1. Introduction
Dioscorea composita Hemsl is a wild species that has its origin in México and Central America [
1], although some authors consider Mexico as the most probable center of origin [
2,
3]. This plant has been historically recognized for the production of secondary metabolites of pharmaceutical importance, including diosgenin [
4]. However, due to advances in the chemical synthesis of these compounds, the relevance of natural diosgenin in medicine has diminished over time [
3]; consequently, the industry’s interest in this plant decreased, which caused the diverse aspects of this plant to remain understudied until now. Nevertheless, recently, the interest in this species has resurfaced, since it has been discovered that it may constitute a promising therapeutic agent against cancer [
5,
6]. In addition,
D. composita is a plant with socioeconomic importance that has great nutritional and ethnobotanical value [
3]. Currently, it can be considered a nutraceutical plant used in states of southeastern México [
7].
Climate change is causing variations in temperature and precipitation patterns, as well as in the frequency of extreme weather events [
8], triggering alterations in the range of species distribution, and modifying the composition and characteristics of ecosystems. México and Central America are some of the most impacted regions by climate change, due to their geographic and orographic characteristics, together with the unequal territorial distribution of natural resources [
9]. Climate scenarios modeled for the middle of this century predict increases in temperature from 1 to 3 °C and a decrease in precipitation of around 10%, causing diverse environmental combinations that provoke particular climatic conditions, which alter the agro-climatic conditions of these regions adversely [
10], which include the central and northern part of México [
11,
12], as well as tropical and subtropical zones [
13]. Central America has experienced the ravages of this phenomenon, with drought mainly in El Salvador, Guatemala, and Honduras in 2014 and 2015, endangering temperate and cloudy forests [
9]. These changes in weather patterns impact the current distribution areas of numerous wild species in México and Central America. In the Mexican occurrence sites of
D. composita, it is estimated that by the year 2050, the annual mean temperature will increase 2–3 °C and annual precipitation will decrease 10–50 mm, in relation to the average climatology in 1961–2010 [
14]; whilst, for the Central American occurrence sites, an increase of 2.5–3 °C and a decrease of 25–70 mm of precipitation are expected by the year 2050 [
15]. These environmental conditions imposed by climate change lead to the need to increasingly use species with greater tolerance to drought and heat; in this regard, recently, the molecular mechanism of WRKY TF (namely, DcWRKY5) was isolated from the plant of
D. composita and proved to act as a positive regulator of the drought and salt tolerance in this plant, which has potential applications in transgenic breeding [
16]. However, many aspects of this species remain unstudied, which limits a comprehensive assessment of its comparative advantages and potential uses. Moreover, the possible effects of climate change on the presence and potential distribution of
D. composita have not yet been assessed. This is why the objectives of this research were to characterize the eco-geography and environmental adaptation of
D. composita, as well as to predict its current and future potential distribution under climate change scenarios in its region of origin.
Different algorithms have been used to evaluate the species distribution, such as the Generalized Additive Model (GAM) and Genetic Algorithm for Rule Set Production (GARP) [
17]. However, MaxEnt stands out as the most widely used algorithm in this field due to its ability to provide accurate and reliable results, its flexibility in handling large datasets, and its efficiency in terms of execution time [
18]. Furthermore, it has allowed for identifying optimal conditions for
Dioscorea species cultivation and production of diosgenin in significant quantities [
19]. The knowledge generated through research with MaxEnt has not only contributed to the development of more efficient production methods but has also laid the foundations for the proper conservation of these species [
20].
