Analysis of Alkali in Bayer Red Mud: Content and Occurrence State in Different Structures
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Raw Materials
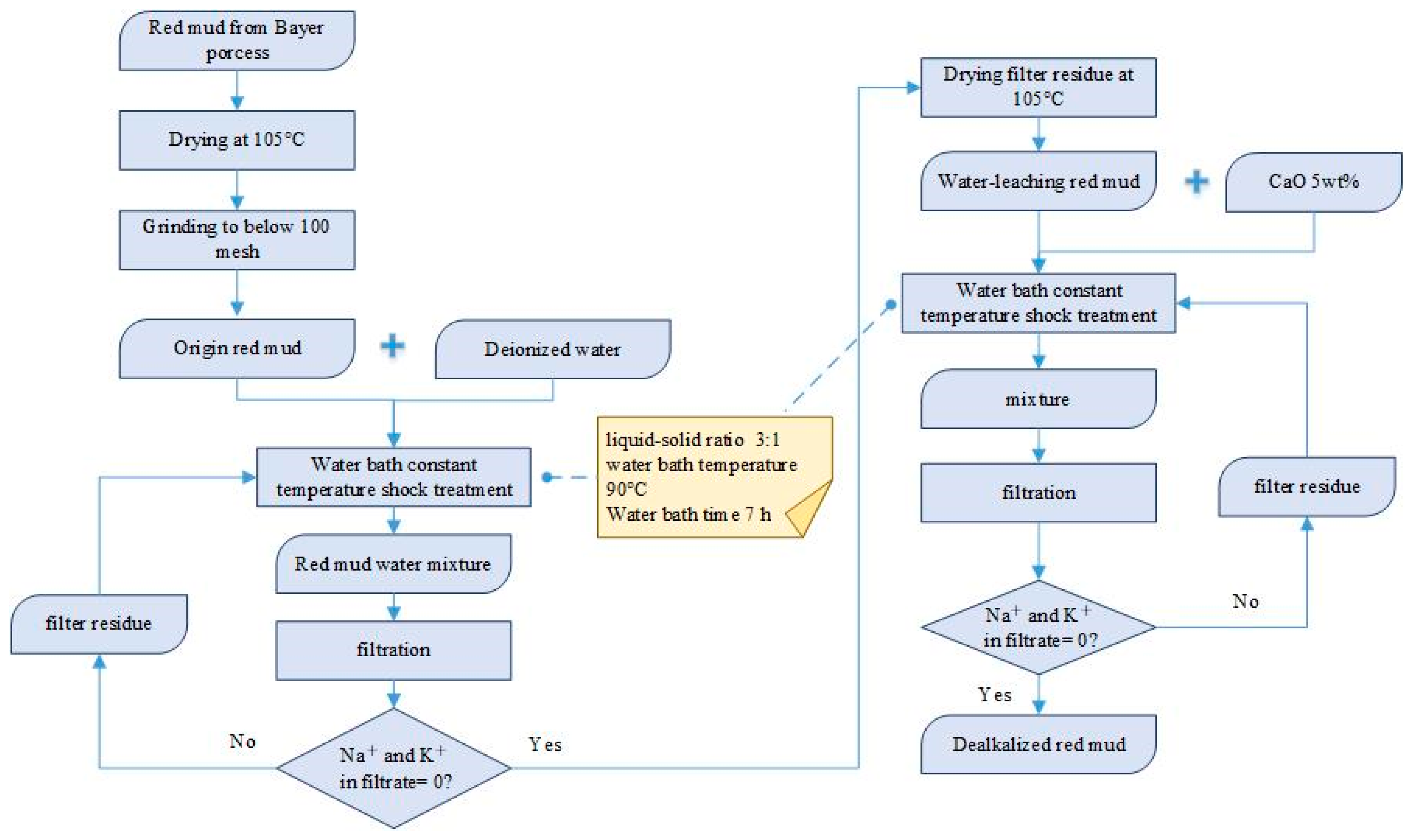
2.2. Dealkalization Experimental Process
2.3. Experimental Instrument
3. Results and Discussion
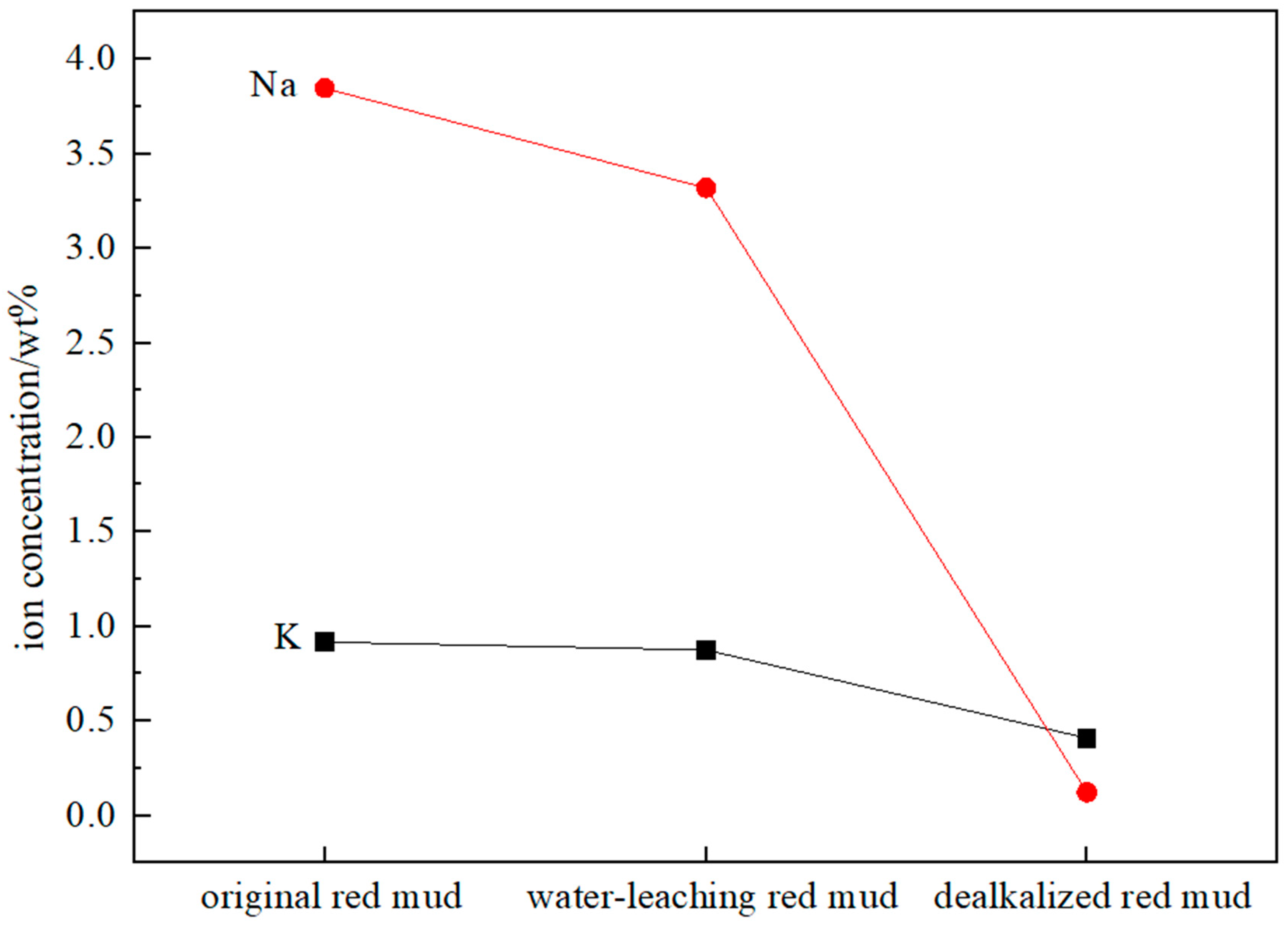
3.1. Inductively Coupled Plasmon Analysis
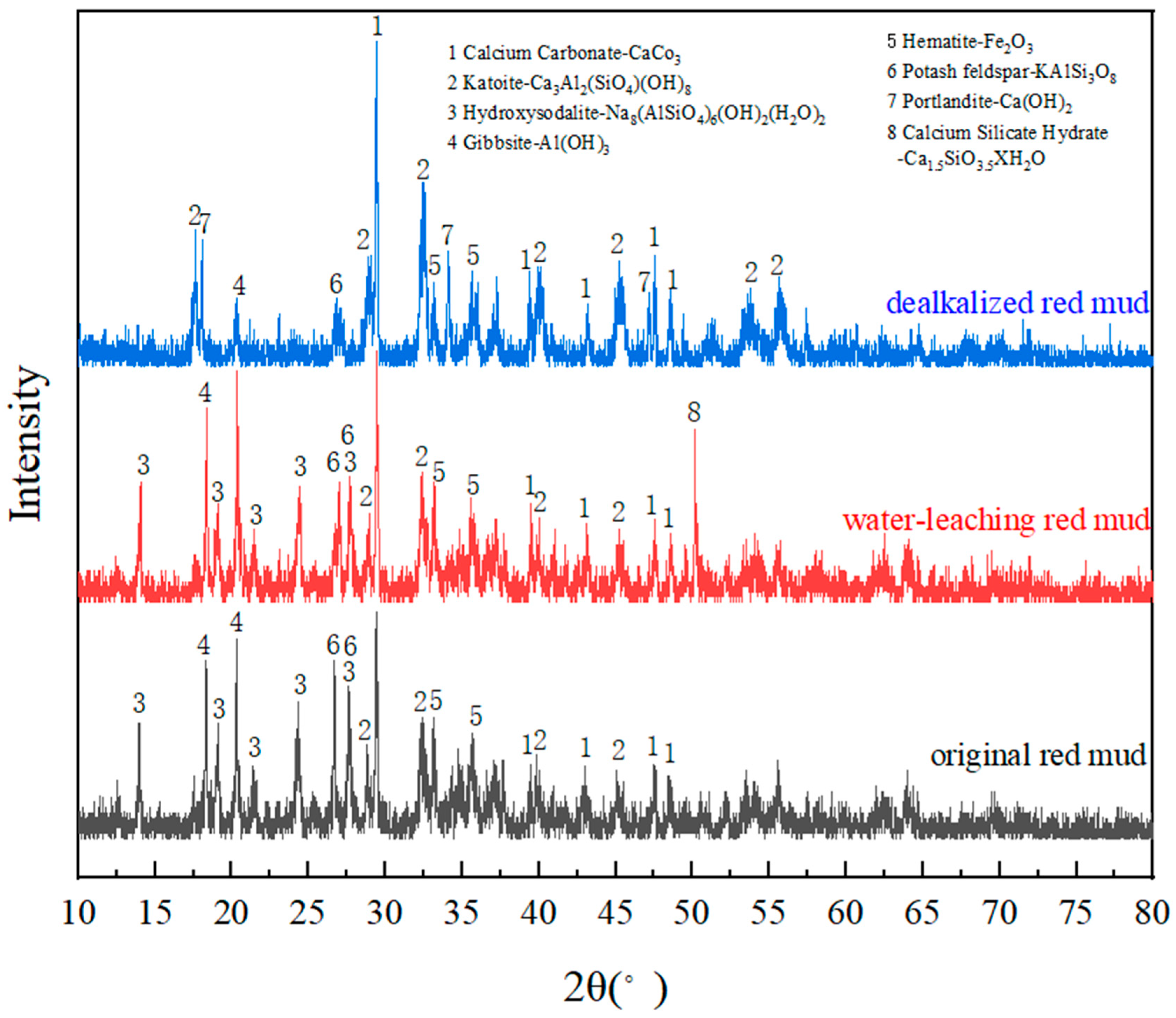
3.2. X-ray Diffraction Analysis
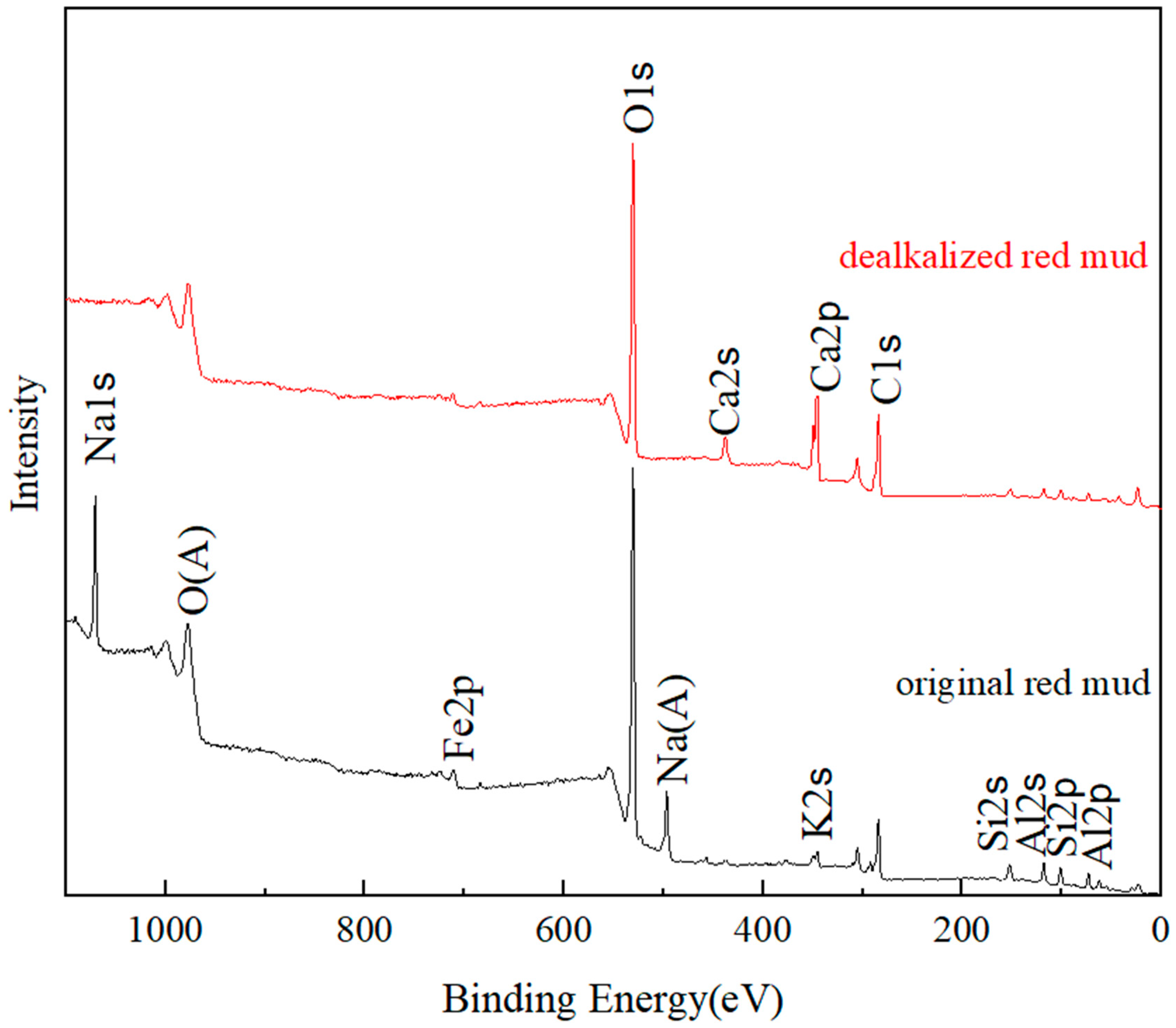
3.3. X-ray Photoelectron Spectroscopy Analysis
3.4. Fourier Transform Infrared Spectroscopy Analysis
3.5. Nuclear Magnetic Resonance Spectroscopy Analysis
3.6. Dealkalization Mechanism Analysis of Bayer Red Mud
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Yan, Y.; Li, Z.; Yang, F.; Hai, R.; Yuan, M. Investigation on the potassium magnesium phosphate cement modified by pretreated red mud: Basic properties, water resistance and hydration heat. Constr. Build. Mater. 2023, 368, 130456. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, J.; Zhang, H.; Bi, Y.; Yue, H.; Xu, R. Preparation and characterization of organic red mud and its application in asphalt modification. Constr. Build. Mater. 2023, 367, 130269. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Qi, Y.; Yu, H.; Tu, G. Preparation of ultra-lightweight ceramsite from red mud and immobilization of hazardous elements. J. Environ. Chem. Eng. 2022, 10, 108157. [Google Scholar] [CrossRef]
