 a |
| T8 | 3.76 bc | 4.67 de | 80 cd | 32.11 b | 4.29 | 5.75 abc |
| T9 | 3.98 bc | 5.18 cd | 80 cd | 30.66 bc | 3.75 | 4.31 bc |
| T10 | 2.26 d | 3.29 f | 100 a | 33.57 ab | 5.09 | 6.74 a |
| T11 | 3.49 bc | 4.49 de | 88 bc | 27.69 cd | 4.83 | 6.34 ab |
| T12 | 3.74 bc | 4.92 cd | 80 cd | 24.37 de | 4.97 | 4.69 abc |
| LSD at α 0.05 | 1.22 | 1.04 | 11.69 | 4.51 | NS | 2.14 |
| T1 = (0 µg/mL NPs + control Pb), T2 = (0 µg/mL NPs +500 mg/L Pb), T3 = (0 µg/mL NPs +1000 mg/L Pb), T4 = (1.0 µg/mL NPs + Control Pb), T5 = (1.0 µg/mL NPs + 500 mg/L Pb), T6 = (1.0 µg/mL NPs + 1000 mg/L Pb), T7 = (5.0 µg/mL NPs + control Pb), T8 = (5.0 µg/mL NPs + 500 mg/L Pb), T9 = (5.0 µg/mL NPs + 1000 mg/L Pb), T10 = (10 µg/mL NPs + control Pb), T11 = (10 µg/mL NPs +_500 mg/L Pb), T12 = (10 µg/mL NPs + 1000 mg/L Pb). | ||||||
| Treatments | Shoot Length (cm) | Root Length (cm) | Leaf Length (cm) | Plant Fresh Biomass (g) | Plant Dry Biomass (g) |
|---|---|---|---|---|---|
| T1 | 12.33 ± 1.22 | 6.31 ± 0.76 | 26.73 ± 1.18 | 4.81 ± 0.54 | 0.71 ± 0.09 |
| T2 | 10.27 ± 1.09 | 6.12 ± 1.04 | 29.14 ± 0.83 | 4.63 ± 0.69 | 0.69 ± 0.11 |
| T3 | 09.59 ± 0.82 | 5.73 ± 1.21 | 26.13 ± 1.24 | 4.18 ± 0.38 | 0.63 ± 0.13 |
| T4 | 13.62 ± 1.56 | 7.18 ± 1.08 | 31.38 ± 1.48 | 5.36 ± 0.77 | 0.78 ± 0.09 |
| T5 | 12.03 ± 1.88 | 6.51 ± 1.19 | 28.81 ± 1.03 | 4.73 ± 0.83 | 0.74 ± 0.12 |
| T6 | 12.16 ± 0.74 | 6.06 ± 0.92 | 27.45 ± 0.91 | 4.85 ± 0.53 | 0.63 ± 0.09 |
| T7 | 12.83 ± 1.65 | 8.28 ± 2.50 | 33.81 ± 1.84 | 5.06 ± 0.92 | 0.73 ± 0.11 |
| T8 | 11.33 ± 1.18 | 7.36 ± 0.88 | 30.08 ± 0.92 | 4.69 ± 0.58 | 0.69 ± 0.07 |
| T9 | 10.96 ± 0.79 | 7.11 ± 1.22 | 27.22 ± 1.36 | 4.57 ± 0.49 | 0.68 ± 0.14 |
| T10 | 10.73 ± 1.22 | 6.59 ± 0.73 | 27.59 ± 0.81 | 4.73 ± 0.82 | 0.67 ± 0.08 |
| T11 | 10.62 ± 0.95 | 6.36 ± 1.26 | 28.18 ± 1.38 | 4.61 ± 0.68 | 0.66 ± 0.16 |
| T12 | 10.50 ± 0.84 | 7.18 ± 1.17 | 27.06 ± 0.83 | 4.69 ± 0.89 | 0.68 ± 0.09 |
| T1 = (0 µg/mL NPs + control Pb), T2 = (0 µg/mL NPs +500 mg/kgPb), T3 = (0 µg/mL NPs +1000 mg/kg Pb), T4 = (1.0 µg/mL NPs + Control Pb), T5 = (1.0 µg/mL NPs + 500 mg/kg Pb), T6 = (1.0 µg/mL NPs + 1000 mg/kg Pb), T7 = (5.0 µg/mL NPs + control Pb), T8 = (5.0 µg/mL NPs + 500 mg/kg Pb), T9 = (5.0 µg/mL NPs + 1000 mg/kg Pb), T10 = (10 µg/mL NPs + control Pb), T11 = (10 µg/mL NPs +_500 mg/kg Pb), T12 = (10 µg/mL NPs + 1000 mg/kg Pb). | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Ullah, Z.; Iqbal, J.; Chalgham, W.; Ahmad, A. Aspartic Acid-Based Nano-Copper Induces Resilience in Zea mays to Applied Lead Stress Via Conserving Photosynthetic Pigments and Triggering the Antioxidant Biosystem. Sustainability 2023, 15, 12186. https://doi.org/10.3390/su151612186
Ullah R, Ullah Z, Iqbal J, Chalgham W, Ahmad A. Aspartic Acid-Based Nano-Copper Induces Resilience in Zea mays to Applied Lead Stress Via Conserving Photosynthetic Pigments and Triggering the Antioxidant Biosystem. Sustainability. 2023; 15(16):12186. https://doi.org/10.3390/su151612186
Chicago/Turabian StyleUllah, Rehman, Zakir Ullah, Javed Iqbal, Wadie Chalgham, and Ajaz Ahmad. 2023. "Aspartic Acid-Based Nano-Copper Induces Resilience in Zea mays to Applied Lead Stress Via Conserving Photosynthetic Pigments and Triggering the Antioxidant Biosystem" Sustainability 15, no. 16: 12186. https://doi.org/10.3390/su151612186






