Assessment of Three Major Shrimp Stocks in Bangladesh Marine Waters Using Both Length-Based and Catch-Based Approaches
Abstract
:1. Introduction
2. Materials and Methods
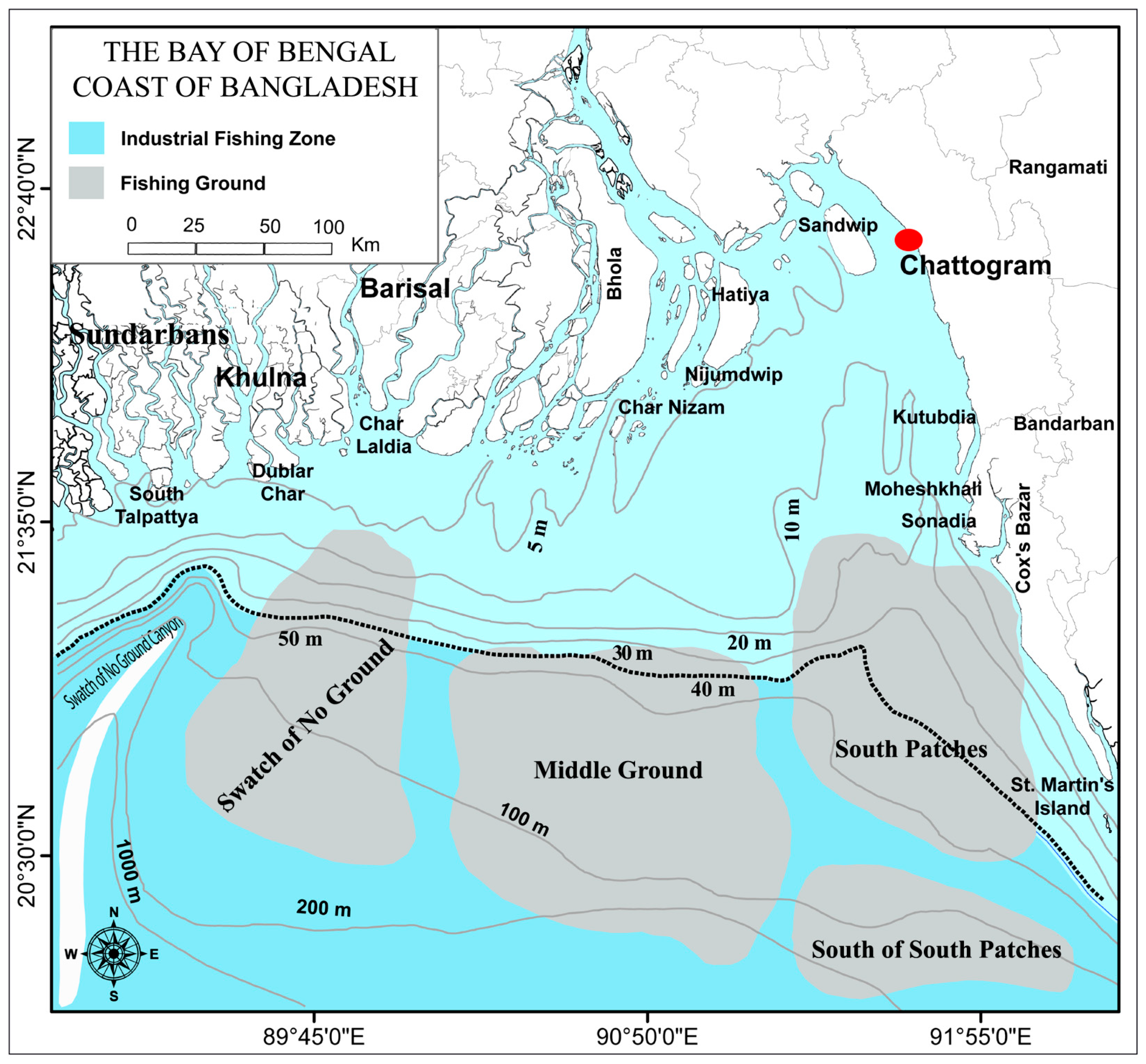
2.1. Study Area
2.2. Data Sources
2.2.1. Length-Based Methods
2.2.2. Catch-Based Methods
2.3. Stock Assessment Indicators
2.3.1. LBB Method
2.3.2. Length-Based Indicators
2.3.3. Fisheries Reference Points from Catch Data
The JABBA Model
Input Fishery Data
Formulation of Input Parameters
3. Results
3.1. Length Distribution
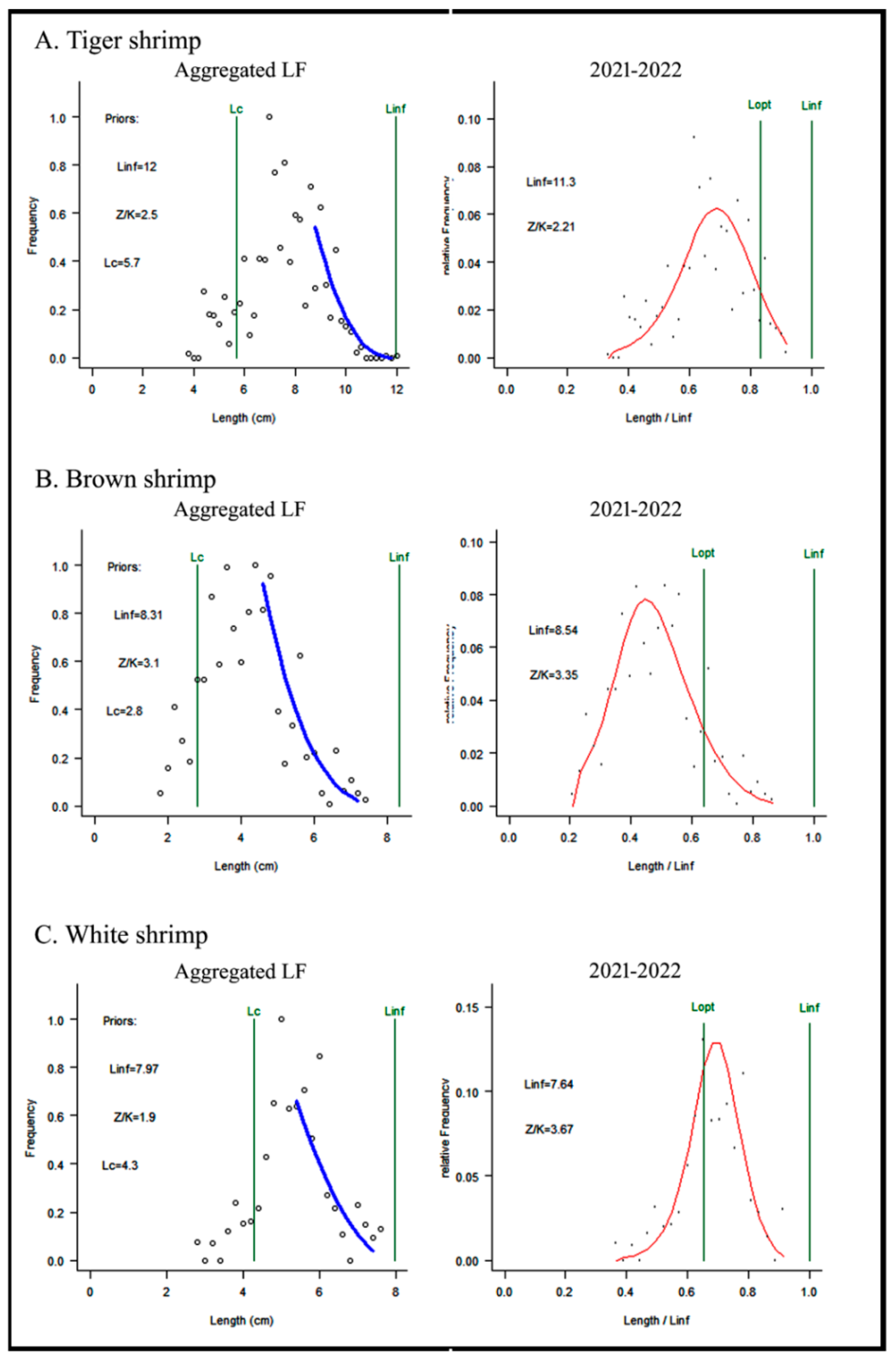
3.2. Shrimp’s Stock Analysis Based on LBB Outputs
3.2.1. Tiger Shrimp
3.2.2. Brown Shrimp
3.2.3. White Shrimp
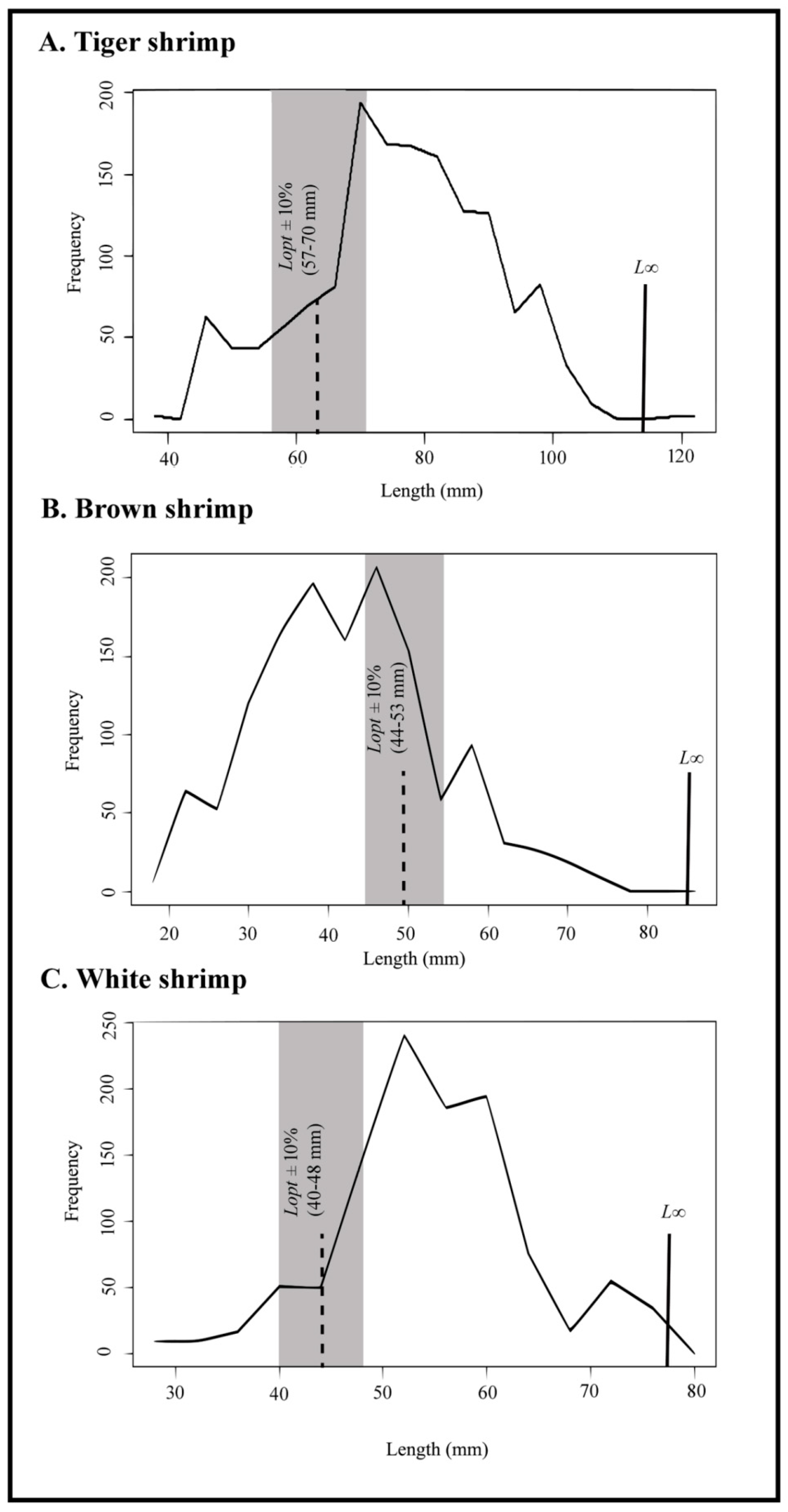
3.3. Results from Length-Based Indicators
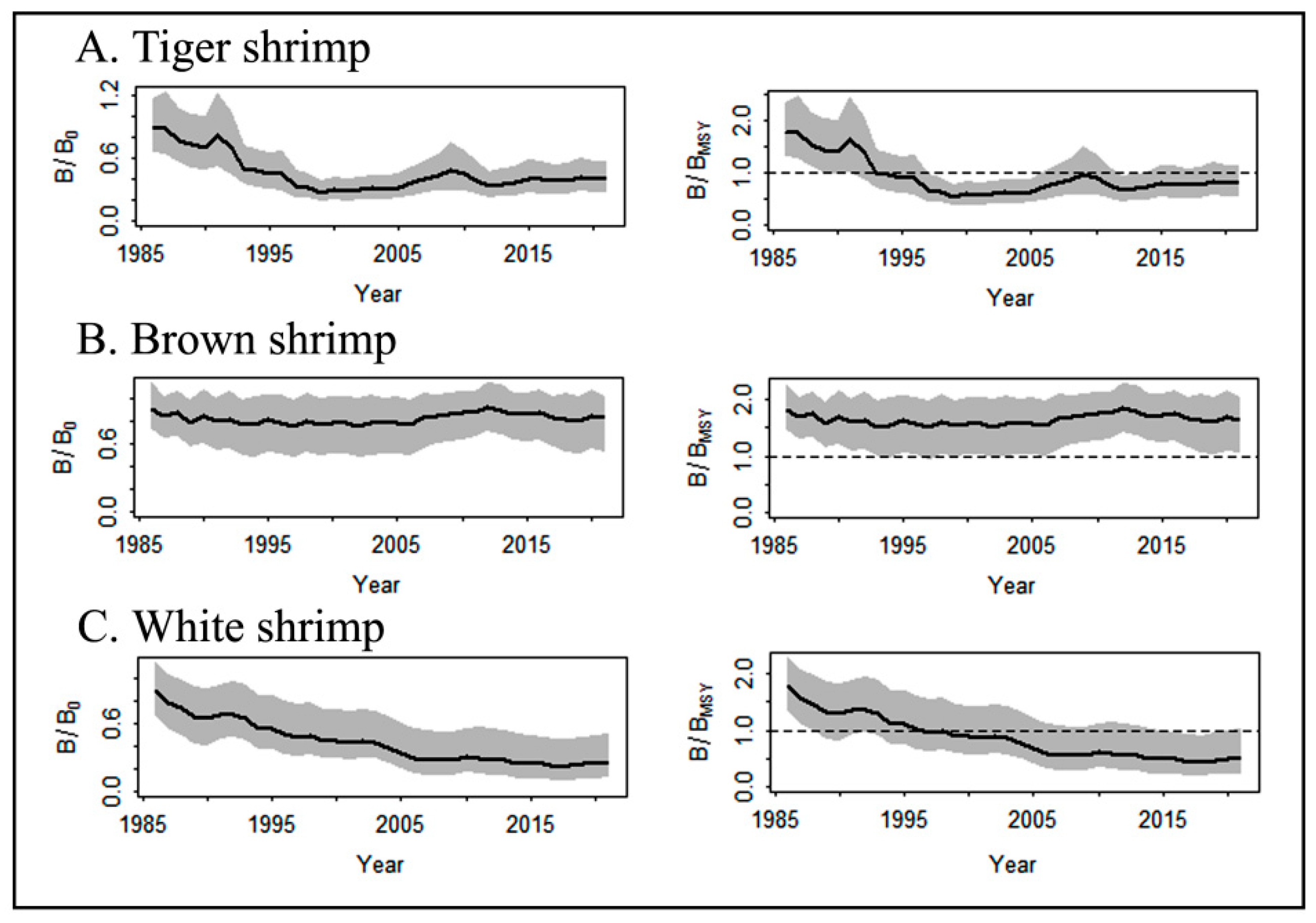
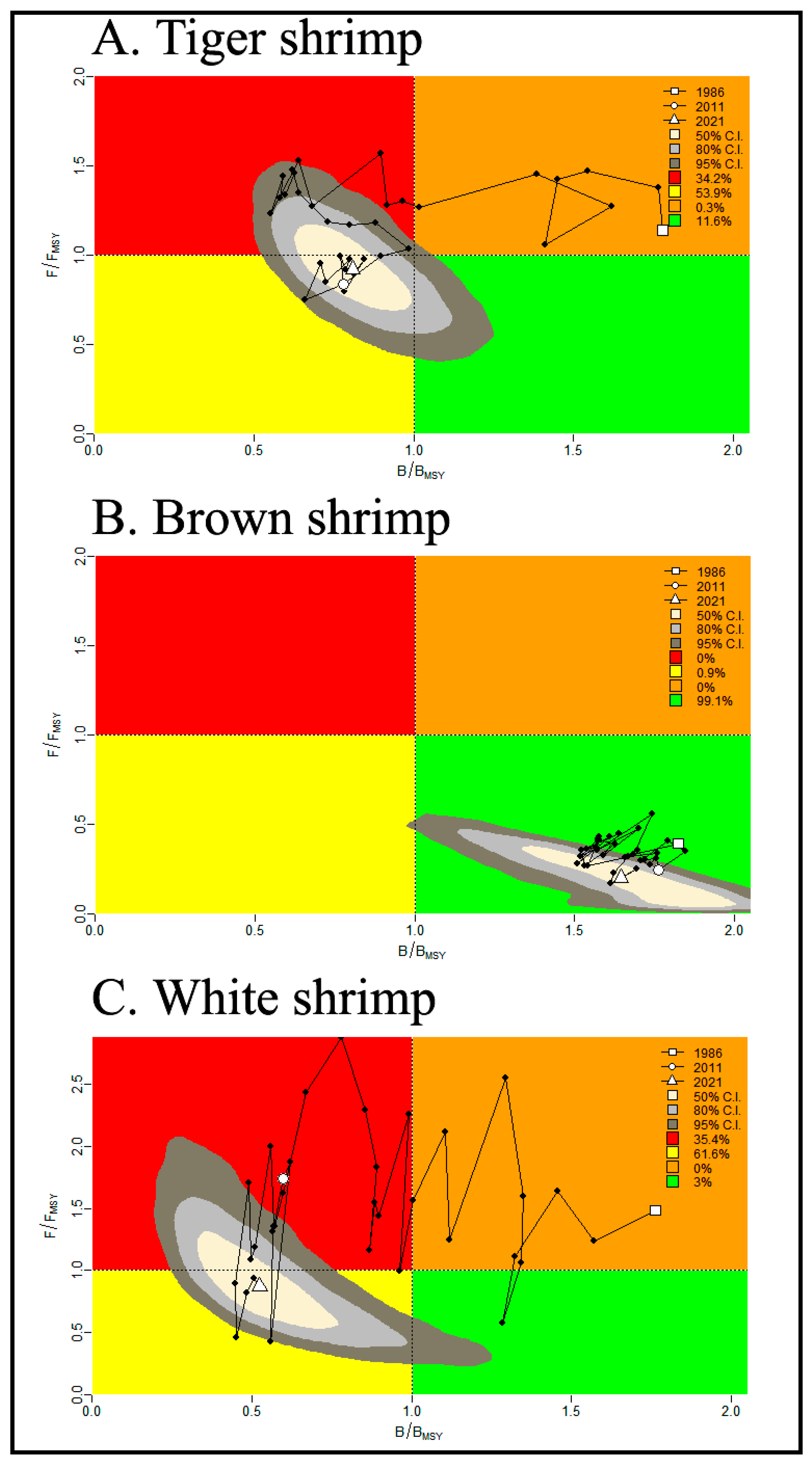
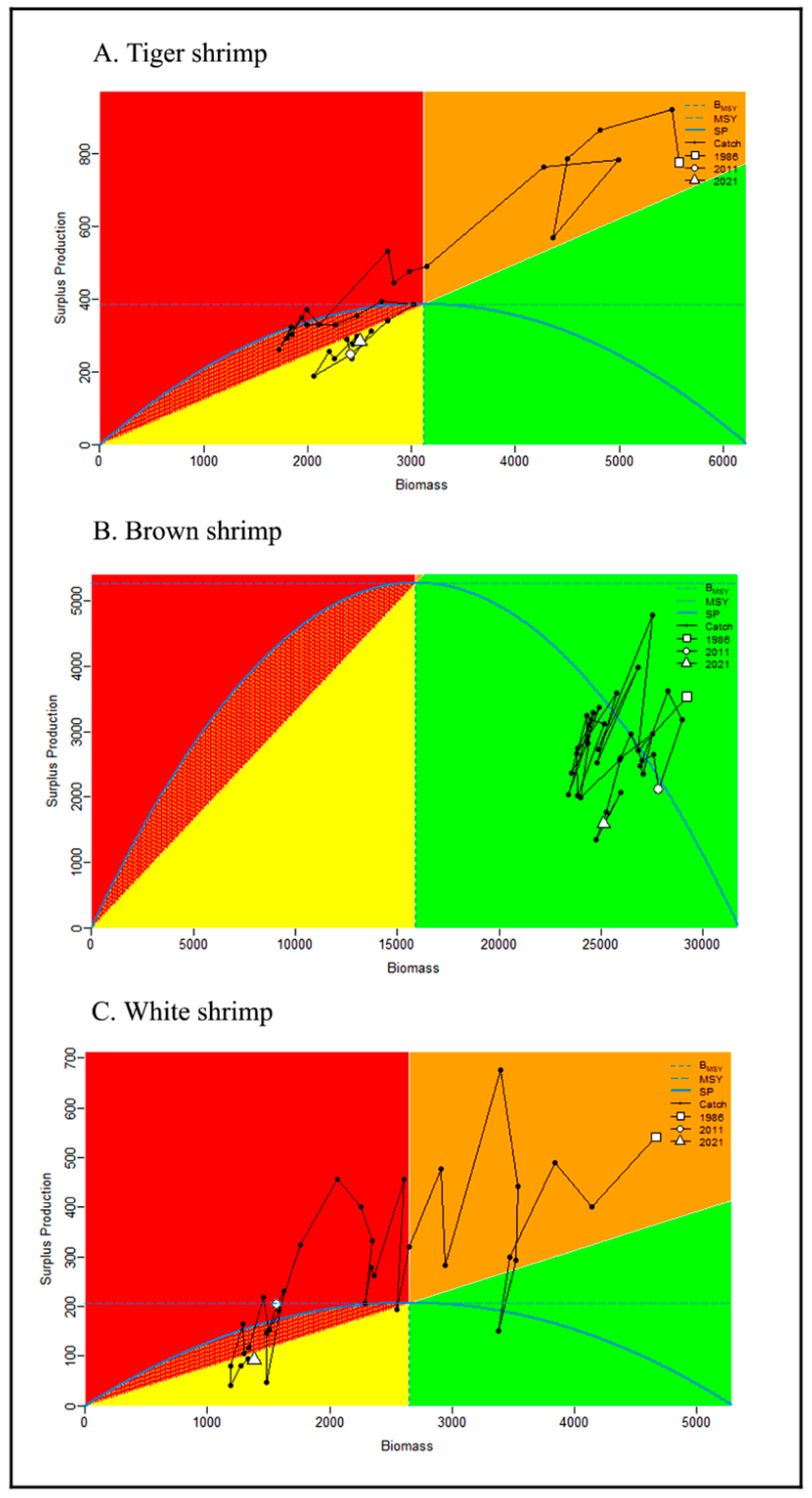
3.4. Shrimp’s Stock Analysis Based on JABBA Outputs
4. Discussion
4.1. Stock Condition Analysis Based on LBB Approaches
4.2. Stock Condition Analysis Based on Length-Based Indicators
4.3. Stock Condition Analysis Based on JABBA Model
5. Conclusions
- The von Bertalanffy Growth Function (VBGF) parameters for tiger, brown, and white shrimps were L∞ = 113.0 mm, 85.4 mm, and 76.4 mm, respectively, for carapace length;
- The relative biomass level (B/BMSY) of the tiger shrimp was 0.43, suggesting an overfishing status, and the values of the brown and white shrimps were 0.84 and 0.96, respectively, indicating that they were fully exploited but not overfished. The estimates of Lc/Lc_opt were less than the unity for tiger and brown shrimps, suggesting that the stocks were suffering from growth overfishing;
- This study recommended an optimum length limit to catch from 57.0–70.0 mm for tiger shrimp, 44.0–53.0 mm for brown shrimp, and 40.0–48.0 mm for white shrimp;
- The estimated maximum sustainable yield (MSY) reference points were optimal: biomass BMSY = 3116 mt, 15,885 mt, and 2649 mt for tiger, brown and white shrimp, respectively, and optimal harvest rate uMSY = 12%, 33%, and 8% for tiger, brown and white shrimp, respectively. The average annual catch for the last ten years was below the estimated MSY values of 389 mt, 4899 mt, and 209 mt for tiger, brown, and white shrimp, respectively;
