Experimental Study on the Solidification of Uranium Tailings and Uranium Removal Based on MICP
Abstract
:1. Introduction
2. Materials and Methods
2.1. MICP Aqueous Solution Tests
2.1.1. Purposes
2.1.2. Materials
2.1.3. Methods
2.2. Grouting Tests on Uranium Tailings
2.2.1. Purposes
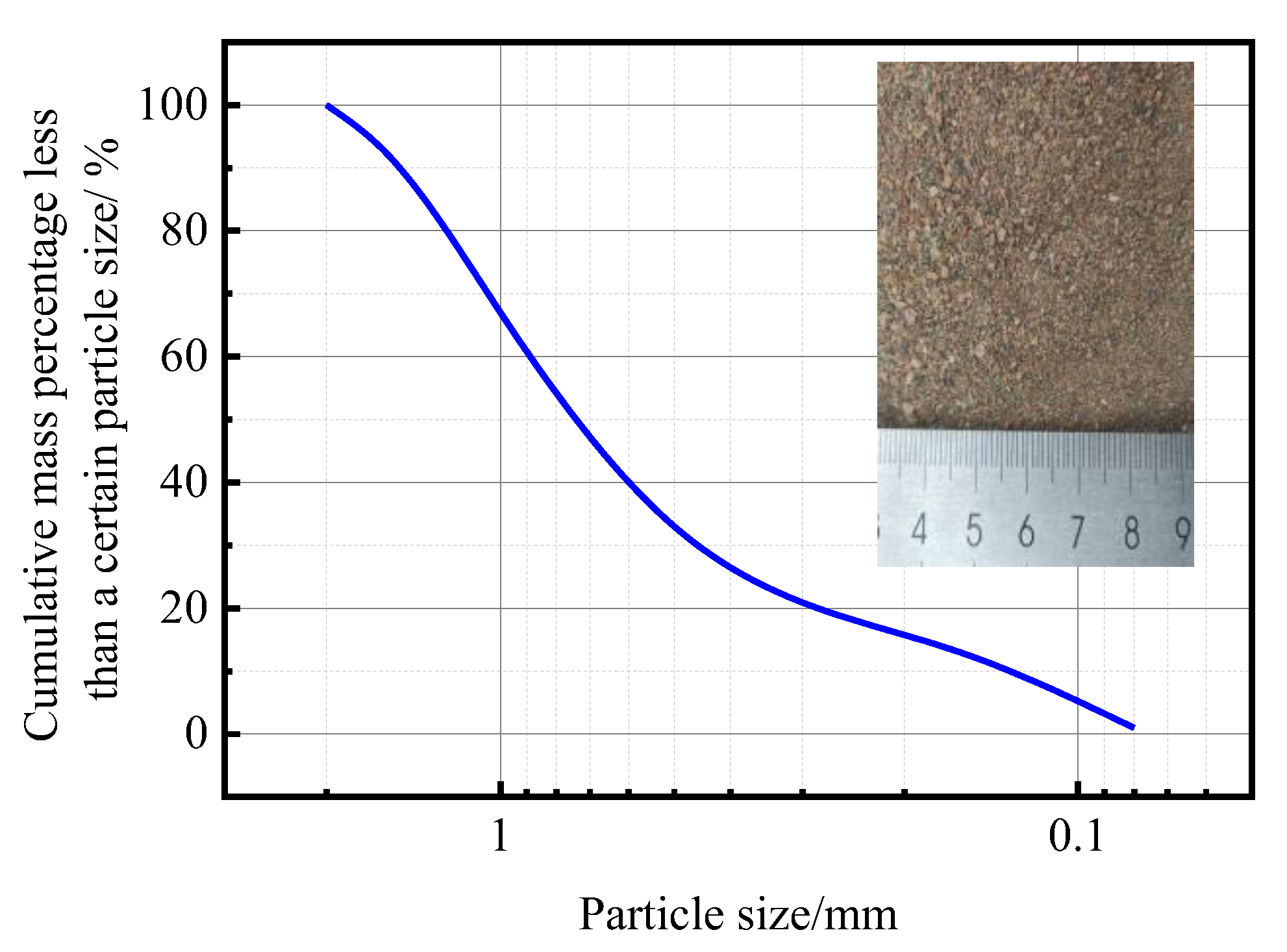
2.2.2. Materials
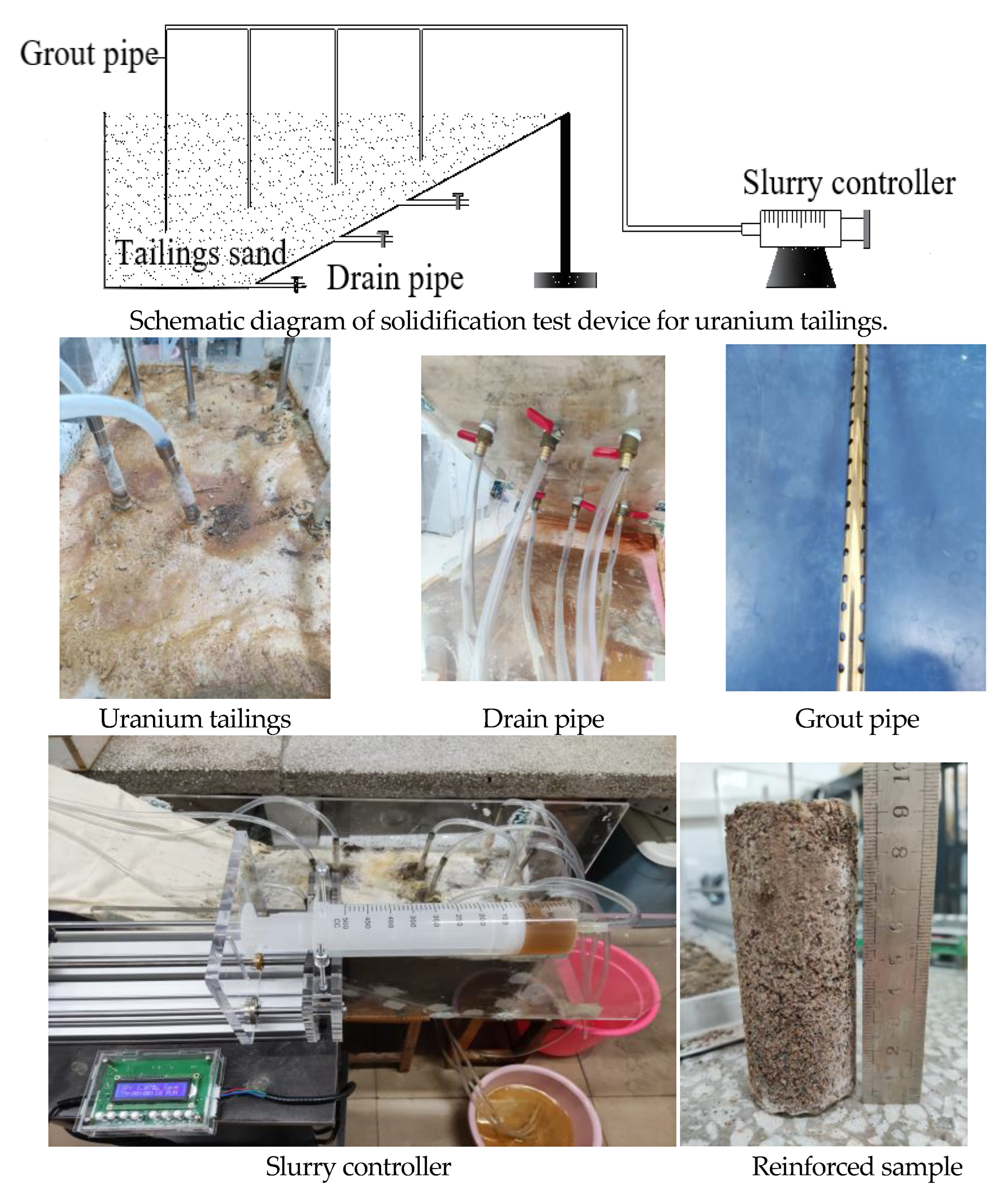
2.2.3. Test Devices and Methods
3. Results and Analysis
3.1. Solution Test Results
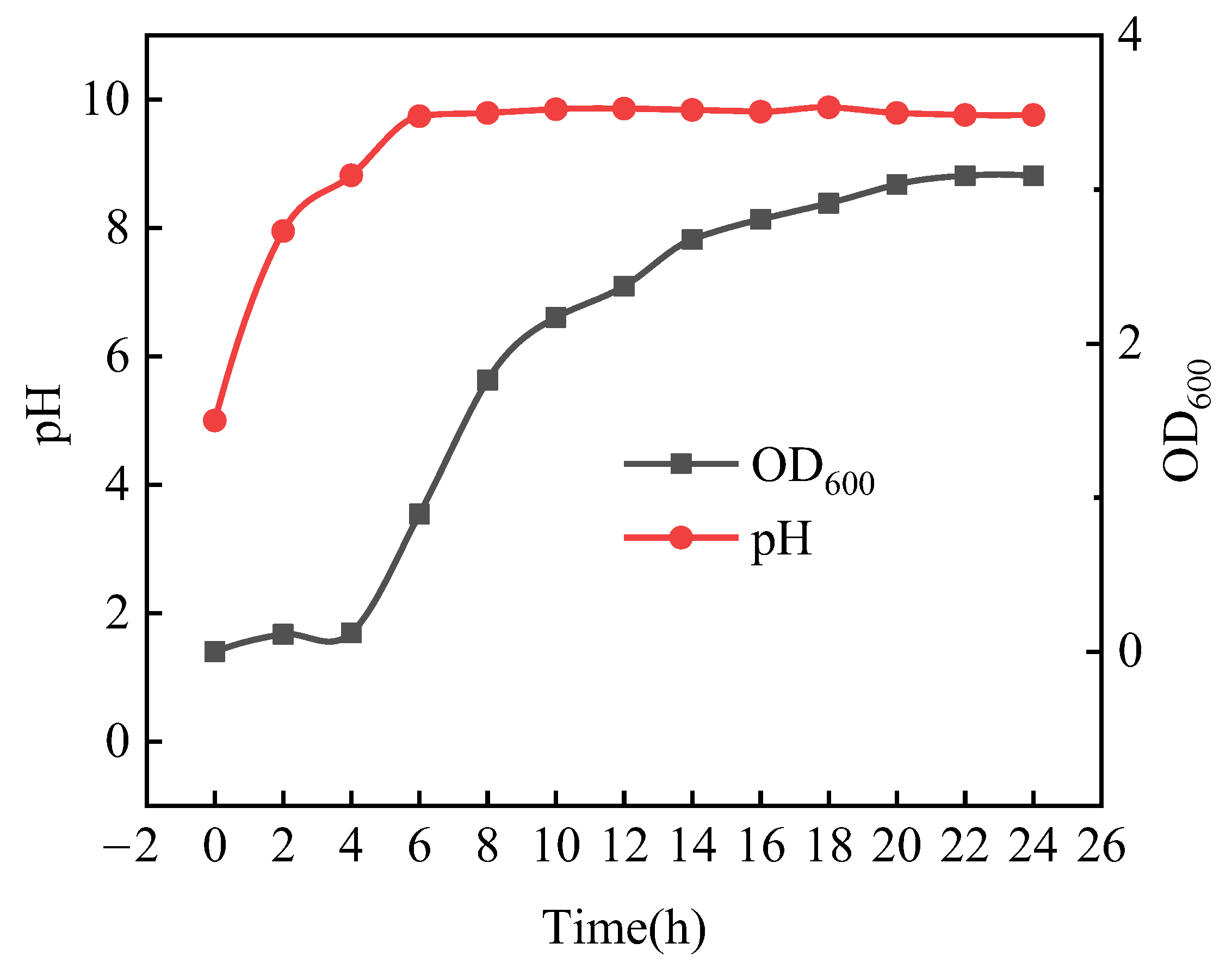
3.1.1. Biochemical Characterization of Bacteria
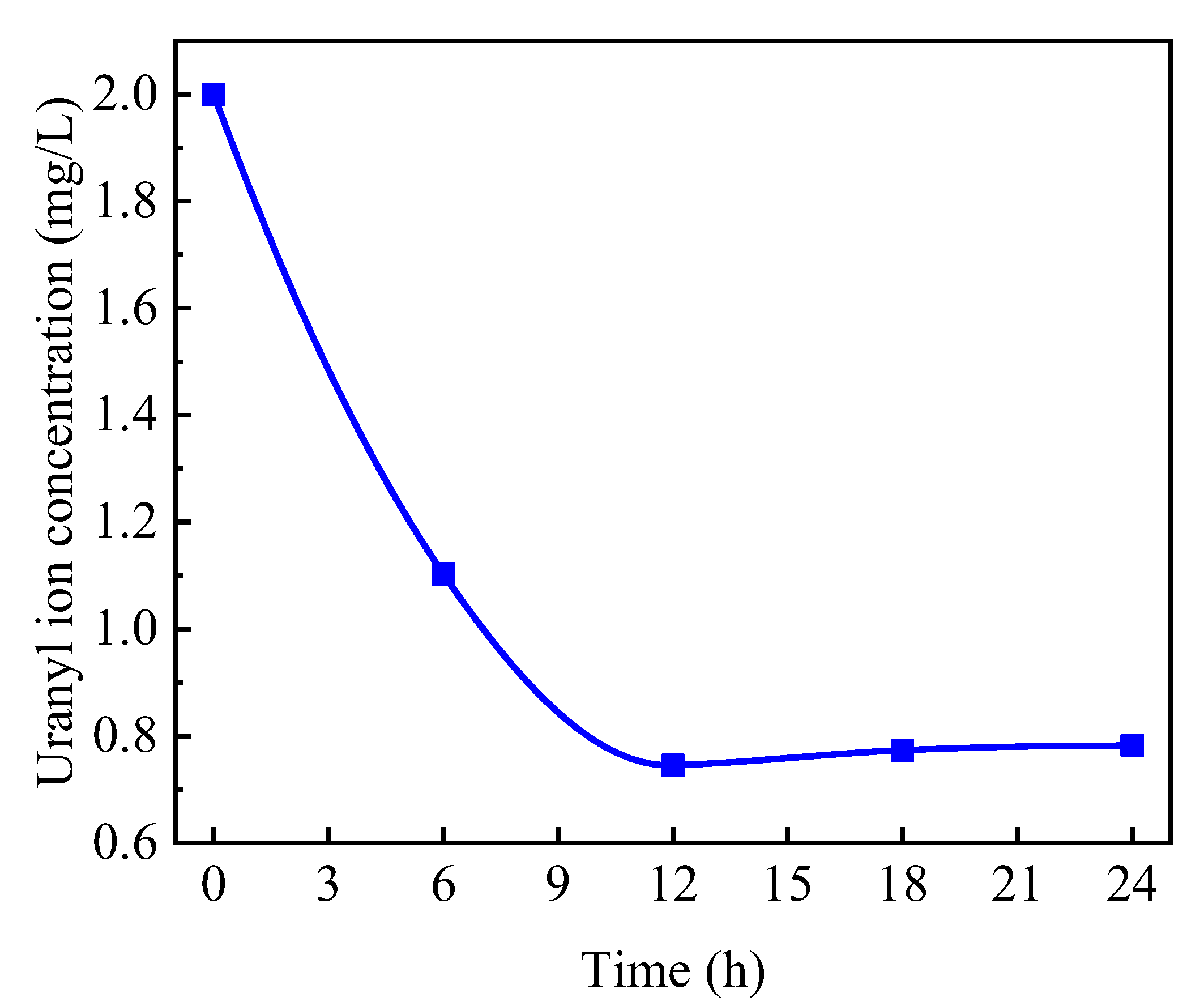
3.1.2. Changes in Uranium Concentration
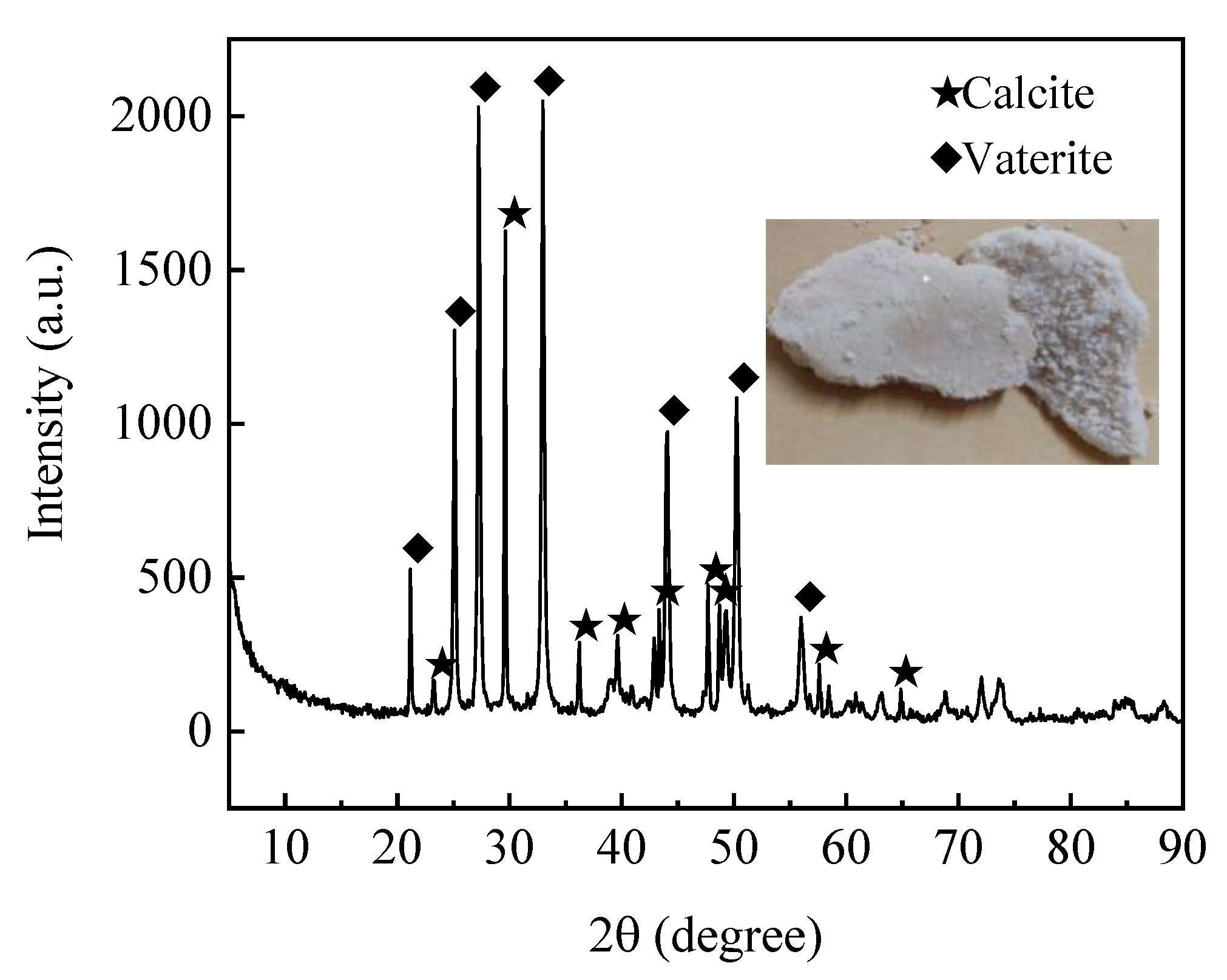
3.1.3. Mineralization Product Test Results
X-ray Diffraction (XRD)
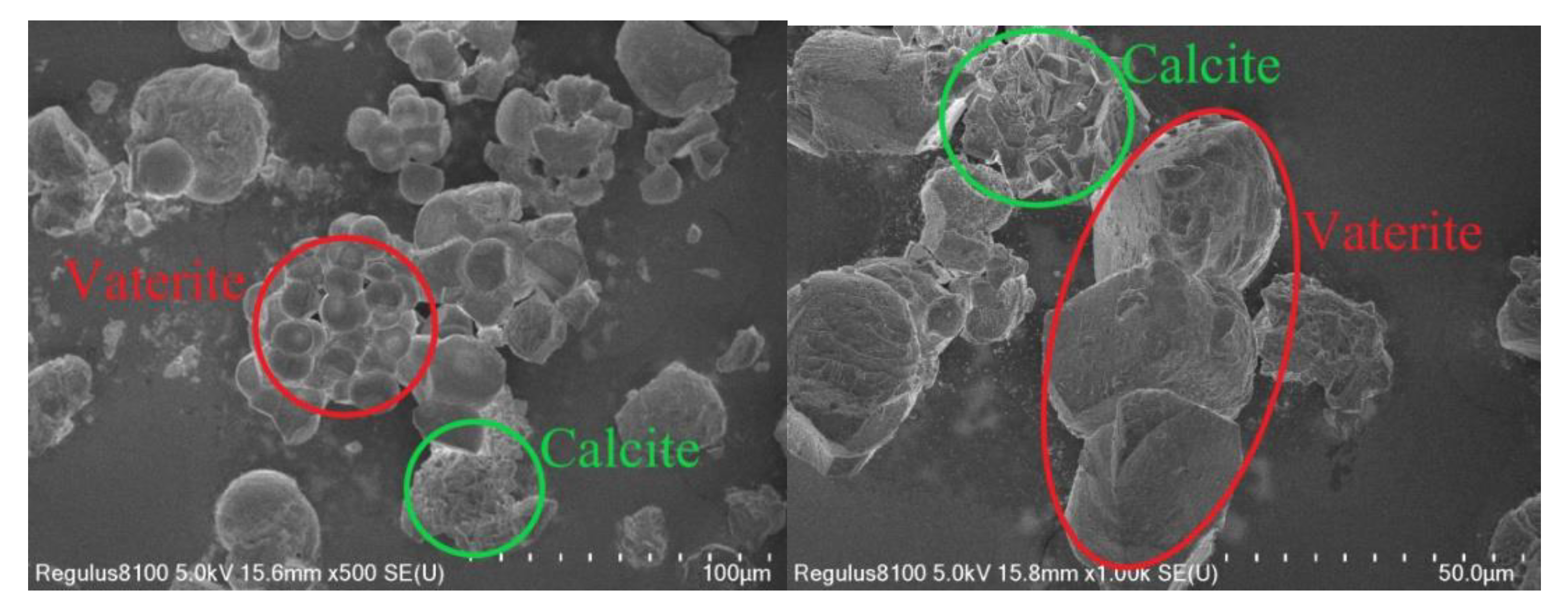
Scanning Electron Microscopy (SEM)
3.2. Test Results Uranium Tailing Sand Grouting