- Gou, M.; Hou, W.; Zhou, L.; Zhao, J.; Zhao, M. Preparation and properties of calcium aluminate cement with Bayer red mud. Constr. Build. Mater. 2023, 373, 130827. [Google Scholar] [CrossRef]
- Luu, T.-T.; Nguyen, D.-K.; Nguyen, T.T.P.; Ho, T.-H.; Dinh, V.-P.; Kiet, H.A.T. The effective Ni(II) removal of red mud modified chitosan from aqueous solution. Environ. Monit. Assess. 2023, 195, 254. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2020, 289, 125136. [Google Scholar] [CrossRef]
- Jiang, G.; Li, H.; Cheng, T.; Tian, Y.; Liu, P.; Guo, J.; Cui, K.; Ma, R.; Ma, X.; Cui, F.; et al. Novel preparation of sludge-based spontaneous magnetic biochar combination with red mud for the removal of Cu2+ from wastewater. Chem. Phys. Lett. 2022, 806, 139993. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, Z. Red mud-based catalysts for the catalytic removal of typical air pollutants: A review. J. Environ. Sci. 2023, 127, 628–640. [Google Scholar] [CrossRef]
- AlBoraikan, R.; Bageri, B.; Solling, T.I. Innovative Applications of Red Mud: Converting an Environmental Challenge to a Drilling Asset. ACS Omega 2022, 8, 614–625. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Chen, P.; Yao, K.; Wang, P.; Ming, Y.; Yi, J.; Zhi, L. The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism. Appl. Sci. 2022, 13, 452. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Xu, D.; Zhang, H. Catalytic activity evaluation and deactivation progress of red mud/carbonaceous catalyst for efficient biomass gasification tar cracking. Fuel 2022, 323, 124278. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Q.; Fan, X.; Zheng, J.; Liu, Y.; Ma, Y.; He, J. Enhanced degradation of sulfamethoxazole by activation of peroxodisulfate with red mud modified biochar: Synergistic effect between adsorption and nonradical activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Bang, K.-H.; Kang, Y.-B. Recycling red mud to develop a competitive desulfurization flux for Kanbara Reactor (KR) desulfurization process. J. Hazard. Mater. 2022, 440, 129752. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Cheng, F.; Meng, J.; Ge, H.; Lu, P.; Song, T. Ni-enhanced red mud oxygen carrier for chemical looping steam methane reforming. Fuel Process. Technol. 2022, 230, 107204. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Li, Z.; Yang, F.; Hai, R. Hardening and microstructural properties of red mud modified magnesium ammonium phosphate cements. J. Build. Eng. 2023, 66, 105849. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Chen, Q.; Xiao, X.; Wang, T.; Cheng, S.; Li, J. Study on the mechanism and reaction characteristics of red-mud-catalyzed pyrolysis of corn stover. Fuel 2023, 338, 127290. [Google Scholar] [CrossRef]
- Chao, X.; Zhang, T.-A.; Lyu, G.; Liang, Z.; Chen, Y. Sustainable application of sodium removal from red mud: Cleaner production of silicon-potassium compound fertilizer. J. Clean. Prod. 2022, 352, 131601. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Zhang, Y.-X.; Wang, L.; Liu, C.-G.; Sun, W.-M.; Wang, Y.-F.; Long, S.-X.; He, X.-T.; Lin, Z.; Liang, J.-L.; et al. Rhizobacteria communities reshaped by red mud based passivators is vital for reducing soil Cd accumulation in edible amaranth. Sci. Total Environ. 2022, 826, 154002. [Google Scholar] [CrossRef]