- Brown shrimp were calculated using the JABBA model to have the highest carrying capacity (31,770 mt) and intrinsic growth rate (66%) compared to tiger and white shrimp. The ratio of fishing mortality for brown shrimp was the lowest (F/FMSY = 0.19) among the three shrimp species. Similarly, the proportion of fishing and natural mortality calculated using the LBB model showed the lowest and prudent estimate for brown shrimp (F/M = 0.99) compared to the tiger (=2.6) and white shrimps (=1.31). Therefore, the stock of brown shrimp was concluded to be in a better state than those of the tiger and white shrimps.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barua, S.; Magnusson, A.; Humayun, N.M. Assessment of offshore shrimp stocks of Bangladesh based on commercial shrimp trawl logbook data. Indian J. Fish. 2018, 65, 1–6. [Google Scholar] [CrossRef]
- DoF. National Fish Week 2022 Compendium (in Bangla); Department of Fisheries, Ministry of Fisheries and Livestock: Dhaka, Bangladesh, 2022; 170p. Available online: www.fisheries.gov.bd (accessed on 20 March 2023).
- Rahman, A.K.A.; Khan, M.G.; Chowdhury, Z.A.; Hussain, M.M. Economically Important Marine Fishes and Shellfishes of Bangladesh; Department of Fisheries: Dhaka, Bangladesh, 1995. [Google Scholar]
- Penn, J.W. The Current Status of Off Shore Marine Fish Stocks in Bangladesh Waters with Special Reference to Penaeid Shrimp Stocks; FAO: Rome, Italy, 1982; 47p. [Google Scholar]
- Fanning, P.; Chowdhury, S.R.; Uddin, M.S.; Al-Mamun, M.A. Marine Fisheries Survey Reports and Stock Assessment 2019; Bangladesh Marine Fisheries Capacity Building Project, Department of Fisheries, Ministry of Fisheries and Livestock: Dhaka, Bangladesh, 2019. Available online: http://mfsmu.fisheries.gov.bd/site/download/03cb42dc-8a4f-4dd3-a08943e5f5bcf61b (accessed on 20 March 2023).
- Lamboeuf, M. Bangladesh Demersal Fish Resources of the Continental Shelf; R/V Anusandhani Trawling Survey Results (September 1984–June 1986); FAO: Rome, Italy, 1987; 26p. [Google Scholar]