4. Discussion
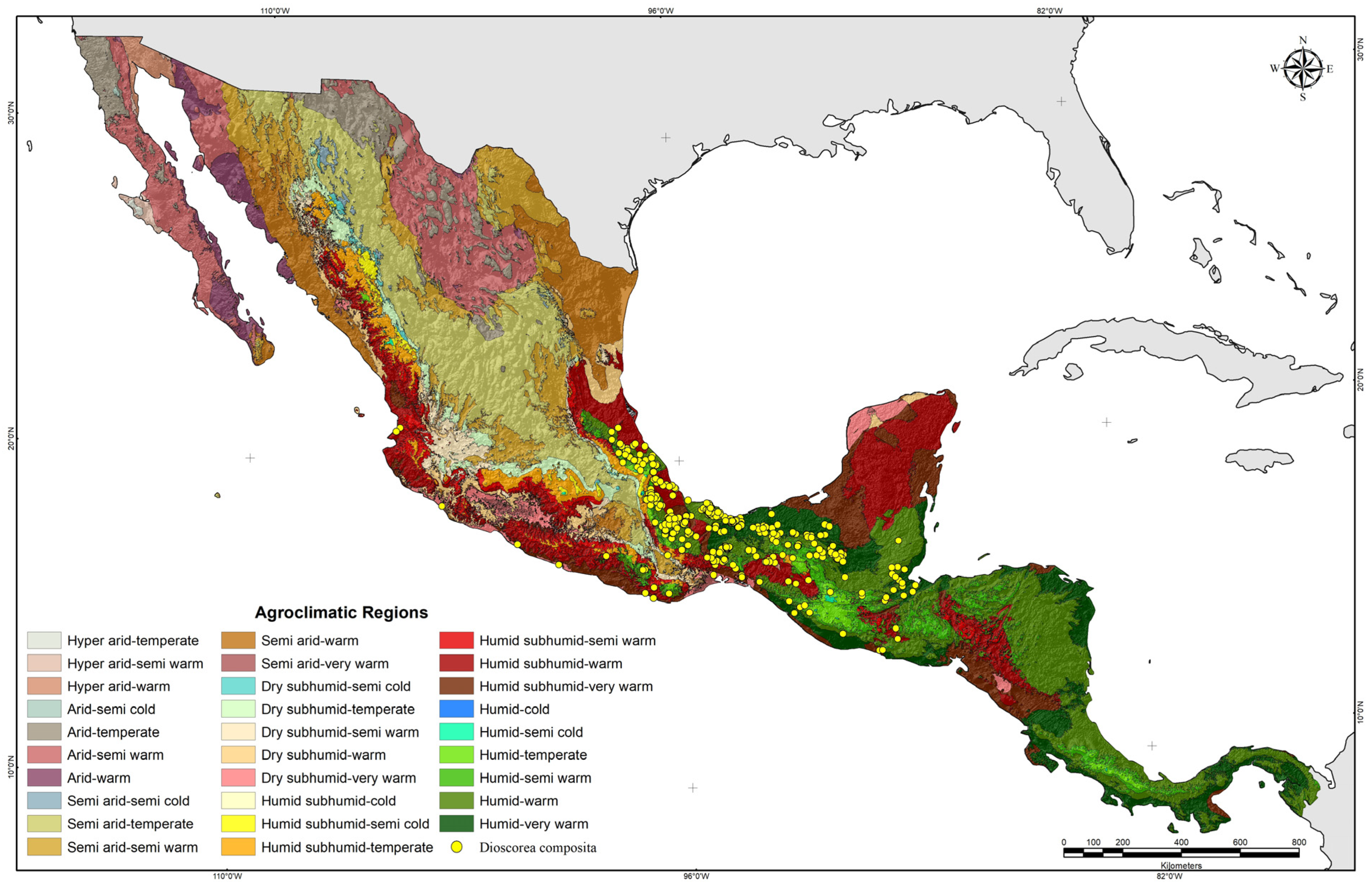
D. composita occurrence sites are concentrated in a region of humid warm climates that goes from the north of Veracruz, México, to the north of Guatemala. Since México–Central America is the most likely center of origin for
D. composita [
1], this geographic area could be suggested as a possible center of origin of this species, although this hypothetical assertion must be proven with phylogenetically targeted genomic, morphological, and Earth system data [
39]. In addition, as expected, niche modeling with MaxEnt showed this region to have the highest environmental suitability for
D. composita, which appends another argument to support such a hypothetical suggestion, since it is known that the centers of the origin of the species meet the best environmental conditions for their growth [
40].
