Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization
Abstract
:1. Introduction
2. Materials and Methods
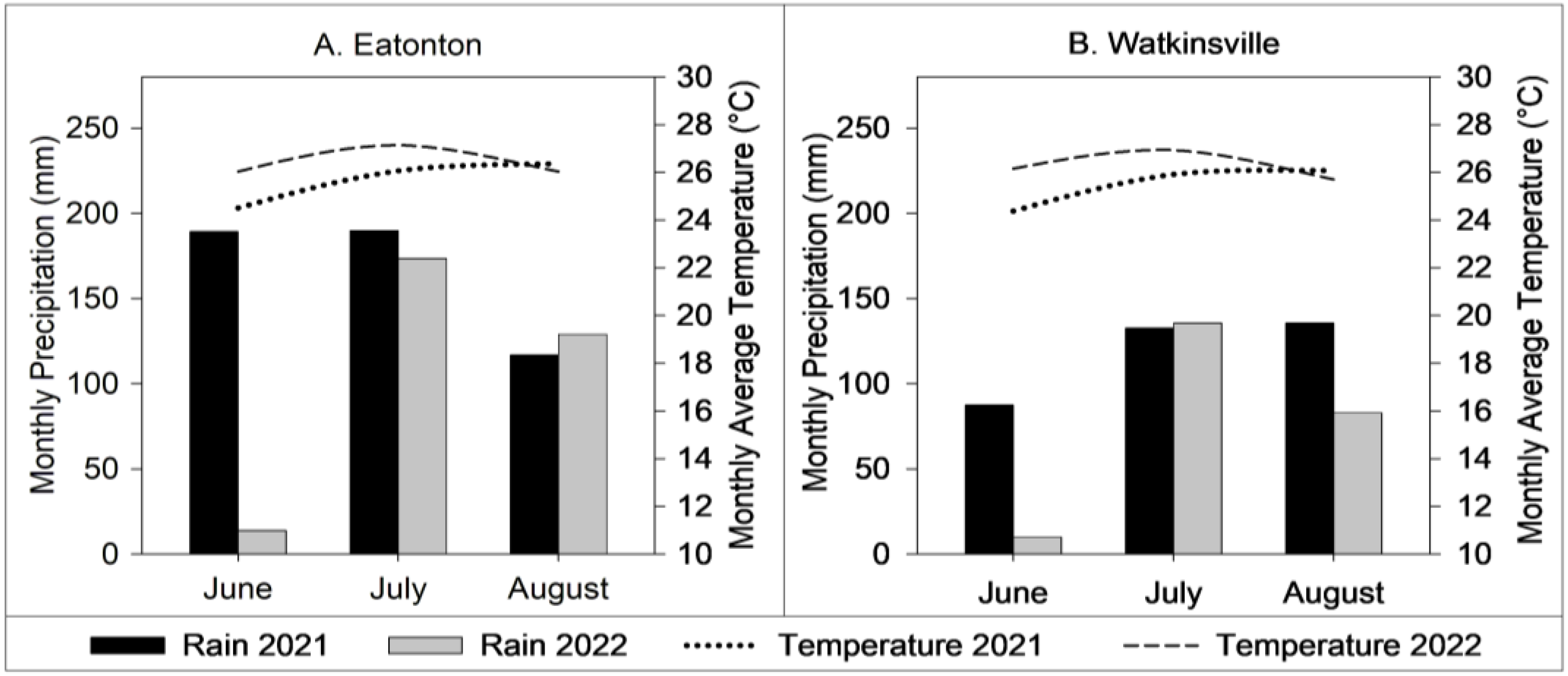
2.1. Characteristics of the Study Site
2.2. Experimental Design and Sampling
2.3. Characterization of Filth Fly Populations
2.4. Characterization of Biodiversity Populations
2.5. Ammonia Volatilization, Nitrous Oxide, and Carbon Dioxide Emissions
2.6. Analysis of Soil and Manure Samples
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Epigeal Arthropods and Filth Fly Populations
3.2. Factors Affecting Filth Fly Development
3.3. Nitrogen Emissions
3.3.1. Ammonia Emissions
3.3.2. Nitrous Oxide Emissions
3.3.3. The Impact of Filth Flies on Nitrogen Emissions in the Environment
3.4. Carbon Dioxide Emissions
3.5. Soil Nitrogen
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT Land Use. Available online: https://www.fao.org/faostat/en/#data/RL (accessed on 9 July 2023).