- Alam, M.S.; Liu, Q.; Schneider, P.; Mozumder, M.M.H.; Uddin, M.M.; Monwar, M.M.; Hoque, M.E.; Barua, S. Stock Assessment and Rebuilding of Two Major Shrimp Fisheries (Penaeus monodon and Metapenaeus monoceros) from the Industrial Fishing Zone of Bangladesh. J. Mar. Sci. Eng. 2022, 10, 201. [Google Scholar] [CrossRef]
- Barua, S.; Al Mamun, M.A.; Nazrul, K.M.S.; Mamun, A.; Das, J. Maximum sustainable yield estimate for Tiger shrimp, Penaeus monodon off Bangladesh coast using trawl catch log. Bangladesh Marit. J. 2020, 4, 135–144. [Google Scholar]
- FRSS. Fisheries Resources Survey System: Fisheries Statistical Report of Bangladesh; Department of Fisheries (DoF), Ministry of Fisheries and Livestock (MoFL): Dhaka, Bangladesh, 2022; Volume 31, pp. 1–57. [Google Scholar]
- Barua, S. Maximum sustainable yield estimate for Brown shrimp, Metapenaeus monoceros (Fabricius 1798) in marine waters of Bangladesh using trawl catch log. Indian J. Geo-Mar. Sci. 2021, 50, 258–261. [Google Scholar]
- West, W.Q.B. Fishery Resources of the Upper Bay of Bengal. Indian Ocean Programme, Indian Ocean Fisheries Commission, IOFC/DEV/73/28; FAO: Rome, Italy, 1973; 44p. [Google Scholar]
- Rashid, M.H. Mitsui Taiyo shrimp survey 1976-77 by the survey research vessels M. V. Santa Monica and M. V. Orion 8 in the marine waters of Bangladesh. Mar. Fish. Bull. 1983, 2, 23. [Google Scholar]
- White, T.F.; Khan, M.G. The marine resources of Bangladesh and their potential for commercial development. In Proceedings of the National Seminar on Fisheries Development in Bangladesh, Dhaka, Bangladesh, 1–4 January 1985; pp. 1–4. [Google Scholar]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment, Choices, Dynamics and Uncertainty; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Khan, M.S.; Hoque, M.S. Bioeconomics modelling: Bangladesh shrimp fishery. In International Seminar in Malaysia; Malaysia Construction Industry Development Board: Kuala Lumpur, Malaysia, 2000. [Google Scholar]
- Kar, T.K.; Chakraborty, K. A bioeconomic assessment of the Bangladesh shrimp fishery. World J. Model. Simul. 2011, 7, 58–69. [Google Scholar]
