Photocatalytic Degradation of Textile Dyeing Wastewater Using Titanium Dioxide on a Fixed Substrate: Optimization of Process Parameters and Continuous Reactor Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiO2 Coated Glass Beads Preparation
2.2. Synthesis of Textile Dyeing Wastewater
2.3. Photodegradation in Batch Experiments
2.4. Photodegradation Efficiency in Continuous Column Experiments
2.5. Analytical Methods
3. Results and Discussion
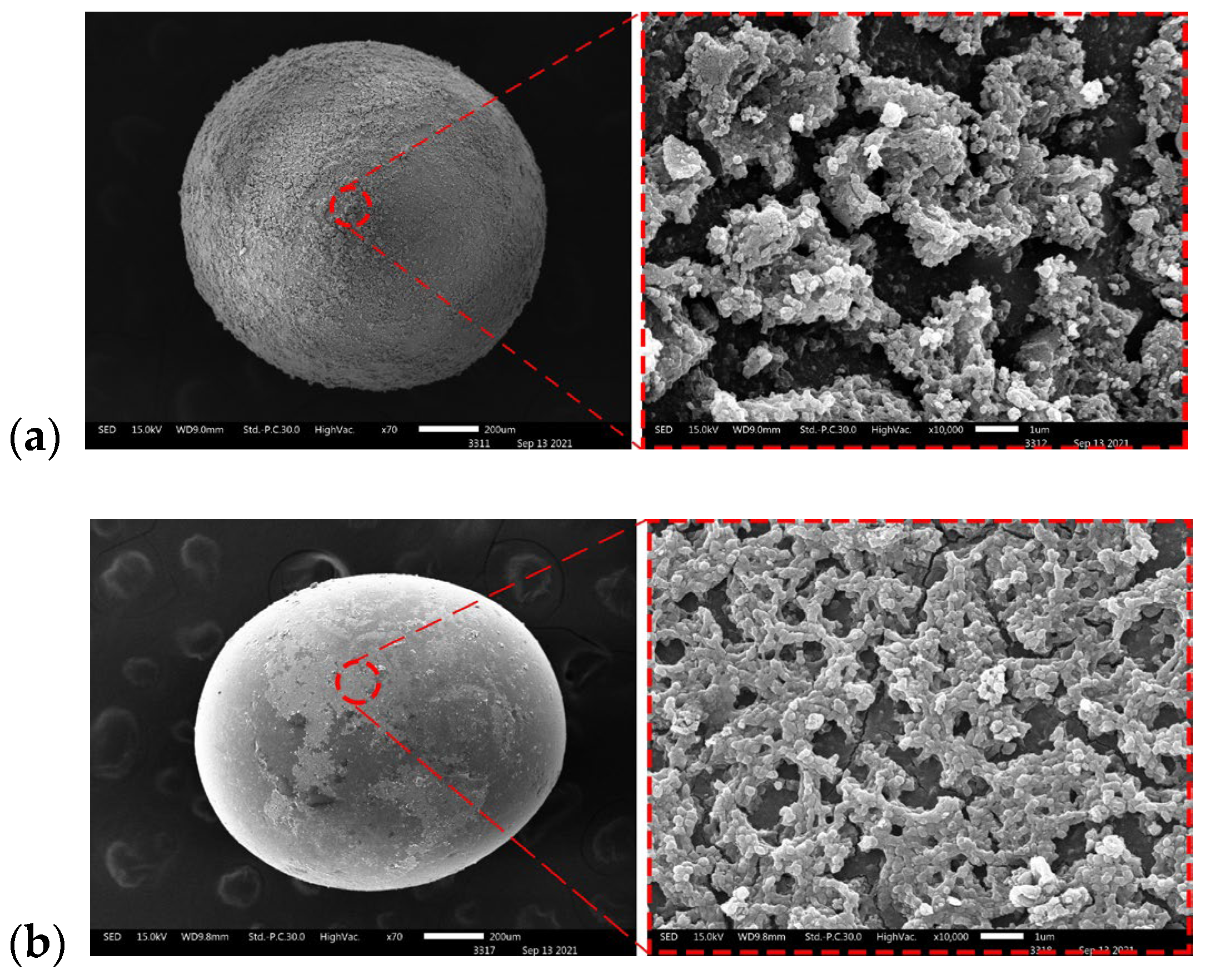
3.1. Characteristics of TiO2-Coated Glass Beads
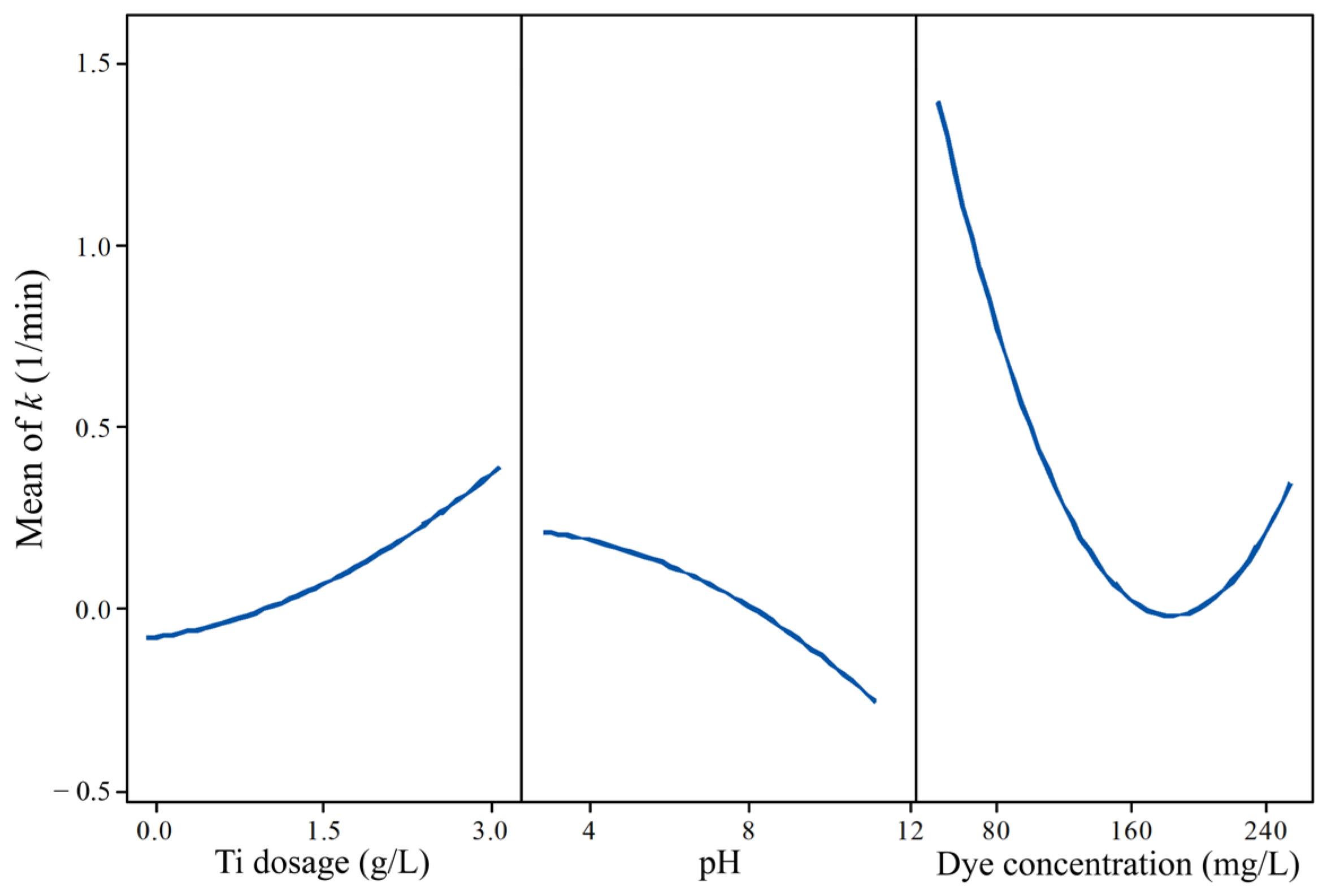
3.2. Batch Experiment for Textile Dyeing Wastewater Treatment Optimization
3.2.1. Optimum Conditions for Photocatalysis of Textile Dyeing Wastewater
+ 0.0196(TiO2 × TiO2) − 0.0204(pH × pH) + 0.1821(Dye × Dye)
− 0.0906(TiO2 × pH) − 0.0806(TiO2 × Dye) − 0.0331(pH × UV)
+ 0.1119(pH × Dye) − 0.0406(UV × Dye)
3.2.2. Validation of the Prediction Equation
3.3. Effect of Light Source on Photodegradation Rate
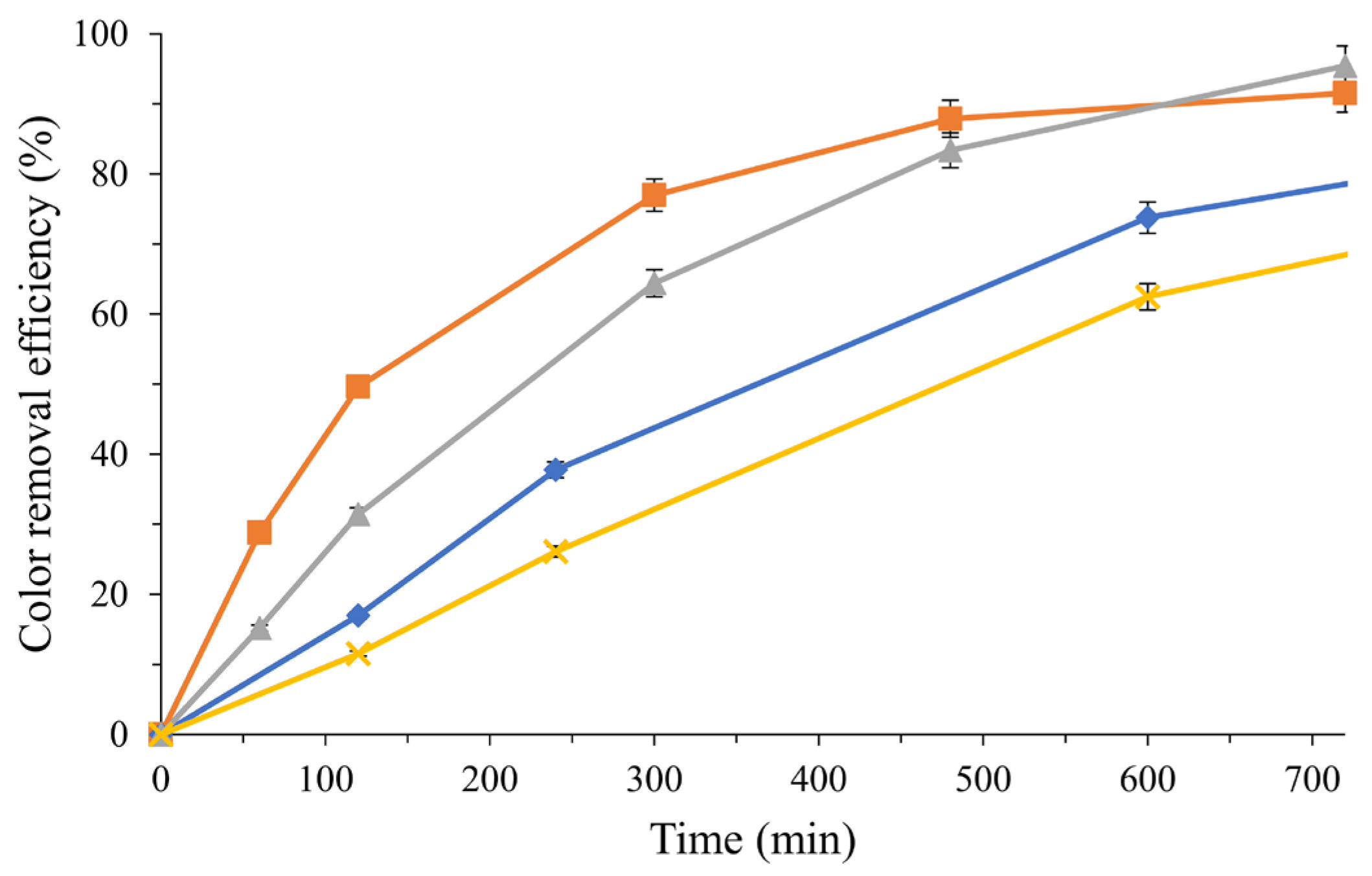
3.4. Fixed-Bed Photocatalysis Reactor for Treatment of Textile Dye Dyeing Wastewater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- India CSR Network. Textile Sector—Second Largest Employer after Agriculture, Accounts 2% of the GDP. Available online: https://indiacsr.in/textile-sector-second-largest-employer-after-agricultureaccounts-2-of-the-gdp/ (accessed on 21 October 2019).
- Artisan Alliance. Artisan Sector. Available online: http://www.artisanalliance.org/sector (accessed on 21 October 2019).