- Luo, M.; Qi, X.; Zhang, Y.; Ren, Y.; Tong, J.; Chen, Z.; Hou, Y.; Yeerkebai, N.; Wang, H.; Feng, S.; et al. Study on dealkalization and settling performance of red mud. Environ. Sci. Pollut. Res. 2016, 24, 1794–1802. [Google Scholar] [CrossRef]
- Liu, X.; Rong, R.; Dai, M.; Bian, H.; Peng, C. Preparation of red mud-based zero-valent iron materials by biomass pyrolysis reduction: Reduction mechanism and application study. Sci. Total Environ. 2023, 864, 160907. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, K.; Zhong, N.; Gao, D. Selective separation of iron and scandium from Bayer Sc-bearing red mud. J. Rare Earths 2023, 41, 1099–1107. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Mao, Q.; Luo, L.; Deng, Y. Removal of Heavy Metals from Acid Mine Drainage by Red Mud–Based Geopolymer Pervious Concrete: Batch and Long–Term Column Studies. Polymers 2022, 14, 5355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.-P.; Ahmad, M.R.; Lao, J.-C.; Dai, J.-G. Recycling of red mud and flue gas residues in geopolymer aggregates (GPA) for sustainable concrete. Resour. Conserv. Recycl. 2023, 191, 106893. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Wu, D.; Liu, X. Engineering and environmental evaluation of red mud amended volcanic ash as a sustainable subgrade material. J. Clean. Prod. 2023, 393, 136353. [Google Scholar] [CrossRef]