- Ritchie, H.; Roser, M. Land Use. Available online: https://ourworldindata.org/land-use (accessed on 9 July 2023).
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production. Available online: https://ourworldindata.org/meat-production (accessed on 9 July 2023).
- USDA. Southern Region News Release Cattle Inventory; United States Department of Agriculture—National Agricultural Statistics Service: Washington, DC, USA, 2022.
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Herrero, M.; Havlik, P.; Valin, H.; Notenbaert, A.; Rufino, M.C.; Thornton, P.K.; Blummel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef] [PubMed]
- Nyameasem, J.K.; Malisch, C.S.; Loges, R.; Taube, F.; Kluss, C.; Vogeler, I.; Reinsch, T. Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions. Atmosphere 2021, 12, 223. [Google Scholar] [CrossRef]
- Bolan, N.S.; Saggar, S.; Luo, J.; Bhandral, R.; Singh, J. Gaseous emissions of nitrogen from grazed pastures: Processes, measurements and modelling, environmental implications and mitigation. Adv. Agron. 2004, 84, 322. [Google Scholar] [CrossRef]
- Brito, L.F.; Azenha, M.V.; Janusckiewicz, E.R.; Cardoso, A.S.; Morgado, E.S.; Malheiros, E.B.; La Scala, N.; Reis, R.A.; Ruggieri, A.C. Seasonal Fluctuation of Soil Carbon Dioxide Emission in Differently Managed Pastures. Agron. J. 2015, 107, 957–962. [Google Scholar] [CrossRef]
- Tierling, J.; Kuhlmann, H. Emissions of nitrous oxide (N2O) affected by pH-related nitrite accumulation during nitrification of N fertilizers. Geoderma 2018, 310, 12–21. [Google Scholar] [CrossRef]
- NASS-USDA. Small Farms; NASS-USDA: Washington, DC, USA, 2007.
- NASS-USDA. Summary by Farm Typology Measured by Gross Cash Farm Income (GCFI) of Family Farm Producers and Non-Family Farms—Georgia: 2017; NASS-USDA: Washington, DC, USA, 2017; p. 14.
- NASS-USDA. Cattle Industry; NASS-USDA: Washington, DC, USA, 2015.
- USDA-APHIS. Small-Scale U.S. Cow-Calf Operations; USDA-APHIS: Washington, DC, USA, 2011.
- Hancock, D.W.; Harris, G.H.; Franks, R.W.; Morgan, S.P.; Green, T.W. Soil and Fertilizer Management Considerations for Forage Systems in Georgia; University of Georgia: Athens, GA, USA, 2014. [Google Scholar]
- Cardoso, A.d.S.; Oliveira, S.C.; Janusckiewicz, E.R.; Brito, L.F.; Morgado, E.d.S.; Reis, R.A.; Ruggieri, A.C. Seasonal effects on ammonia, nitrous oxide, and methane emissions for beef cattle excreta and urea fertilizer applied to a tropical pasture. Soil Tillage Res. 2019, 194, 104341. [Google Scholar] [CrossRef]
- Butler, J.F.; Escher, R.; Hogsette, J.A. Natural parasite levels in house flies, stable flies, and horn flies in Florida. In Status of Biological Control of Filth Flies; Patterson, R.S., Ed.; U. S. Department of Agriculture Science and Education Administration: New Orleans, LA, USA, 1981; Volume 771, p. 212. [Google Scholar]
- Foil, L.D.; Hogsette, J.A. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. Oie 1994, 13, 1125–1158. [Google Scholar] [CrossRef]
- Brewer, G.J.; Boxler, D.J.; Domingues, L.N.; Trout Fryxell, R.T.; Holderman, C.; Loftin, K.M.; Machtinger, E.; Smythe, B.; Talley, J.L.; Watson, W. Horn Fly (Diptera: Muscidae)—Biology, Management, and Future Research Directions. J. Integr. Pest Manag. 2021, 12, 42. [Google Scholar] [CrossRef]
- Hogsette, J.A.; Prichard, D.L.; Ruff, J.P. Economic effects of horn fly (Diptera: Muscidae) populations on beef cattle exposed to three pesticide treatment regimes. J. Econ. Entomol. 1991, 84, 1270–1274. [Google Scholar] [CrossRef]
- Kuramochi, K.; Nishijima, Y. Measurement of the Meal Size of the Horn Fly, Hematobia irritans (L) (Diptera: Muscidae), by the Use of Amaranth. Appl. Entomol. Zool. 1980, 15, 262–269. [Google Scholar] [CrossRef]