- Ramesh Babu, B.; Parande, A.K.; Raghu, S.; Prem Kumar, T. Textile technology cotton textile processing: Waste generation and effluent treatment. J. Cotton Sci. 2007, 11, 141–153. [Google Scholar]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile Dyeing Wastewater Treatment; Huazhong University of Science and Technology: Wuhan, China, 2011. [Google Scholar]
- Ariza-Tarazona, M.C.; Siligardi, C.; Carreón-López, H.A.; Valdéz-Cerda, J.E.; Pozzi, P.; Kaushik, G.; Villarreal-Chiu, J.F.; Cedillo-Gonz’alez, E.I. Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C, N-TiO2/SiO2 photocatalysts. Mar. Pollut. Bull. 2023, 193, 115206. [Google Scholar] [CrossRef]
- Torres, N.H.; Souza, B.S.; Ferreira, L.F.R.; Lima, A.S.; Nicolau, G.; Cavalcanti, E.B. Real textile effluents treatment using coagulation/flocculation followed by electrochemical oxidation process and ecotoxicological assessment. Chemosphere 2019, 236, 124309. [Google Scholar] [CrossRef]
- Chimupala, Y.; Phromma, C.; Yimklan, S.; Semakul, N.; Ruankham, P. Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv. 2020, 10, 28567–28575. [Google Scholar] [CrossRef]
- Chula Unisearch Academic Services. Wastewater Management for Textile Dyeing Factories Guidelines Handbook; Chula Unisearch Academic Services: Bangkok, Thailand, 2013. (In Thai) [Google Scholar]
- Zangeneh, H.; Zinatizadeh, A.A.; Nazari, S.; Joshaghani, M.; Zinadini, S.; Sibali, L.; Feyzi, M. Highly efficient azo dye degradation in a photocatalytic rotating disc reactor with deposited L-histidine-TiO2-CdS. Mater. Sci. Semicond. Process. 2022, 152, 107071. [Google Scholar] [CrossRef]
- Hoai, P.T.T.; Lam, T.D.; Huong, N.T.M.; Anh, M.T.V. Removal of ethylene by synthesized Ag/TiO2 photocatalyst under visible light irradiation. Chemosphere 2023, 329, 138607. [Google Scholar] [CrossRef] [PubMed]