- Zhu, F.; Zhou, J.; Xue, S.; Hartley, W.; Wu, C.; Guo, Y. Aging of bauxite residue in association of regeneration: A comparison of methods to determine aggregate stability & erosion resistance. Ecol. Eng. 2016, 92, 47–54. [Google Scholar] [CrossRef]
- Wu, J.; Lei, T.; Wang, B.; Ma, S.; Lin, Y.; Lu, X.; Ye, Z. An Eco-Friendly Acid Leaching Strategy for Dealkalization of Red Mud by Controlling Phase Transformation. Materials 2022, 15, 580. [Google Scholar] [CrossRef]
- Yang, W.; Ma, W.; Li, P.; Liu, Z.; Yan, H. Alkali Recovery of Bauxite Residue by Calcification. Minerals 2022, 12, 636. [Google Scholar] [CrossRef]
- Hu, G.; Lyu, F.; Khoso, S.A.; Zeng, H.; Sun, W.; Tang, H.; Wang, L. Staged leaching behavior of red mud during dealkalization with mild acid. Hydrometallurgy 2020, 196, 105422. [Google Scholar] [CrossRef]
- Leiva, C.; Arroyo-Torralvo, F.; Luna-Galiano, Y.; Villegas, R.; Vilches, L.F.; Pereira, C.F. Valorization of Bayer Red Mud in a Circular Economy Process: Valuable Metals Recovery and Further Brick Manufacture. Processes 2022, 10, 2367. [Google Scholar] [CrossRef]
- Zeng, K.; Quan, X.; Jiang, Q.; Jiang, Z.; Qiu, F. An Efficient Dealkalization of Red Mud Through Microwave Roasting and Water Leaching. JOM 2022, 74, 3221–3231. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, S.; Ma, S.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Gao, J.; Qu, X.; Guo, Z. An environmental-friendly method for recovery of soluble sodium and harmless utilization of red mud: Solidification, separation, and mechanism. Resour. Conserv. Recycl. 2022, 186, 106543. [Google Scholar] [CrossRef]