- Iwasa, M.; Moki, Y.; Takahashi, J. Effects of the Activity of Coprophagous Insects on Greenhouse Gas Emissions from Cattle Dung Pats and Changes in Amounts of Nitrogen, Carbon, and Energy. Environ. Entomol. 2015, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, A. Horn Fly Breeding, Nitrogen Loss and Nutrient Immobilization Associated with Cattle Dung in the Southern Interior of British Columbia; Simon Fraser University: Burnaby, BC, Canada, 1973. [Google Scholar]
- Macqueen, A.; Doube, B.M. Emergence, Host-Finding and Longevity of Adult Haematobia irritans exigua Demeijere (Diptera, Muscidae). J. Aust. Entomol. Soc. 1988, 27, 167–174. [Google Scholar] [CrossRef]
- deCastro-Arrazola, I.; Andrew, N.R.; Berg, M.P.; Curtsdotter, A.; Lumaret, J.-P.; Menéndez, R.; Moretti, M.; Nervo, B.; Nichols, E.S.; Sánchez-Piñero, F.; et al. A trait-based framework for dung beetle functional ecology. J. Anim. Ecol. 2023, 92, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.D. Insect population dynamics meets ecosystem ecology: Effects of herbivory on soil nutrient dynamics. Agric. Forest Entomol. 2001, 3, 77–84. [Google Scholar] [CrossRef]
- Lafleur, B.; Bradley, R.L.; Francoeur, A. Soil modifications created by ants along a post-fire chronosequence in lichen-spruce woodland. Ecoscience 2002, 9, 63–73. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kai, H.; Koga, T.; Aibe, T. Nitrogen mineralization and microbial populations in cow dung, dung balls and underlying soil affected by paracoprid dung beetles. Soil Biol. Biochem. 1991, 23, 649–653. [Google Scholar]
- Hu, G.Y.; Frank, J.H. Predation on the Horn Fly (Diptera: Muscidae) by Five Species of Philonthus (Coleoptera: Staphylinidae). Environ. Entomol. 1997, 26, 1240–1246. [Google Scholar] [CrossRef]
- Summerlin, J.W.; Petersen, H.D.; Harris, R.L. Red Imported Fire Ant (Hymenoptera: Formicidae): Effects on the Horn Fly (Diptera: Muscidae) and Coprophagous Scarabs. Environ. Entomol. 1984, 13, 1405–1410. [Google Scholar] [CrossRef]
- Dewes, T. Effect of pH, temperature, amount of litter and storage density on ammonia emissions from stable manure. J. Agric. Sci. 1996, 127, 501–509. [Google Scholar] [CrossRef]
- Longhini, V.Z.; Cardoso, A.D.; Berca, A.S.; Boddey, R.M.; Reis, R.A.; Dubeux, J.C.B.; Ruggieri, A.C. Nitrogen supply and rainfall affect ammonia emissions from dairy cattle excreta and urea applied on warm-climate pastures. J. Environ. Qual. 2020, 49, 1453–1466. [Google Scholar] [CrossRef]
- Hu, E.Z.; Sutitarnnontr, P.; Tuller, M.; Jones, S.B. Modeling temperature and moisture dependent emissions of carbon dioxide and methane from drying dairy cow manure. Front. Agric. Sci. Eng. 2018, 5, 280–286. [Google Scholar] [CrossRef]
- Brumme, R.; Borken, W.; Finke, S. Hierarchical control on nitrous oxide emission in forest ecosystems. Glob. Biogeochem. Cycles 1999, 13, 1137–1148. [Google Scholar] [CrossRef]
- Saggar, S.; Andrew, R.M.; Tate, K.R.; Hedley, C.B.; Rodda, N.J.; Townsend, J.A. Modelling nitrous oxide emissions from dairy-grazed pastures. Nutr. Cycl. Agroecosystems 2004, 68, 243–255. [Google Scholar] [CrossRef]
- Krewer, G.; Cline, B.; NeSmith, D.S. Southeast Regional Blueberry Horticulture and Growth Regulator Guide. 2007. Available online: https://www.uaex.uada.edu/farm-ranch/crops-commercial-horticulture/horticulture/commercial-fruit-production/Blueberry%20Hort%20Guide.pdf (accessed on 9 July 2023).
- Chang, F.; Fabian-Wheeler, E.; Richard, T.L.; Hile, M. Compaction effects on greenhouse gas and ammonia emissions from solid dairy manure. J. Environ. Manag. 2023, 332, 117399. [Google Scholar] [CrossRef] [PubMed]
- USDA. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 9 July 2023).