- Mustafa, M.G.; Ali, M.S.; Azadi, M.A. Some aspect of population dynamics of three penaeid shrimps (Penaeus monodon, Penaeus semisulcatus and Metapenaeus monoceros) from the Bay of Bengal, Bangladesh. Chittagong Univ. J. Sci. 2006, 30, 97–102. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Hordyk, A.; Ono, K.; Sainsbury, K.; Loneragan, N.; Prince, J. Some explorations of the life history ratios to describe length composition, spawning-per-recruit, and the spawning potential ratio. ICES J. Mar. Sci. 2015, 72, 204–216. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Sparre, P. Can we use traditional length-based fish stock assessment when growth is seasonal? Fishbyte 1990, 8, 29–32. [Google Scholar]
- Smith, D.; Punt, A.; Dowling, N.; Smith, A.; Tuck, G.; Knuckey, I. Reconciling approaches to the assessment and management of data-poor species and fisheries with Australia’s harvest strategy policy. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2009, 1, 244–254. [Google Scholar] [CrossRef]
- Agnew, D.J.; Gutiérrez, N.L.; Butterworth, D.S. Fish catch data: Less than what meets the eye. Mar. Policy 2013, 42, 268–269. [Google Scholar] [CrossRef]
- Branch, T.A.; Jensen, O.P.; Ricard, D.; Ye, Y.; Hilborn, R. Contrasting Global Trends in Marine Fishery Status Obtained from Catches and from Stock Assessments. Conserv. Biol. 2011, 25, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Prager, M.H. ASPIC: A Surplus-Production Model Incorporating Covariates. Coll. Vol. Sci. Pap. Int. Comm. Conserv. Atl. Tunas 1992, 28, 218–229. [Google Scholar]
- Martell, S.; Froese, R. A simple method for estimating MSY from catch and resilience. Fish Fish. 2013, 14, 504–514. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Fogarty, M.J.; Cooper, A.B.; Dickey-Collas, M.; Fulton, E.A.; Gutiérrez, N.L.; Hyde, K.J.W.; Kleisner, K.M.; Kristiansen, T.; Longo, C.; et al. Developing New Approaches to Global Stock Status Assessment and Fishery Production Potential of the Seas; FAO Fisheries and Aquaculture Circular No. 1086; FAO: Rome, Italy, 2014; 175p. [Google Scholar]