- Chairungsri, W.; Subkomkaew, A.; Kijjanapanich, P.; Chimupala, Y. Direct dye wastewater photocatalysis using immobilized titanium dioxide on fixed substrate. Chemosphere 2022, 286, 131762. [Google Scholar] [CrossRef] [PubMed]
- Yuzer, B.; Aydin, M.I.; Con, A.H.; Inan, H.; Can, S.; Selcuk, H.; Kadmi, Y. Photocatalytic, self-cleaning and antibacterial properties of Cu(II) doped TiO2. J. Environ. Manag. 2022, 302, 114023. [Google Scholar] [CrossRef]
- Ali, A.; Shoeb, M.; Baoan, L.; Khan, M.A. Photocatalytic degradation of antibiotic drug and dye pollutants under visible-light irradiation by reduced grapheneoxide decorated MoO3/TiO2 nanocomposite. Mater. Sci. Semicond. Process. 2022, 150, 106974. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chimupala, Y.; Drummond-Brydson, R. Hydrothermal synthesis and phase formaton mechanism of TiO2(B) nanorods via alkali metal titanate phase. Solid State Phenom. 2018, 283, 23–36. [Google Scholar] [CrossRef]
- Hathaisamit, K.; Sutha, W.; Kamruang, P.; Pudwat, S.; Teekasap, S. Decolorization of Cationic Yellow X-Gl 200% from textile dyes by TiO2 films-coated rotor. Procedia Eng. 2012, 32, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, H.S.; Parmesh, G.; Vaish, R.; Varma, K.B.R. TiO2 microcrystallized glass plate mediated photocatalytic degradation of estrogenic pollutant in water. J. Non-Cryst. Solids. 2015, 408, 13–17. [Google Scholar] [CrossRef]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B 2019, 200, 111655. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.M.; Stojiljkovic, S.T.; Savic, I.M.; Stojanovic, S.B.; Moder, K. Modeling and optimization of Fe(III) adsorption from water using bentonite clay: Comparison of central composite design and artificial neural network. Chem. Eng. Technol. 2012, 35, 2007–2014. [Google Scholar] [CrossRef]