- UGA. University of Georgia Weather Network. Available online: http://weather.uga.edu/ (accessed on 9 July 2023).
- Lee, C.N.; Toyama, G.M. Ovipositional Preference Exhibited by Musca sorbens (Diptera: Muscidae) to Feces of Cows Fed Different Rations. Environ. Entomol. 1990, 19, 1296–1298. [Google Scholar] [CrossRef]
- Laub, C.; Youngman, R.R.; Love, K.; Mize, T. Using Pitfall Traps to Monitor Insect Activity. In Virginia Cooperative Extension; Virginia State University: Petersburg, VA, USA, 2019. [Google Scholar]
- Roeder, K.A.; Prather, R.M.; Paraskevopoulos, A.W.; Roeder, D.V. The Economics of Optimal Foraging by the Red Imported Fire Ant. Environ. Entomol. 2020, 49, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvanery, C.S. Nitrogen-Total 1; Wiley Online Library: Hoboken, NJ, USA, 1982. [Google Scholar]
- Maynard, D.G.; Kalra, Y.P.; Crumbaugh, J.A. Nitrate and Exchangeable ammonium nitrogen. In Soil Sampling and Mehtos of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Taylor & Francis Group: Abingdon, UK, 2008; p. 199. [Google Scholar]
- Doane, T.A.; Horwath, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Kempers, A.J.; Zweers, A. Ammonium Determination in Soil Extracts by the Salicylate Method. Commun. Soil Sci. Plan. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Picone, L.I.; Cabrera, M.L.; Franzluebbers, A.J. A rapid method to estimate potentially mineralizable nitrogen in soil. Soil Sci. Soc. Am. J. 2002, 66, 1843–1847. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Determination of Total Nitrogen in Plant Material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Larrain, P.; Salas, C. House fly (Musca domestica L.) (Diptera: Muscidae) development in different types of manure. Chil. J. Agric. Res. 2008, 68, 192–197. [Google Scholar] [CrossRef]
- Hu, G.Y.; Frank, J.H. Effect of the Arthropod Community on Survivorship of Immature Haematobia irritans (Diptera: Muscidae) in North Central Florida. Fla. Entomol. 1996, 79, 497–503. [Google Scholar] [CrossRef]
- Bussink, D.W. Ammonia volatilization from grassland receiving nitrogen fertilizer and rotationally grazed by dairy cattle. Fert. Res. 1992, 33, 257–265. [Google Scholar] [CrossRef]
- Nichols, K.L.; Del Grosso, S.J.; Derner, J.D.; Follett, R.F.; Archibeque, S.L.; Delgado, J.A.; Paustian, K.H. Nitrous Oxide and Ammonia Emissions from Cattle Excreta on Shortgrass Steppe. J. Environ. Qual. 2018, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Drees, B.B.M.; Summerlin, B.; Vinson, S.B. Foraging activity and temperature relationship for the red imported fire ant. Southwest Entomol. 2007, 32, 149–155. [Google Scholar] [CrossRef]
- Porter, S.D.; Tschinkel, W.R. Foraging in Solenopsis invicta (Hymenoptera: Formicidae): Effects of Weather and Season. Environ. Entomol. 1987, 16, 802–808. [Google Scholar] [CrossRef]
- Trout Fryxell, R.T.; Moon, R.D.; Boxler, D.J.; Watson, D.W. Face Fly (Diptera: Muscidae)—Biology, Pest Status, Current Management Prospects, and Research Needs. J. Integr. Pest Manag. 2021, 12, 5. [Google Scholar] [CrossRef]
- Yamulki, S.; Jarvis, S.C.; Owen, P. Nitrous oxide emissions from excreta applied in a simulated grazing pattern. Soil Biol. Biochem. 1998, 30, 491–500. [Google Scholar] [CrossRef]
- Clayton, H.; McTaggart, I.P.; Parker, J.; Swan, L.; Smith, K.A. Nitrous oxide emissions from fertilised grassland: A 2-year study of the effects of N fertiliser form and environmental conditions. Biol. Fert. Soils 1997, 25, 252–260. [Google Scholar] [CrossRef]
- Waldrip, H.M.; Parker, D.B.; Miller, S.; Miller, D.N.; Casey, K.D.; Todd, R.W.; Min, B.R.; Spiehs, M.J.; Woodbury, B. Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification. Atmosphere 2020, 11, 1056. [Google Scholar] [CrossRef]
- Baral, K.R.; Arthur, E.; Olesen, J.E.; Petersen, S.O. Predicting nitrous oxide emissions from manure properties and soil moisture: An incubation experiment. Soil Biol. Biochem. 2016, 97, 112–120. [Google Scholar] [CrossRef]
- Bollmann, A.; Conrad, R. Influence of O-2 availability on NO and N2O release by nitrification and denitrification in soils. Glob. Change Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Natural Resources Conservation Service. Agricultural Waste Management Field Handbook; Natural Resources Conservation Service: Washington, DC, USA, 2008; p. 40.