3.2.1. The Triaxial Test Results
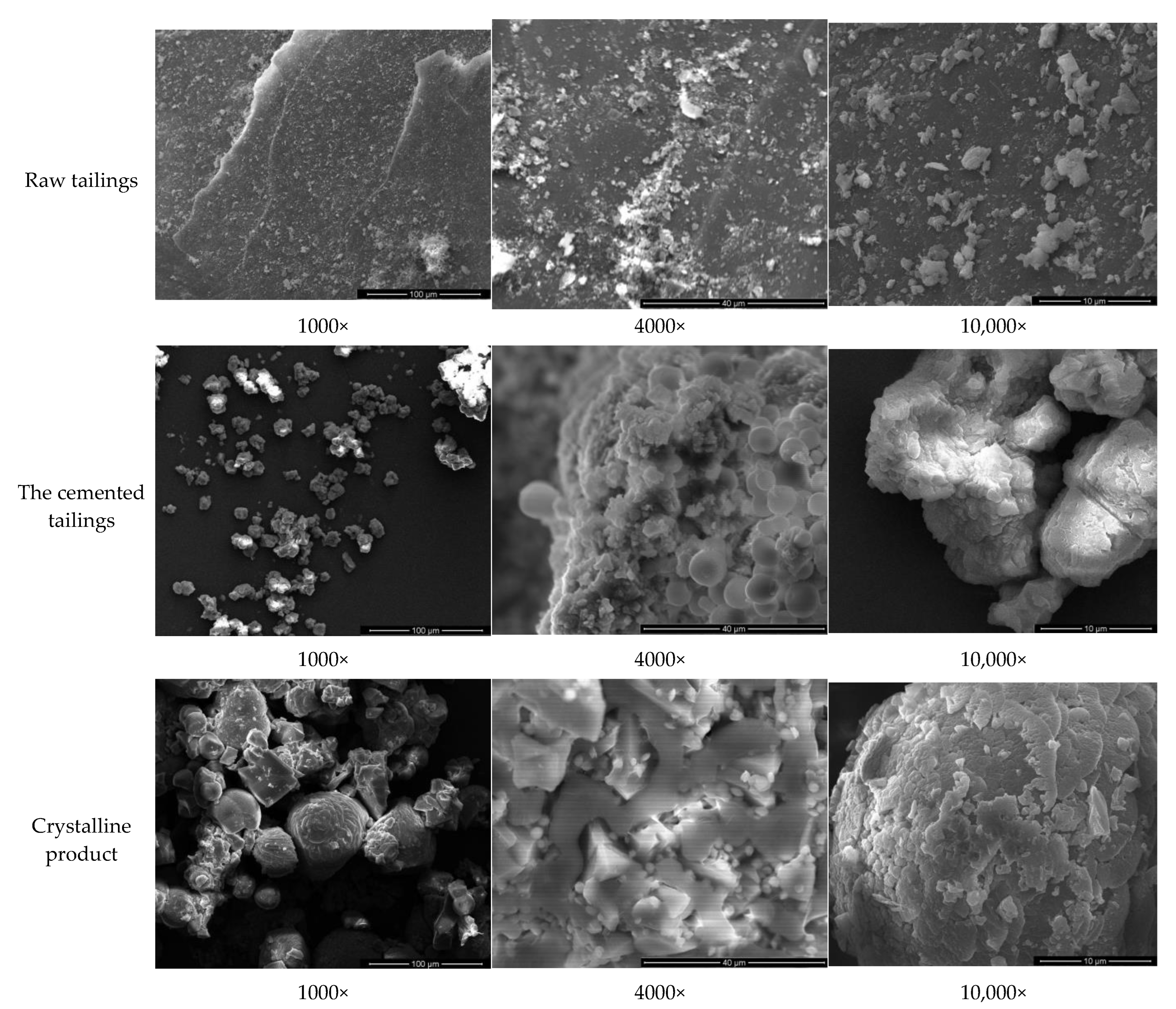
3.2.2. Comparative Analysis of the Micro-Morphology of Cement
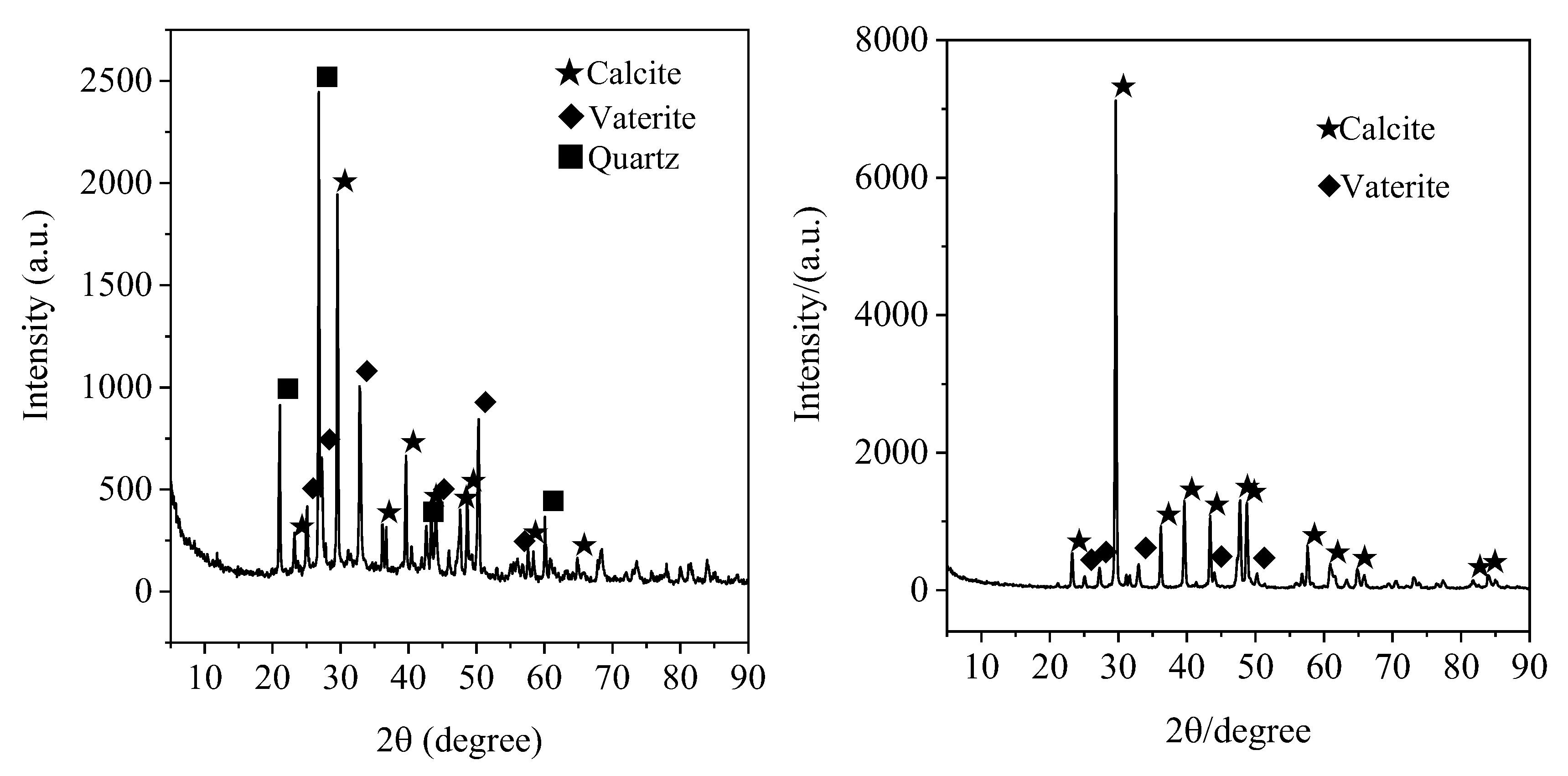
3.2.3. XRD Detection
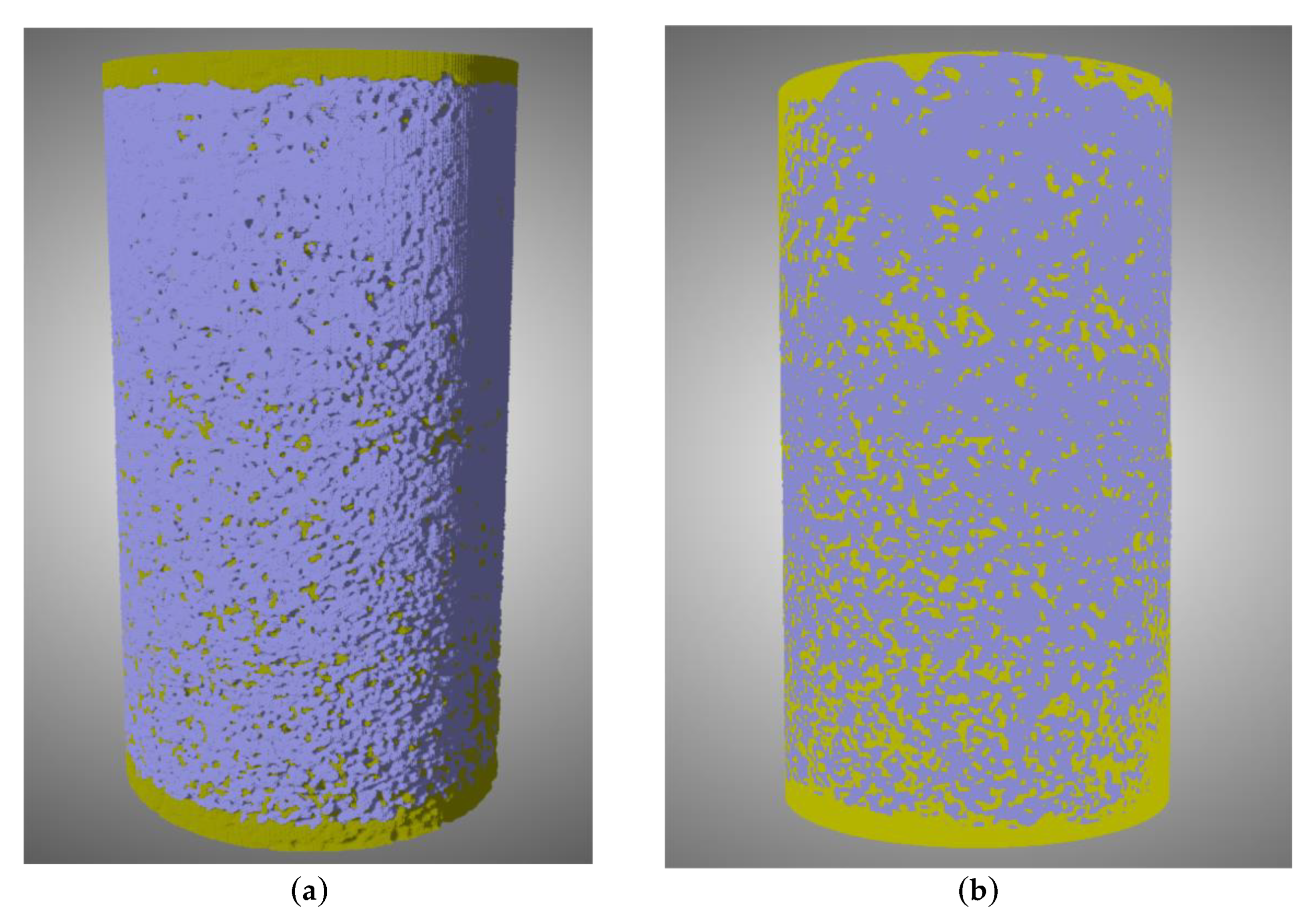
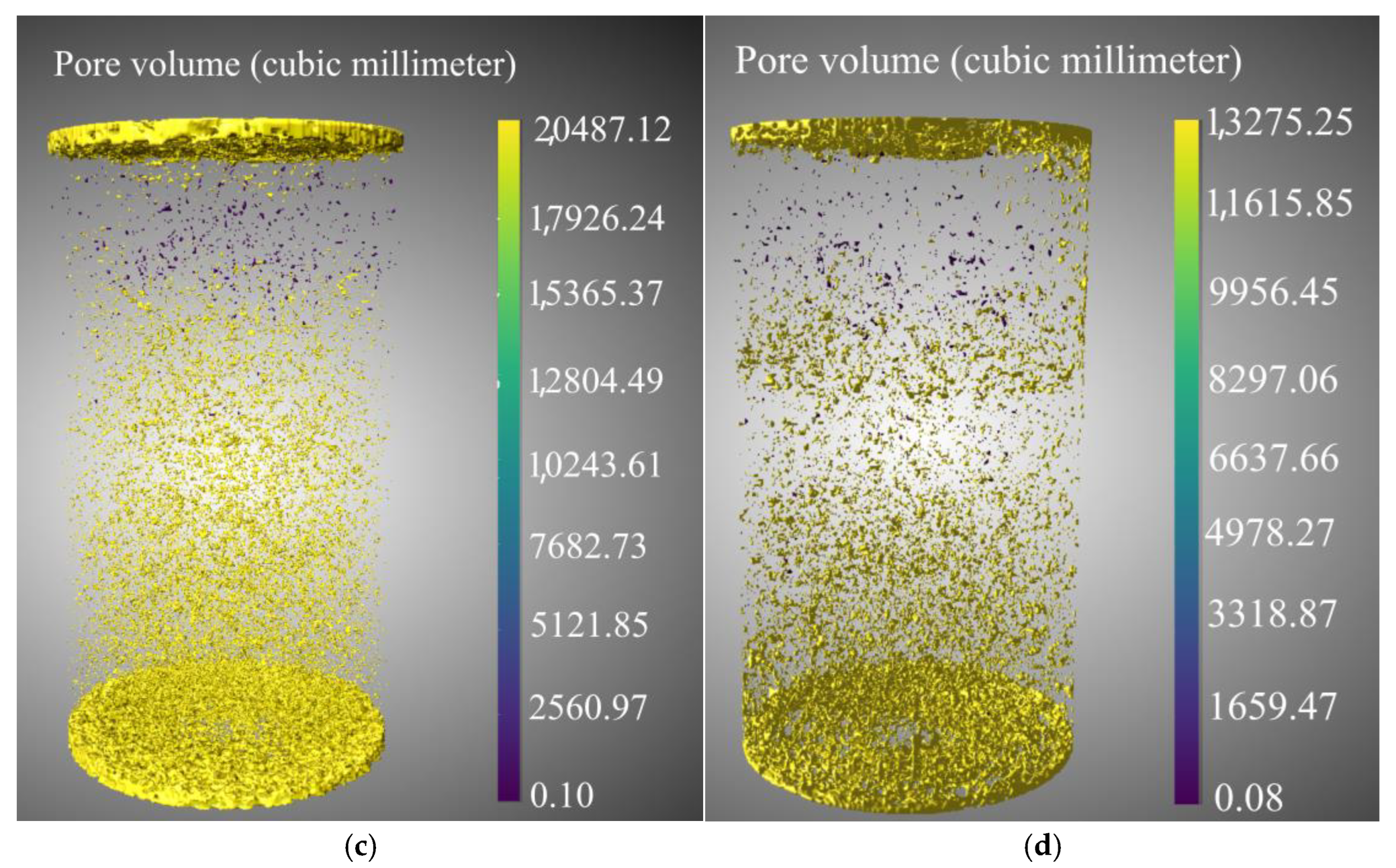
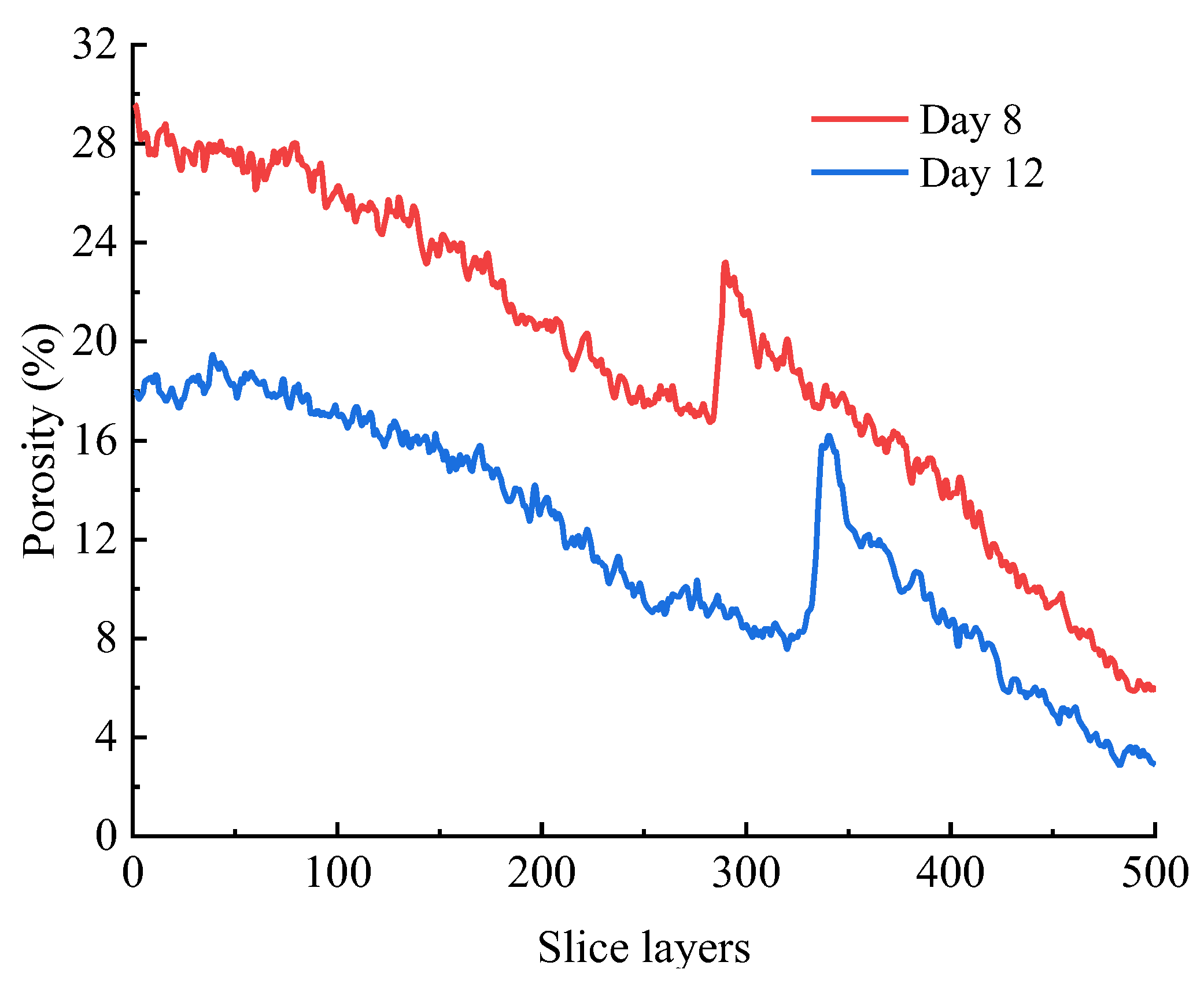
3.2.4. Quantitative Characterization of Pore Structure Based on CT Scanning
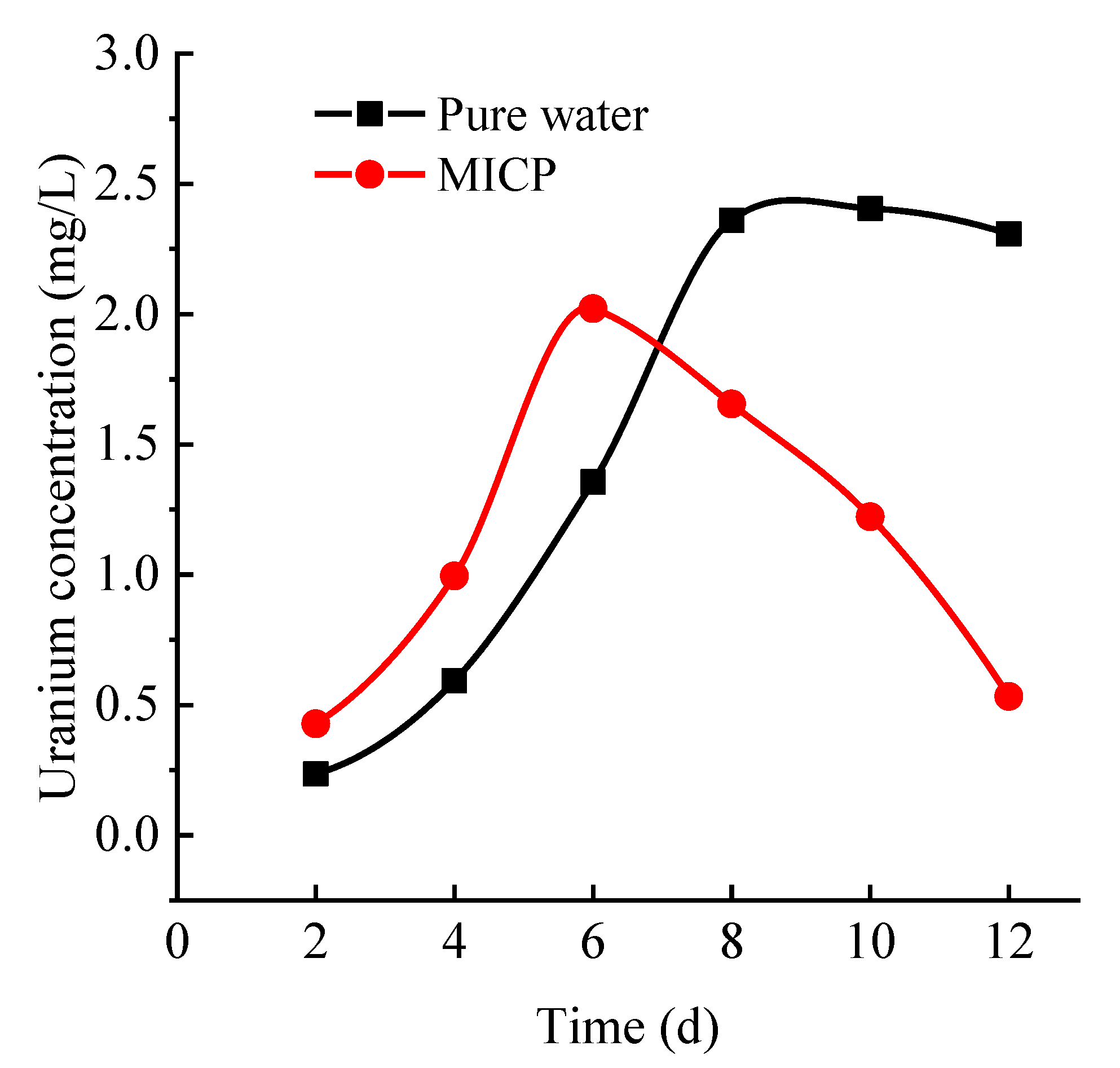
3.2.5. Determination of Changes in Uranium Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.S.; Yin, L.-Y.; Jiang, N.-J.; Zhu, C.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Lv, C.; Tang, C.S.; Zhang, J.Z.; Pan, X.H.; Liu, H. Effects of calcium sources and magnesium ions on the mechanical behavior of MICP-treated calcareous sand: Experimental evidence and precipitated crystal insights. Acta Geotech. 