- Menzies, N.W.; Fulton, I.M.; Morrell, W.J. Seawater Neutralization of Alkaline Bauxite Residue and Implications for Revegetation. J. Environ. Qual. 2004, 33, 1877–1884. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Zhu, X. Clean dealkalization technology from aluminum industry hazardous tailings—Red mud by displacement with Mg-based agent. Environ. Sci. Pollut. Res. 2022, 29, 55957–55970. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T.; Zhang, B.; Chao, X. Research on Bayer Red Mud Slurry Electrolysis. Bull. Environ. Contam. Toxicol. 2022, 109, 101–109. [Google Scholar] [CrossRef]
- Feng, H.; She, X.-F.; You, X.-M.; Zhang, G.-Q.; Wang, J.-S.; Xue, Q.-G. Carbothermal reduction of red mud for iron extraction and sodium removal. High Temp. Mater. Process. 2022, 41, 161–171. [Google Scholar] [CrossRef]
- Atan, E.; Sutcu, M.; Cam, A.S. Combined effects of bayer process bauxite waste (red mud) and agricultural waste on technological properties of fired clay bricks. J. Build. Eng. 2021, 43, 103194. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Sun, H. Research on technology of alkali recovery in red mud. Inorg. Chem. Ind. 2014, 46, 46–48. (In Chinese) [Google Scholar]
- Zhang, D.-R.; Chen, H.-R.; Xia, J.-L.; Nie, Z.-Y.; Zhang, R.-Y.; Pakostova, E. Efficient dealkalization of red mud and recovery of valuable metals by a sulfur-oxidizing bacterium. Front. Microbiol. 2022, 13, 973568. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, J.; Hu, P.; Meng, K.; Lv, F.; Tong, W.; Chu, P.K. Dealkalization of Red Mud by Carbide Slag and Flue Gas. Clean-Soil Air Water 2018, 46, 1700634. [Google Scholar] [CrossRef]