- Hanafiah, M.M.; Ibraheem, A.J.; Razman, K.K. Emissions of carbon dioxide and methane from dairy cattle manure. IOP Conf. Ser. Earth Environ. Sci. 2021, 880, 012037. [Google Scholar] [CrossRef]
- Mazzetto, A.M.; Barneze, A.S.; Feigl, B.J.; Van Groenigen, J.W.; Oenema, O.; Cerri, C.C. Temperature and moisture affect methane and nitrous oxide emission from bovine manure patches in tropical conditions. Soil Biol. Biochem. 2014, 76, 242–248. [Google Scholar] [CrossRef]
- Vidal-Beaudet, L.; Charpentier, S. Percolation theory and hydrodynamics of soil-peat mixtures. Soil Sci. Soc. Am. J. 2000, 64, 827–835. [Google Scholar] [CrossRef]
- Lin, X.W.; Wang, S.P.; Ma, X.Z.; Xu, G.P.; Luo, C.Y.; Li, Y.N.; Jiang, G.M.; Xie, Z.B. Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods. Soil Biol. Biochem. 2009, 41, 718–725. [Google Scholar] [CrossRef]
- Iqbal, J.; Hu, R.G.; Lin, S.; Hatano, R.; Feng, M.L.; Lu, L.; Ahamadou, B.; Du, L.J. CO2 emission in a subtropical red paddy soil (Ultisol) as affected by straw and N-fertilizer applications: A case study in Southern China. Agric. Ecosyst. Environ. 2009, 131, 292–302. [Google Scholar] [CrossRef]
- Fogal, W.H.; Slansky, F. Contribution of feeding by European pine sawfly larvae to litter production and element flux in Scots pine plantations. Can. J. Forest Res. 1985, 15, 484–487. [Google Scholar] [CrossRef]
- Tukey, H.B.; Morgan, J.V. Injury to Foliage and its Effect Upon the Leaching of Nutrients from Above-Ground Plant Parts. Physiol. Plant 1963, 16, 557–564. [Google Scholar] [CrossRef]
- Swank, W.T.; Waide, J.B.; Crossley, D.A.; Todd, R.L. Insect Defoliation Enhances Nitrate Export from Forest Ecosystems. Oecologia 1981, 51, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; de Ruiter, P.; Didden, W.; Janssen, M.; Schouten, T.; Verhoef, H. Community food web, decomposition and nitrogen mineralisation in a stratified Scots pine forest soil. Oikos 2001, 94, 130–142. [Google Scholar] [CrossRef]
- Osler, G.H.R.; Sommerkorn, M. Toward a complete soil C and N cycle: Incorporating the soil fauna. Ecology 2007, 88, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, A.; Beirne, B.P. Influence of Some Dipterous Larvae on Nitrogen Loss from Cattle Dung. Environ. Entomol. 1975, 4, 868–870. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Barbercheck, M.E. Linking invertebrate communities to decomposition rate and nitrogen availability in pine forest soils. Appl. Soil Ecol. 2012, 54, 14–23. [Google Scholar] [CrossRef]














| Replication | NH3 Captured with 24 h Trap (mg N) | NH3 Captured with Fresh Trap (mg N) | NH3 Remaining in Desiccator (mg N) | Trap Capacity (%) |
|---|---|---|---|---|
| 1 | 20.6 | 0.08 | 38.4 | 34.9% |
| 2 | 18.1 | 0.07 | 33.8 | 34.9% |
| 3 | 18.9 | 0.09 | 40.1 | 32.0% |
| 4 | 20.7 | 0.09 | 38.8 | 34.7% |
| Mean ± STD | 19.6 ± 1.1 | 0.08 ± 0.01 | 37.8 ± 2.4 | 34.1 ± 1.2% |
| Species | Eatonton Research Farm | Garmon Farm | Hollowed Hawk Farm | Total Number of Individuals per Species | Percentage of Total | |||