- Ping-feng, F.; Zhuo, Z.; Peng, P.; Xue-gang, D. Photodegradation of methylene blue in a batch fixed bed photoreactor using activated carbon fibers supported TiO2 photocatalyst. Guocheng Gongcheng Xuebao 2008, 8, 66–71. [Google Scholar]
- Tai, A.; (Textile Dyeing Factory, Chiang Mai, Thailand). Personal Communication, 2021.
- Pantelis, A.P.; Nikolaos, P.X.; Dionissios, M. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar]
- Subramani, A.K.; Byrappa, K.; Ananda, S.; Lokanatha Rai, K.M.; Ranganathaiah, C.; Yoshimura, M. Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull. Mater. Sci. 2007, 30, 37–41. [Google Scholar] [CrossRef]
- Liu, C.; Hsieh, Y.; Lai, P.; Li, C.; Kao, C. Photodegradation treatment of azo dye wastewater by UV/TiO2 process. Dyes Pigm. 2006, 68, 191–195. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, R.; Farooq Khan, A.; Farooq, R. Improved photocatalytic activity of TiO2 coupling ultrasound for reactive blue 19 degradation. J. Chem. Soc. Pak. 2014, 36, 37. [Google Scholar]
- Huang, D.M.Y.; Matsumoto, T.; Tojo, T.; Fan, T.; Ding, J.; Guo, Q.; Zhang, D. Preparation and characterization of high-surface-area TiO2/activated carbon by low-temperature impregnation. Sep. Purif. Technol. 2011, 78, 9–15. [Google Scholar] [CrossRef]