Even when
D. composita mostly distributes in humid warm climates, the occurrence sites in other environmental conditions evidences the species’ capabilities to adapt to an ampler climatic scope. This provides
D. composita the possibility of better adaptation to novel climates brought on by global warming [
19,
41,
42].
The former results match with previous reports that state that the species of the genus
Dioscorea are distributed in warm climates as well as in temperate zones [
43], even at elevations above 2200 m [
44]. Likewise, our results agree with previous findings that
Dioscorea species are also adapted to both tropical and subtropical zones [
45,
46].
According to the annual mean precipitation range characterization for 18 species of
Dioscorea genus with a presence in India [
4], they distribute in environments with annual precipitation ranging from 2165 to 3778 mm, reinforcing the wetland plants’ character. In our research, we obtained a range of 737 to 4874 annual millimeters; the value of 737 mm corresponds with an accession located on the borderline of Mexican states Guerrero and Oaxaca, near the Pacific coast. In fact, 12 of 27 occurrence sites located near the Pacific coastline register annual precipitation lower than 1500 mm, indicating that
D. composita has adapted to drier environments. These accessions could be of interest for a possible breeding program focused on giving rise to varieties better capable of coping with climate change drought episodes.
The photoperiod was also determined to be an important variable for
D. composita distribution. Short days have been reported to favor
Dioscorea species tubers, whereas a photoperiod of more than 12 h has been shown to promote the growth of leaf area, long stems, and vigorous vines [
47,
48].
Results evidenced that annual thermal oscillation (ATO) and annual thermal range (ATR) contributed to explaining
D. composita presence and distribution; the optimal intervals determined for
D. composita are 10.1–12.9 °C and 16.9–20.2 °C, respectively (
Table 4). These values are indicative of a species not typically from thermo-regulated climates, as might be expected due to its presence in mostly humid and warm climates, but rather of a species that tolerates the occurrence of extreme temperatures. According to the Köppen-García Climate Classification System [
49], these environments correspond to extreme thermal climates (ATO = 7–14 °C).
Growth of the
Dioscorea species requires temperatures in a range from 25 to 30 °C to exhibit normal development; we obtained a very similar range (23–28 °C,
Table 4) as an optimal interval in this parameter [
45]. This author also reports that the growth of
Dioscorea species is restricted by temperatures below 20 °C; such conditions are present in the occurrence sites located in the original region of the distribution of
D. composita (yellow color area in map of
Figure 3). However, for the rest of the distribution sites, temperatures keep below 20 °C many days during the year (
Supplemental File), denoting that
D. composita is subject to adaptation in other environments.
Warm temperatures favor foliage growth, but they also favor high respiration rates, which retard tuber growth and alter the production of diosgenin [
50]. However, the optimal range determined in this research for the mean maximum temperature in the hot season (May–October) was 29–35 °C, which supposes certain comfort status of
D. composita even under temperatures considered extreme (>32 °C) for many plant species [
51,
52].
A niche model with an AUC value greater than 0.7 can make good estimations [
53]. We classify the accuracy of the models according to their AUC value in five categories: 0.50–0.60—insufficient model; 0.60–0.70—poor model; 0.70–0.80—average acceptable model; 0.80–0.90—good model, and 0.90–1.00—excellent model [
54,
55]. According to this classification, the obtained models for all climatic scenarios studied are excellent to describe the potential distribution of
D. composita and constitute an adequate tool to derive the eco-geographic characterization of the territories where this species is distributed [
21].