|---|---|---|---|---|---|---|---|---|
| Total | Median ± QD | Total | Median ± QD | Total | Median ± QD | |||
| Horn fly (Haematobia irritans Linnaeus) | 384 | 21.0 ± 12.8 | 215 | 10.0 ± 7.9 | 380 | 11.5 ± 9.6 | 979 | 80.2% |
| Bottle fly (Lucilia sericata Meigen) | 32 | 1.0 ± 1.5 | 6 | 0.0 ± 0.4 | 48 | 1.0 ± 1.5 | 115 | 9.4% |
| Face fly (Musca autumnalis DeGeer) | 3.0 | 0.0 ± 0.0 | 3 | 0.0 ± 0.0 | 109 | 0.0 ± 1.0 | 86 | 7.0% |
| Eye gnat (Liohippelates spp.) | 5 | 0.0 ± 0.0 | 0 | 0.0 ± 0.0 | 35 | 0.0 ± 0.0 | 40 | 3.3% |
| Total filth flies per farm | 424 | 25 ± 11.0 | 224 | 10.0 ± 8.0 | 572 | 23.5 ± 10.8 | 1220 | |
| Species | Eatonton Research Farm | Garmon Farm | Hollowed Hawk Farm | Total Number of Individuals per Species | Percentage of Total | |||
|---|---|---|---|---|---|---|---|---|
| Total | Median ± QD | Total | Median ± QD | Total | Median ± QD | |||
| Ants (Solenopsis invicta Buren) * | 92 | 9.5 ± 10.0 | 112 | 21.0 ± 29.5 | 184 | 1.5 ± 12.3 | 388.0 | 68.9% |
| Spiders (Agelenopsis spp.) | 51 | 3.0 ± 2.5 | 10 | 0.0 ± 0.5 | 18 | 0.0 ± 0.5 | 79.0 | 14.0% |
| Tiger beetles (Cicindelinae subfamily) | 21 | 1.0 ± 1.0 | 24 | 0.0 ± 0.8 | 19 | 0.0 ± 0.5 | 64.0 | 11.4% |
| Paper wasp (Polistinae subfamily) | 5 | 0.0 ± 0.0 | 6 | 0.0 ± 0.4 | 7 | 0.0 ± 0.0 | 18.0 | 3.2% |
| Rove beetles (Staphylinidae family) | 5 | 0.0 ± 0.0 | 4 | 0.0 ± 0.0 | 5 | 0.0 ± 0.0 | 14.0 | 2.5% |
| Total epigeal arthropods per farm | 174 | 5.0 ± 6.3 | 156 | 4.0 ± 3.4 | 233 | 2.5 ± 2.6 | 563.0 | |
| Rain Range (mm) | N Produced by Filth Flies (mg N · Day−1 Fly−1 · kg Dry Manure−1) * | N Emitted per Cow (g N · Day−1) | N Emitted per Farm (kg N · Month−1) | N Emitted in Georgia (ton N · Month−1) | |||
|---|---|---|---|---|---|---|---|
| When 9 Flies per Manure Pat | When 31 Flies per Manure Pat | When 9 Flies per Manure Pat | When 31 Flies per Manure Pat | When 9 Flies per Manure Pat | When 31 Flies per Manure Pat | ||
| 10 to 31 | 1.04 | 0.5 | 1.6 | 0.7 | 2.5 | 7.9 | 27.3 |
| 32 to 81 | 1.39 | 0.6 | 2.1 | 1.0 | 3.3 | 10.6 | 36.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, N.; Franklin, D.H.; Cabrera, M.; Hinkle, N.C.; Stewart, L.; Subedi, A. Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization. Sustainability 2023, 15, 12572. https://doi.org/10.3390/su151612572
Espinoza N, Franklin DH, Cabrera M, Hinkle NC, Stewart L, Subedi A. Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization. Sustainability. 2023; 15(16):12572. https://doi.org/10.3390/su151612572
Chicago/Turabian StyleEspinoza, Natalia, Dorcas H. Franklin, Miguel Cabrera, Nancy C. Hinkle, Lawton Stewart, and Anish Subedi. 2023. "Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization" Sustainability 15, no. 16: 12572. https://doi.org/10.3390/su151612572
APA StyleEspinoza, N., Franklin, D. H., Cabrera, M., Hinkle, N. C., Stewart, L., & Subedi, A. (2023). Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization. Sustainability, 15(16), 12572. https://doi.org/10.3390/su151612572