- Raza, H.; Liu, Q.; Alam, M.S.; Han, Y. Length based stock assessment of five fish species from the marine water of Pakistan. Sustainability 2022, 14, 1587. [Google Scholar] [CrossRef]
- Barua, S.; Thordarson, G.; Jonsdottir, I.G. Comparison of catch and survey data for assessing northern shrimp (Pandalus borealis) from Arnarfjordur (NW-Iceland) using a stock production model. Turk. J. Fish. Aquat. Sci. 2018, 18, 359–366. [Google Scholar] [CrossRef]
- MFA. 2020. Marine Fisheries Act 2020. Ministry of Fisheries and Livestock, SRO 211-AIN/2019, 24 June 2019. Available online: http://bdlaws.minlaw.gov.bd/act-1347.html (accessed on 10 June 2022).
- MFO. Marine Fisheries Ordinance 1983 (Bangladesh). 1983. Available online: http://www.fisheries.gov.bd/sites/default/files (accessed on 10 June 2022).
- IOTC. IOTC National Report 2020 (Bangladesh part). 2020; National Report of Bangladesh for IOTC; IOTC: Dhaka, Bangladesh, 2020. [Google Scholar]
- Barua, S.; Karim, E.; Humayun, N.M. Present status and species composition of commercially important finfish in landed trawl catch from Bangladesh marine waters. J. Pure Appl. Zool. 2014, 2, 150–159. [Google Scholar]
- Islam, M.S. Perspectives of the coastal and marine fisheries of the Bay of Bengal, Bangladesh. Ocean Coast. Manag. 2003, 46, 763–796. [Google Scholar] [CrossRef]
- Barua, S.; Liu, Q.; Alam, M.S.; Schneider, P.; Mozumder, M.M.H.; Rouf, M.A. Population dynamics and stock assessment of two major eels (Muraenesox bagio and Congresox talabonoides) from the marine waters of Bangladesh. Front. Mar. Sci. 2023, 10, 1134343. [Google Scholar] [CrossRef]
- Froese, R. Keep it simple: Three indicators to deal with overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Cope, J.M.; Punt, A.E. Length-based reference points for data-limited situations: Applications and restrictions. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2009, 1, 169–186. [Google Scholar] [CrossRef]
- Winker, H.; Carvalho, F.; Kapur, M. JABBA: Just another bayesian biomass assessment. Fish. Res. 2018, 204, 275–288. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A Simple User Guide for LBB (LBB_33a.R). 2019. Available online: http://oceanrep.geomar.de/44832/ (accessed on 11 October 2020).