The results of the Jackknife test revealed that the variables that mainly determine the presence and disribution of
D. composita are AMAI, NAPH, ATO, MOMAI, NAMAI, Bio14, and Bio11 (
Table 7). These results partially match with previous reports [
37], which mention that the most important variables in the growth and development of the species, especially in the production of diosgenin, are precipitation and solar radiation. For
Solanum tuberosum, with which the species of the genus
Dioscorea are compared for the production of tubers, the most important environmental variables are annual precipitation and average soil temperature, in such a way that at higher soil temperature and lower soil moisture, the distribution of the species that produce tubers is limited [
56].
Most studies about the effects of climate change on the environmental suitability for species report more contraction than expansion areas for their potential distribution [
57]. However, these types of climate change effects should not be considered as generalizations, since the new environmental conditions brought about by climate change can represent comparative advantages for diverse species, mostly causing the expansion of their potential distribution areas [
58]; Moreover, some species could remain in their current distribution areas without being significantly affected by climate change. In this way, species of origin and adaptation to temperate environments would observe their potential distribution reduced by the year 2050, as in the case of
Solanum tuberosum [
56]; while species of tropical origin and adaptation to warm environments would benefit from the expansion of their potential distribution areas; an example is
Dioscorea alata, which is predicted to have a significant increase in production and potential distribution area by the 2040s [
57].
Environmental factors are the main drivers of changes in the distribution of
Dioscorea species [
59]. Based on the values in
Table 8, which describe the dynamics of environmental suitability for
D. composita under different climate change scenarios, it is concluded that scenario RCP 4.5 would have a negative impact on the species, with a decrease in its potential distribution area. Loss of biodiversity, as a consequence of climate change, has been observed in similar environments in other species such as passion fruit, which, being a climbing species, will also be affected in scenario RCP 4.5, decreasing its potential area due to increased temperature and decreased precipitation (and hence, reduced water availability), and new physico-chemical soil characteristics [
60].
Based on the results, the year 2050 RCP 8.5 climatology would have a positive effect on the environmental suitability of
D. composita. However, studies on the effects of climate change indicate mainly impacts on areas of environmental suitability, resulting in contraction areas being greater than expansion areas [
57]. Notwithstanding the foregoing, the response of each plant species may differ, with some decreasing their distribution, others changing or expanding it, and perhaps some not being affected at all [
58]. While climate change has the potential to negatively affect the distribution and biodiversity of plant species, specific effects may vary depending on the species and the scenario considered. In the case of
D. composita, the RCP 8.5 scenario suggests a positive effect on its potential distribution area. Furthermore, the variation in the impacts of climate change on population growth rates is mainly due to differences in the climatic response of the species populations [
61].
It is important to consider the inherent biases in the algorithms of both the ecological niche model and climate change models, which may affect the accuracy and reliability of the results. The understanding and adequate mitigation of these biases are essential to improve the usefulness and reliability of these models in decision-making related to the conservation and management of plant species and ecosystems [
62].
5. Conclusions
D. composita is predominantly distributed in a concentrated region of southern México and Central America characterized by warm and humid climates. The modeling of environmental suitability has identified a potential center of origin for D. composita; spanning from northern Veracruz, México to northern Guatemala. Several key parameters, including moisture availability indices, photoperiod, thermal oscillation, precipitation, and temperature, significantly influence its distribution.
Considering climate change scenarios for 2050, the study indicates a decrease in envi-ronmental suitability under the RCP 4.5 scenario and an increase under the RCP 8.5 scenario, suggesting that D. composita could become a favorable crop option under the latter emissions scenario.
The knowledge derived from this research contributes to a better understanding of the plant–environment interactions of D. composita, enabling the determination of its potential as a crop in the current and future climates, as well as the development of strategies for the conservation of its natural populations in México and Central America. These findings are particularly valuable given the growing public interest in D. composita for its nutraceutical properties. This knowledge will facilitate the effective utilization of this plant resource, both in the development of new pharmaceutical products and the implementation of appropriate conservation measures.
It is recommended to continue the morphological and genetic characterization of the populations of this species, to identify traits of tolerance to drought, excess water and heat, in the process of a possible genetic improvement program to obtain possible varieties’ adaptable to new climatic environments derived from climate change.