- Duan, G.; Wei, G.; Li, Q.; Zhu, Y.; Zhang, L.; Liang, L.; Huang, Z.; He, S.; Li, B. Insight into catalytic activation of bisulfite for lomefloxacin degradation by simple composite of calcinated red mud. Environ. Sci. Pollut. Res. 2022, 30, 103030. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Pang, Y.; Peng, D.; Huang, T.; Chen, J. Red mud as a magnesium carrier for enhanced N and P recovery from wastewater by the struvite method. Environ. Technol. Innov. 2023, 30, 103030. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Y.; Hu, Y.; Pan, Q.; Luo, L.; Li, F.; Xiao, H.; Wu, Y.; Chen, Z.; Wang, X.; et al. The preparation and properties of the C-doped modified layers on uranium surfaces by pulsed laser irradiating. J. Nucl. Mater. 2019, 529, 151921. [Google Scholar] [CrossRef]
- Du, Z.; Sheng, S.; Guo, J. Effect of composite activators on mechanical properties, hydration activity and microstructure of red mud-based geopolymer. J. Mater. Res. Technol. 2023, 24, 8077–8085. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, T. Comparison of the Effects of Ultrasonic and Ball Milling on Red Mud Desulfurization. Metals 2022, 12, 1887. [Google Scholar] [CrossRef]
- Haouas, M.; Taulelle, F.; Martineau, C. Recent advances in application of 27Al NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94–95, 11–36. [Google Scholar] [CrossRef]