- Tiwari, H.; Sonwani, R.K.; Singh, R.S. A comprehensive evaluation of the integrated photocatalytic-fixed bed bioreactor system for the treatment of Acid Blue 113 dye. Bioresour. Technol. 2022, 364, 128037. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Gaware, G.J.; Chinthala, M. Heterojunction photocatalysts for the removal of nitrophenol: A systematic review. Chemosphere 2023, 310, 136853. [Google Scholar] [CrossRef]
- Yang, L.; Fu, Y.; Sun, F.; Deng, M.; Zhang, C.; Li, N.; Hao, D.; Want, Q.; Zhuang, G. Preparation of novel diperylene-cored polyimide photocatalyst with broad-spectra response and high stability. J. Colloid Interface Sci. 2023, 639, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Liu, X.; Wang, H.; Liu, Z.; Xing, H.; You, L. Preparation and characterization of TiO2 photocatalytic composites supported by blast furnace slag fibres for wastewater degradation. Ceram. Int. 2023, 49, 5180–5188. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Mishra, J.; Nanda, J.; Sahoo, P.K.; Kumar, R.; Sahoo, N.K. Photocatalytic degradation of cyanide using polyurethane foam immobilized Fe-TCPP-S-TiO2-rGO nano-composite. J. Environ. Manag. 2021, 297, 113312. [Google Scholar] [CrossRef]





| Parameters | Uncoded Value | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| TiO2 dosage (g/L) | 0.00 | 0.75 | 1.50 | 2.25 | 3.00 |
| Initial pH | 3.0 | 5.0 | 7.0 | 9.0 | 11.0 |
| UV-A intensity (µW/cm2) | 1000 | 1750 | 2500 | 3250 | 4000 |
| Dye concentration (mg/L) | 50 | 100 | 150 | 200 | 250 |
| Type of Runs | No. | Parameters | Direct Dye Removal Efficiency (%) | |||
|---|---|---|---|---|---|---|
| TiO2 (g/L) | pH | UV Intensity (µW/cm2) | Dye Concentration (mg/L) | |||
| Full-factorial experiments | 1 | 0.75 | 5.0 | 1750 | 100 | 24.6 |
| 2 | 2.25 | 5.0 | 1750 | 100 | 67.4 | |
| 3 | 0.75 | 5.0 | 3250 | 100 | 37.4 | |