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. ii). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment—Part 1: Manual. FAO Fish. Tech. Pap. 1998, 306, 1407. [Google Scholar]
- Holt, S.J. The evaluation of fisheries resources by the dynamic analysis of stocks, and notes on the time factors involved. In International Commission for the Northwest Atlantic Fisheries; ICNAF Special Publication: Vancouver, BC, Canada, 1958; pp. 77–95. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Ministry of Agriculture, Fisheries and Food: London, UK, 1957. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. Manual of methods for fish stock assessment, Part II—Tables of yield functions. In FAO Fisheries Technical Paper No. 38; FAO: Rome, Italy, 1966; p. 10. [Google Scholar]
- Amorim, P.; Sousa, P.; Jardim, E.; Menezes, G.M. Sustainability status of data-limited fisheries: Global challenges for snapper and grouper. Front. Mar. Sci. 2019, 6, 654. [Google Scholar] [CrossRef]
- Pella, J.J.; Tomlinson, P.K. A generalized stock production model. InterAmerican Trop. Tuna Comm. Bull. 1969, 13, 421–458. [Google Scholar]
- Fox, W.W., Jr. An exponential surplus-yield model for optimizing exploited fish populations. Trans. Am. Fish. Soc. 1970, 99, 80–88. [Google Scholar] [CrossRef]
- Thorson, J.T.; Cope, J.M.; Branch, T.A.; Jensen, O.P. Spawning biomass reference points for exploited marine fishes, incorporating taxonomic and body size information. Can. J. Fish. Aquat. Sci. 2012, 69, 1556–1568. [Google Scholar] [CrossRef]
- Wang, S.P.; Maunder, M.N.; Aires-da-Silva, A. Selectivity’s distortion of the production function and its influence on management advice from surplus production models. Fish. Res. 2014, 158, 181–193. [Google Scholar] [CrossRef]
- Barrowman, N.J.; Myers, R.A. Still more spawner–recruitment curves: The hockey stick and its generalizations. Can. J. Fish. Aquat. Sci. 2000, 57, 665–676. [Google Scholar] [CrossRef]
- ICCAT. Report of the 2017 ICCAT Atlantic Swordfish Stock Assessment Session; Retrieved from Collection of Volume Scientific Papers; ICCAT: Madrid, Spain; Volume 74, pp. 841–967.