2022, 18, 2703–2717. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wang, G.; Zhong, Y.H.; Shao, J.G.; Zhao, J.C.; Li, D.B. Comparison of different treatment methods on macro-micro characteristics of Yellow River silt solidified by MICP technology. Mar. Georesources Geotechnol. 2022, 41, 425–435. [Google Scholar] [CrossRef]
- Song, C.; Elsworth, D.; Jia, Y.; Lin, J. Permeable rock matrix sealed with microbially-induced calcium carbonate precipitation: Evolutions of mechanical behaviors and associated microstructure. Eng. Geol. 2022, 304, 106697. [Google Scholar] [CrossRef]
- Banik, N.; Sarkar, R.; Uddin, M.E. Assessment of strength and low-strain shear modulus of bio-cemented sand considering MICP treatment. Environ. Earth Sci. 2023, 82, 1–20. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, Y.; Tong, H.; Yuan, J.; Liu, J. Study on calcareous sand treated by MICP in different NaCl concentrations. Eur. J. Environ. Civ. Eng. 2022, 27, 3137–3156. [Google Scholar] [CrossRef]
- Xiao, R.; Liang, B.Y.; Wu, F.; Huang, L.C.; Lai, Z.S. Biocementation of coral sand under seawater environment and an improved three-stage biogrouting approach. Constr. Build. Mater. 2023, 362, 129758. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J.; He, Z.; Zheng, C.; Pan, X. Study on soil physical structure after the bioremediation of Pb pollution using microbial-induced carbonate precipitation methodology. J. Hazard. Mater. 2021, 411, 125103. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X.L.; Zhang, D.Y. Remediation of copper-con taminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.H.; Guo, H.X. Heavy metal removal by