| 4 | 2.25 | 5.0 | 3250 | 100 | 75.8 | |
| 5 | 0.75 | 9.0 | 1750 | 100 | 13.1 | |
| 6 | 2.25 | 9.0 | 1750 | 100 | 15.4 | |
| 7 | 0.75 | 9.0 | 3250 | 100 | 14.7 | |
| 8 | 2.25 | 9.0 | 3250 | 100 | 37.2 | |
| 9 | 0.75 | 5.0 | 1750 | 200 | 1.9 | |
| 10 | 2.25 | 5.0 | 1750 | 200 | 3.3 | |
| 11 | 0.75 | 5.0 | 3250 | 200 | 2.5 | |
| 12 | 2.25 | 5.0 | 3250 | 200 | 21.0 | |
| 13 | 0.75 | 9.0 | 1750 | 200 | 1.6 | |
| 14 | 2.25 | 9.0 | 1750 | 200 | 3.5 | |
| 15 | 0.75 | 9.0 | 3250 | 200 | 4.1 | |
| 16 | 2.25 | 9.0 | 3250 | 200 | 10.6 | |
| Central composite experiments | 17 | 0.00 | 7.0 | 2500 | 150 | 0.8 |
| 18 | 3.00 | 7.0 | 2500 | 150 | 50.2 | |
| 19 | 1.50 | 3.0 | 2500 | 150 | 21.3 | |
| 20 | 1.50 | 11.0 | 2500 | 150 | 1.0 | |
| 21 | 1.50 | 7.0 | 1000 | 150 | 19.0 | |
| 22 | 1.50 | 7.0 | 4000 | 150 | 32.3 | |
| 23 | 1.50 | 7.0 | 2500 | 50 | 93.2 | |
| 24 | 1.50 | 7.0 | 2500 | 250 | 2.7 | |
| Center point experiments | 25 | 1.50 | 7.0 | 2500 | 150 | 12.1 |
| 26 | 1.50 | 7.0 | 2500 | 150 | 11.4 | |
| 27 | 1.50 | 7.0 | 2500 | 150 | 12.7 | |
| 28 | 1.50 | 7.0 | 2500 | 150 | 11.7 | |
| 29 | 1.50 | 7.0 | 2500 | 150 | 12.0 | |
| 30 | 1.50 | 7.0 | 2500 | 150 | 10.6 | |
| 31 | 1.50 | 7.0 | 2500 | 150 | 10.9 | |
| 32 | 1.50 | 7.0 | 2500 | 150 | 11.3 | |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 11 | 3.510 | 0.319 | 5.81 | 0.001 |
| Linear effects | 3 | 2.094 | 0.698 | 12.71 | 0.000 |
| TiO2 | 1 | 0.302 | 0.302 | 5.49 | 0.032 |
| pH | 1 | 0.297 | 0.297 | 5.41 | 0.034 |
| Dye conc. | 1 | 1.495 | 1.495 | 27.22 | 0.000 |
| Square effects | 3 | 0.936 | 0.312 | 5.68 | 0.008 |
| TiO2 × TiO2 | 1 | 0.010 | 0.010 | 0.18 | 0.678 |
| pH × pH | 1 | 0.011 | 0.011 | 0.19 | 0.666 |