- Meyer, R.; Millar, R.B. BUGS in Bayesian stock assessments. Can. J. Fish. Aquat. Sci. 1999, 56, 1078–1086. [Google Scholar] [CrossRef]
- Maunder, M.N.; Piner, K.R. Dealing with data conflicts in statistical inference of population assessment models that integrate information from multiple diverse data sets. Fish. Res. 2017, 192, 16–27. [Google Scholar] [CrossRef]
- Cowles, M.K.; Carlin, B.P. Markov chain Monte Carlo convergence diagnostics: A comparative review. J. Am. Stat. Assoc. 1996, 91, 883–904. [Google Scholar] [CrossRef]
- O’Hara, R.B.; Sillanpää, M.J. A review of Bayesian variable selection methods: What, how and which. Bayesian Anal. 2009, 4, 85–117. [Google Scholar] [CrossRef]
- Haddon, M. Modelling and Quantitative Methods in Fisheries, 2nd ed.; Chapman and Hall: New York, NY, USA, 2010. [Google Scholar]
- Palomares, M.L.D.; Froese, R.; Derrick, B.; Nöel, S.-L.; Tsui, G.; Woroniak, J.; Pauly, D. A preliminary global assessment of the status of exploited marine fish and invertebrate populations. In A Report Prepared by the Sea Around Us for OCEANA; OCEANA: Washington, DC, USA, 2018; p. 64. [Google Scholar]
- Barua, S. Maximum Sustainable Yield (MSY) estimates for industrial finfish fishery in marine waters of Bangladesh using trawl catch log. Bang. J. Fish. 2019, 31, 313–324. [Google Scholar]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef]
- FAO; Garcia, S.M.; Ye, Y.; Rice, J.; Charles, A. Rebuilding of marine fisheries Part 1: Global review. FAO Fish. Aquac. Tech. Pap. 2018, 630, I-274. [Google Scholar]
- Costello, C.; Ovando, D.; Hilborn, R.; Gaines, S.D.; Deschenes, O.; Lester, S.E. Status and solutions for the world’s unassessed fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef]
- Liang, C.; Xian, W.; Liu, S.; Pauly, D. Assessments of 14 exploited fish and Invertebrate stocks in Chinese waters using the LBB method. Front. Mar. Sci. 2020, 7, 314. [Google Scholar] [CrossRef]
- Nadon, M.O.; Ault, J.S.; Williams, I.D.; Smith, S.G.; DiNardo, G.T. Length-based assessment of coral reef fish populations in the main and northwestern Hawaiian Islands. PLoS ONE 2015, 10, e0133960. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.; Sibert, J.R.; Kleiber, P.; Maunder, M.N.; Harley, S.J. Changes in abundance of large pelagic predators in the Pacific Ocean. Nature 2005, 434, E2–E3. [Google Scholar] [PubMed]
- Hinton, M.G.; Maunder, M.N. Methods for standardizing CPUE and how to select among them. Col. Vol. Sci. Pap. ICCAT 2004, 56, 169–177. [Google Scholar]
- Branch, T.A.; Hilborn, R.; Haynie, A.C.; Fay, G.; Flynn, L.; Griffiths, J.; Marshall, K.N.; Randall, J.K.; Scheuerell, J.M.; Ward, E.J.; et al. Fleet dynamics and fishermen behavior: Lessons for fisheries managers. Can. J. Fish. Aquat. Sci. 2006, 63, 1647–1668. [Google Scholar] [CrossRef]
- MoFL. Circular No. 160; Ministry of Fisheries and Livestock, Bangladesh Secretariat: Dhaka, Bangladesh, 2003. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase 2000: Concepts, Design, and Data Sources; WorldFish: Penang, Malaysia, 2000; 344p. [Google Scholar]
- Rao, G.S. Studies on the reproductive biology of the brown prawn Metapenaeus monoceros (Fabricius, 1798) along the Kakinada coast. Indian J. Fish. 1989, 36, 107–123. [Google Scholar]
- Khan, R.N.; Aravindan, N.; Kalavati, C. Distribution of two post larvae species of commercial prawns (Fenneropenaeus indicus and Penaeus monodon) in a coastal tropical estuary. J. Aquat. Sci. 2001, 16, 99–104. [Google Scholar] [CrossRef]



| Species | Month | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jul’21 | Aug’21 | Sep’21 | Oct’21 | Nov’21 | Dec’21 | Jan’ 22 | Feb’22 | Mar’22 | Apr’22 | May’22 | ||
| Tiger shrimp | 100 | 90 | 105 | 180 | 351 | 125 | 129 | 130 | 100 | 113 | 73 | 1496 |
| Brown shrimp | 90 | 90 | 105 | 183 | 183 | 122 | 119 | 155 | 100 | 138 | 80 | 1365 |
| White shrimp | 98 | 90 | 105 | 90 | 219 | 102 | 90 | 95 | 67 | 64 | 64 | 1084 |
| Parameter | Tiger Shrimp | Brown Shrimp | White Shrimp |
|---|---|---|---|
| (mm) | 120.0 | 74.0 | 75.0 |