biomin-eralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian Mine cal careous soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Waggitt, P. A Review of Worldwide Practices for Disposal of Uranium Mill Tailings; Australian Government Publishing Service: Canberra, Australia, 1994. [Google Scholar]
- Ting, L.; Qiaofeng, L. Uranium Resources Present Situation and Development Trend of Nuclear Power Around World. Mod. Min. 2017, 4, 98–103. (In Chinese) [Google Scholar]
- Li, G.; Liu, Y.; Zhao, G.; Zang, X. Analysis and Application of Environmental Stability of Decommissioning Uranium Tailings Pond. Ind. Saf. Environ. Prot. 2016, 42, 68–71. (In Chinese) [Google Scholar]
- Lui, R.; Chen, J.; Li, J.; Wang, X.; Huang, M. Study on Countermeasures of Supervision and Radiation Safety of Uranium Mine and Mill in North China. Uranium Min. Metall. 2017, 36, 151–154. [Google Scholar]
- Howe, S.E.; Davidson, C.M.; McCartney, M. Determination of uranium concentration and isotopic composition by means of ICP-MS in sequential extracts of sediment from the vicinity of a uranium enrichment plant. J. Anal. At. Spectrom. 2002, 17, 497–501. [Google Scholar] [CrossRef]
- ASTM. Standard Classification of Soils for Engineering Purposes (Unified Soil Classification System); American Society for Testing and Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Zhang, Z.-J.; Tong, K.-W.; Hu, L.; Yu, Q.; Wu, L.-L. Experimental study on solidification of tailings by MICP under the regulation of organic matrix. Constr. Build. Mater. 2020, 265, 120303. [Google Scholar] [CrossRef]