| Dye conc. × Dye conc. | 1 | 0.849 | 0.849 | 15.46 | 0.001 |
| Two-Way Interactions | 5 | 0.480 | 0.096 | 1.75 | 0.181 |
| TiO2 × pH | 1 | 0.131 | 0.131 | 2.39 | 0.141 |
| TiO2 × Dye | 1 | 0.104 | 0.104 | 1.89 | 0.188 |
| pH × UV | 1 | 0.018 | 0.018 | 0.32 | 0.580 |
| pH × Dye conc. | 1 | 0.200 | 0.200 | 3.65 | 0.074 |
| UV × Dye conc. | 1 | 0.026 | 0.026 | 0.48 | 0.498 |
| Error | 16 | 0.878 | 0.055 | ||
| Lack-of-fit | 13 | 0.878 | 0.068 | ||
| Pure error | 3 | 0.000 | 0.000 | ||
| Total | 27 | 4.388 | |||
| Model summary | |||||
| S | R2 | R2(adj) | R2 (pred) | ||
| 0.234358 | 79.98% | 66.21% | 12.51% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chairungsri, W.; Pholchan, P.; Sumitsawan, S.; Chimupala, Y.; Kijjanapanich, P. Photocatalytic Degradation of Textile Dyeing Wastewater Using Titanium Dioxide on a Fixed Substrate: Optimization of Process Parameters and Continuous Reactor Tests. Sustainability 2023, 15, 12418. https://doi.org/10.3390/su151612418
Chairungsri W, Pholchan P, Sumitsawan S, Chimupala Y, Kijjanapanich P. Photocatalytic Degradation of Textile Dyeing Wastewater Using Titanium Dioxide on a Fixed Substrate: Optimization of Process Parameters and Continuous Reactor Tests. Sustainability. 2023; 15(16):12418. https://doi.org/10.3390/su151612418
Chicago/Turabian StyleChairungsri, Woottikrai, Patiroop Pholchan, Sulak Sumitsawan, Yothin Chimupala, and Pimluck Kijjanapanich. 2023. "Photocatalytic Degradation of Textile Dyeing Wastewater Using Titanium Dioxide on a Fixed Substrate: Optimization of Process Parameters and Continuous Reactor Tests" Sustainability 15, no. 16: 12418. https://doi.org/10.3390/su151612418