| (mm) | 90.8 | 45.2 | 57.1 |
| (mm) | 113.0 (111.0–116.0) | 85.4 (84.0–87.0) | 76.4 (74.0–78.6) |
| (mm) | 72.6 (71.2–74.0) | 33.3 (32.5–34.0) | 51.0 (50.3–51.6) |
| 0.85 | 0.73 | 1.2 | |
| 0.9 | 0.84 | 1.2 | |
| 0.64 (0.63–0.65) | 0.39 (0.38–0.40) | 0.66 (0.65–0.67) | |
| 0.92 | 0.87 | 0.92 | |
| 0.61 (0.35–0.83) | 1.68 (1.4–1.97) | 1.59 (1.35–1.86) | |
| 2.6 (1.5–5.1) | 0.99 (0.7–1.4) | 1.31 (0.74–1.94) | |
| 2.1 (1.8–2.6) | 3.3 (3.1–3.6) | 3.7 (2.9–4.4) | |
| 0.18 (0.06–0.37) | 0.3 (0.18–0.45) | 0.35 (0.14–0.56) | |
| 0.43 (0.14–0.87) | 0.84 (0.5–1.2) | 0.96 (0.39–1.6) | |
| alpha | 1.28 (1.24–1.32) | 2.0 (1.92–2.09) | 2.89 (2.79–2.99) |
| Status | Grossly overexploited | Fully exploited but not overfished | Fully exploited but not overfished |
| Species | Lm (mm) | Lopt (mm) | Pmat | Popt | Pmega | Pobj | Stock Condition | Probability of Being SB < RP |
|---|---|---|---|---|---|---|---|---|
| Tiger shrimp | 113.0 | 63.43 | 86.09 | 25.13 | 63.03 | 1.74 | SB < RP | 44% for TRP 22% for LRP |
| Brown shrimp | 85.4 | 48.67 | 35.53 | 30.69 | 13.19 | 0.79 | SB ≥ RP | 0% for TRP 0% for LRP |
| White shrimp | 76.4 | 43.8 | 92.06 | 20.02 | 73.89 | 1.86 | SB < RP | 44% for TRP 22% for LRP |
| Parameters | Tiger Shrimp | Brown Shrimp | White Shrimp |
|---|---|---|---|
| K (year−1) | 6232.89 (4003.64–12,361.32) | 31,770.30 (15,214.16–90,873.27) | 5298.48 (2816.63–9344.75) |
| r (year−1) | 0.24 (0.12–0.41) | 0.66 (0.27–1.93) | 0.15 (0.07–0.37) |
| q | 0.000024 (0.000011–0.000040) | 0.000018 (0.000005–0.000042) | 0.000017 (0.000007–0.000043) |
| MSY (mt) | 388.84 (275.87–552.85) | 4899.24 (2791.25–23,536.08) | 208.68 (128.20–301.46) |
| BMSY (mt) | 3116.45 (2001.82–6180.66) | 15,885.15 (7607.08–45,436.64) | 2649.24 (1408.31–4672.38) |
| FMSY (year−1) | 0.12 (0.06–0.20) | 0.33 (0.14–0.97) | 0.08 (0.03–0.18) |
| B/B0 | 0.89 (0.74–1.07) | 0.91 (0.76–1.09) | 0.88 (0.73–1.08) |
| B2021/BMSY | 0.81 (0.57–1.14) | 1.64 (1.09–2.01) | 0.52 (0.27–1.03) |
| F2021/FMSY | 0.92 (0.53–1.39) | 0.19 (0.04–0.49) | 0.87 (0.37–1.79) |
| Species Name | K (mt) | r (Year−1) | MSY (mt) | BMSY (mt) | *BCurrent (mt) | FMSY (year−1) | Model Used | Reference |
|---|---|---|---|---|---|---|---|---|
| Penaeus monodon | 4720 (3350–6650) | 0.45 (0.32–0.62 | 527 (388–717) | 2360 (1670–3320) | 1250 (885–1550) | 0.22 (0.16–0.31) | CMSY | [8] |
| 5015 (3635–5808) | - | 203 (166–250) | 2062 (1451–2694) | 1429 (626–2458) | 0.13 (0.08–0.23) | DB-SRA | [7] | |
| 6233 (4004–12,361) | 0.24 (0.12–0.41) | 389 (276–553) | 3116 (2002–6181) | 2524 | 0.12 (0.06–0.20) | JABBA | Present study | |
| Metapenaeus monoceros | 10,000 (8380–12,200) | 1.22 (1.03–1.45) | 3090 (2920–3260) | 5060 (4990–6110) | 5960 (4760–6830) | 0.61 (0.51–0.73) | CMSY | [10] |
| 35,871 (26,192–40,750) | - | 1408 (1155–1715) | 15,140 (10,795–19,320) | 9470 (4200–17,097) | 0.12 (0.07–0.20) | DB-SRA | [7] | |
| 31,770 (15,214–90,873) | 0.66 (0.27–1.93) | 4899 (2791–23,536) | 15,885 (7607–45,437) | 26,051 | 0.33 (0.14–0.97) | JABBA | Present study | |
| Fenneropenaeus indicus | 5298 (2817–9345) | 0.15 (0.07–0.37) | 209 (128–301) | 2649 (1408–4672) | 1377 | 0.08 (0.03–0.18) | JABBA | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, S.; Liu, Q.; Alam, M.S.; Schneider, P.; Chowdhury, S.K.; Mozumder, M.M.H. Assessment of Three Major Shrimp Stocks in Bangladesh Marine Waters Using Both Length-Based and Catch-Based Approaches. Sustainability 2023, 15, 12835. https://doi.org/10.3390/su151712835
Barua S, Liu Q, Alam MS, Schneider P, Chowdhury SK, Mozumder MMH. Assessment of Three Major Shrimp Stocks in Bangladesh Marine Waters Using Both Length-Based and Catch-Based Approaches. Sustainability. 2023; 15(17):12835. https://doi.org/10.3390/su151712835
Chicago/Turabian StyleBarua, Suman, Qun Liu, Mohammed Shahidul Alam, Petra Schneider, Shoukot Kabir Chowdhury, and Mohammad Mojibul Hoque Mozumder. 2023. "Assessment of Three Major Shrimp Stocks in Bangladesh Marine Waters Using Both Length-Based and Catch-Based Approaches" Sustainability 15, no. 17: 12835. https://doi.org/10.3390/su151712835