- Saxena, N.; Hofmann, R.; Alpak, F.O.; Dietderich, J.; Hunter, S.; Day-Stirrat, R.J. Effect of image segmentation & voxel size on micro-CT computed effective transport & elastic properties. Mar. Pet. Geol. 2017, 86, 972–990. [Google Scholar]

| Experimental Instrument | Company | Model |
|---|---|---|
| pH | Mettler Toledo, Shanghai, China | S220-K |
| OD600 | Eppendorf, Hamburg, Germany | Biophotometer |
| ICP-MS | Agilent, Santa Clara, CA, USA | 7700× |
| XRD | Hitachi, Tokyo, Japan | Regulus8100 |
| SEM | Bruker, Karlsruhe, Germany | Bruker D8 ADVANCE |
| CT | Zeiss Xradia, Turingen, Germany | 410Versa |
| Triaxial testing | Teco, Nanjing, China | TKA-TTS-IS |
| Test Group | Confining Pressure (kPa) | Deviatoric Stress (kPa) | Cohesion (kPa) | Internal Friction Angle (°) |
|---|---|---|---|---|
| original tail sand | 50 | 112.15928 | 5.564 | 17.891 |
| 100 | 200.29918 | |||
| 200 | 393.86521 | |||
| Reinforcement (12 days) | 50 | 199.62468 | 21.906 | 26.284 |
| 100 | 329.99297 | |||
| 200 | 588.21775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Zhang, Z.; Wu, L.; Yu, Q.; Zheng, H.; Tian, Y.; He, G. Experimental Study on the Solidification of Uranium Tailings and Uranium Removal Based on MICP. Sustainability 2023, 15, 12387. https://doi.org/10.3390/su151612387
Hu L, Zhang Z, Wu L, Yu Q, Zheng H, Tian Y, He G. Experimental Study on the Solidification of Uranium Tailings and Uranium Removal Based on MICP. Sustainability. 2023; 15(16):12387. https://doi.org/10.3390/su151612387
Chicago/Turabian StyleHu, Lin, Zhijun Zhang, Lingling Wu, Qing Yu, Huaimiao Zheng, Yakun Tian, and Guicheng He. 2023. "Experimental Study on the Solidification of Uranium Tailings and Uranium Removal Based on MICP" Sustainability 15, no. 16: 12387. https://doi.org/10.3390/su151612387