- Prasanphan, S.; Hemra, K.; Wannagon, A.; Kobayashi, T.; Onutai, S.; Jiemsirilers, S. 29Si and 27Al NMR study of the structural transformation of calcined kaolin residue-based geopolymer using low alkali activator content for sustainable construction materials. J. Build. Eng. 2023, 70, 106332. [Google Scholar] [CrossRef]

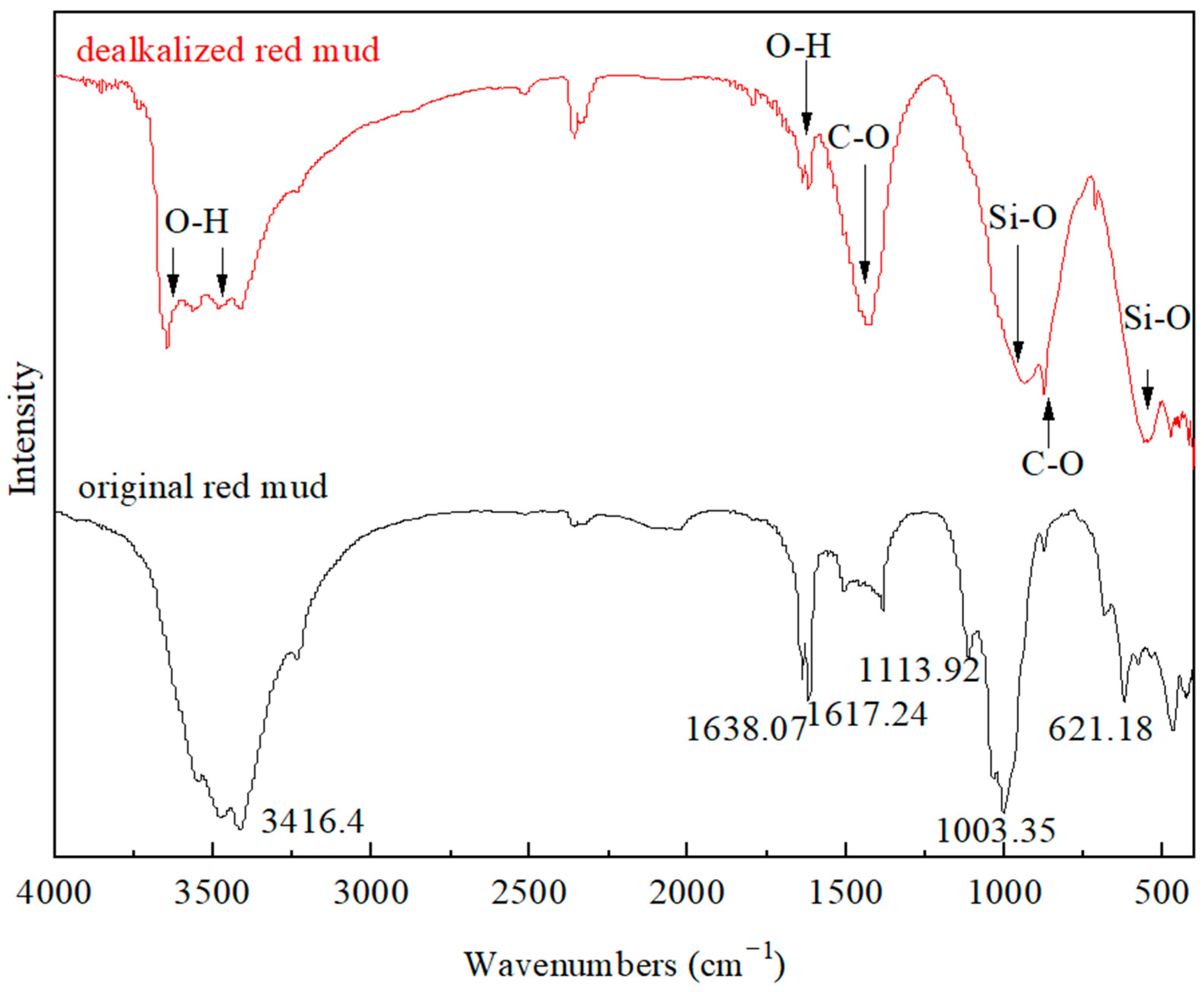
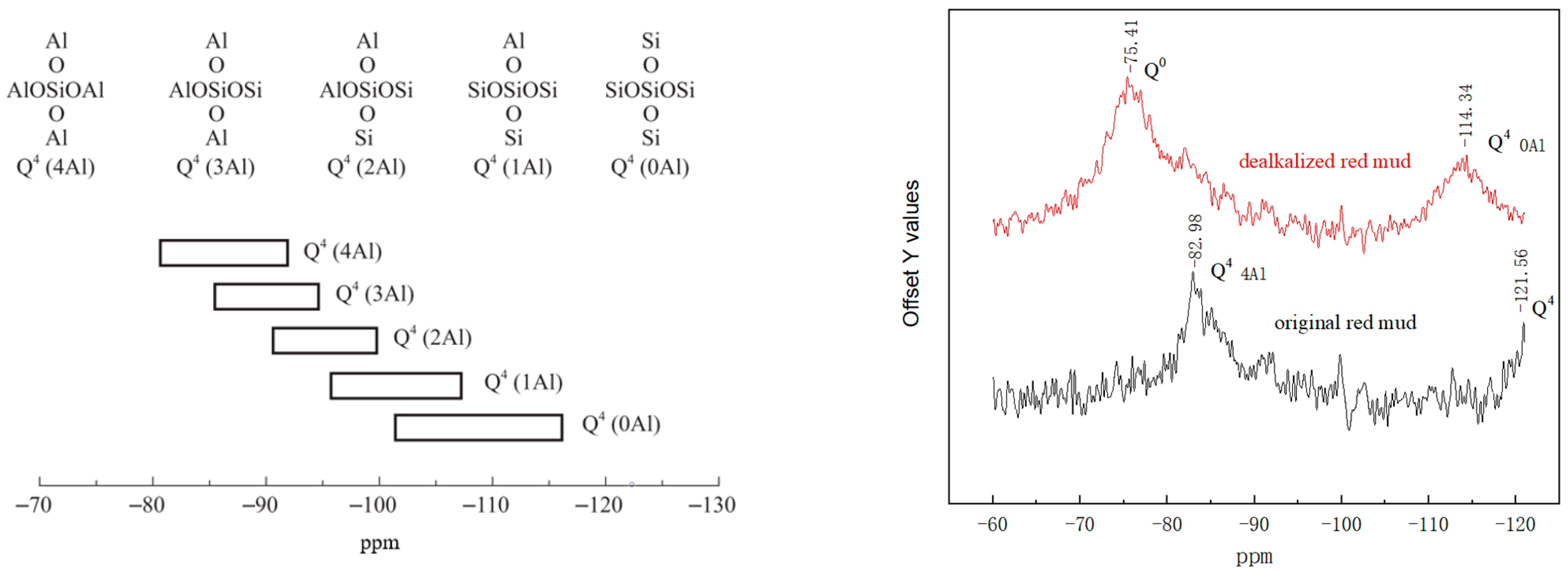
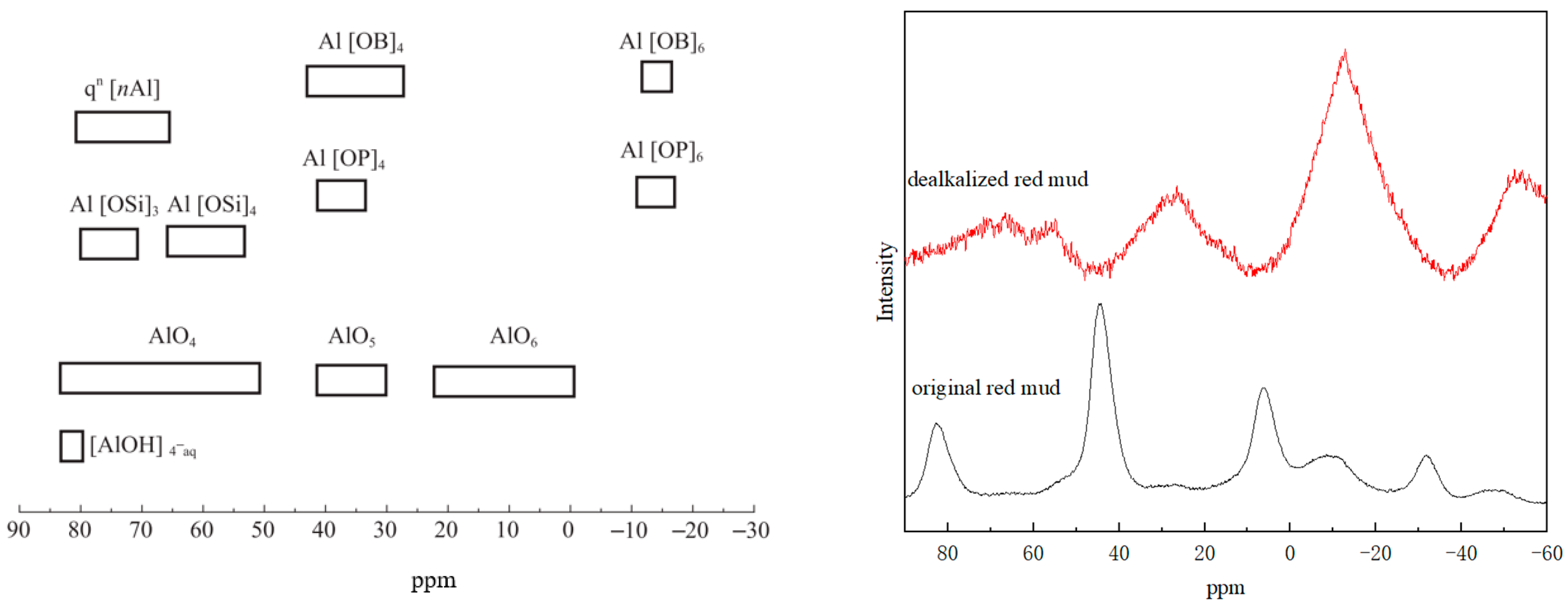
| Oxide (wt%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | Others | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| Bayer red mud | 19.1 | 22.8 | 16.3 | 13.2 | 0.41 | 1.37 | 6.97 | 3.29 | 0.95 | 15.6 |
| Sample | Na | K |
|---|---|---|
| origin red mud | 3.84 | 0.92 |
| water-leaching red mud | 3.32 | 0.88 |
| dealkalized red mud | 0.12 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jing, H.; Zhang, M.; Li, J.; Ma, Y.; Yan, L. Analysis of Alkali in Bayer Red Mud: Content and Occurrence State in Different Structures. Sustainability 2023, 15, 12686. https://doi.org/10.3390/su151712686
Wang X, Jing H, Zhang M, Li J, Ma Y, Yan L. Analysis of Alkali in Bayer Red Mud: Content and Occurrence State in Different Structures. Sustainability. 2023; 15(17):12686. https://doi.org/10.3390/su151712686
Chicago/Turabian StyleWang, Xiao, Haowen Jing, Maoliang Zhang, Jianwei Li, Yan Ma, and Liang Yan. 2023. "Analysis of Alkali in Bayer Red Mud: Content and Occurrence State in Different Structures" Sustainability 15, no. 17: 12686. https://doi.org/10.3390/su151712686




