Permeable Pavement in the Northwestern United States: Pollution Source or Treatment Option?
Abstract
:1. Introduction
2. Materials and Methods
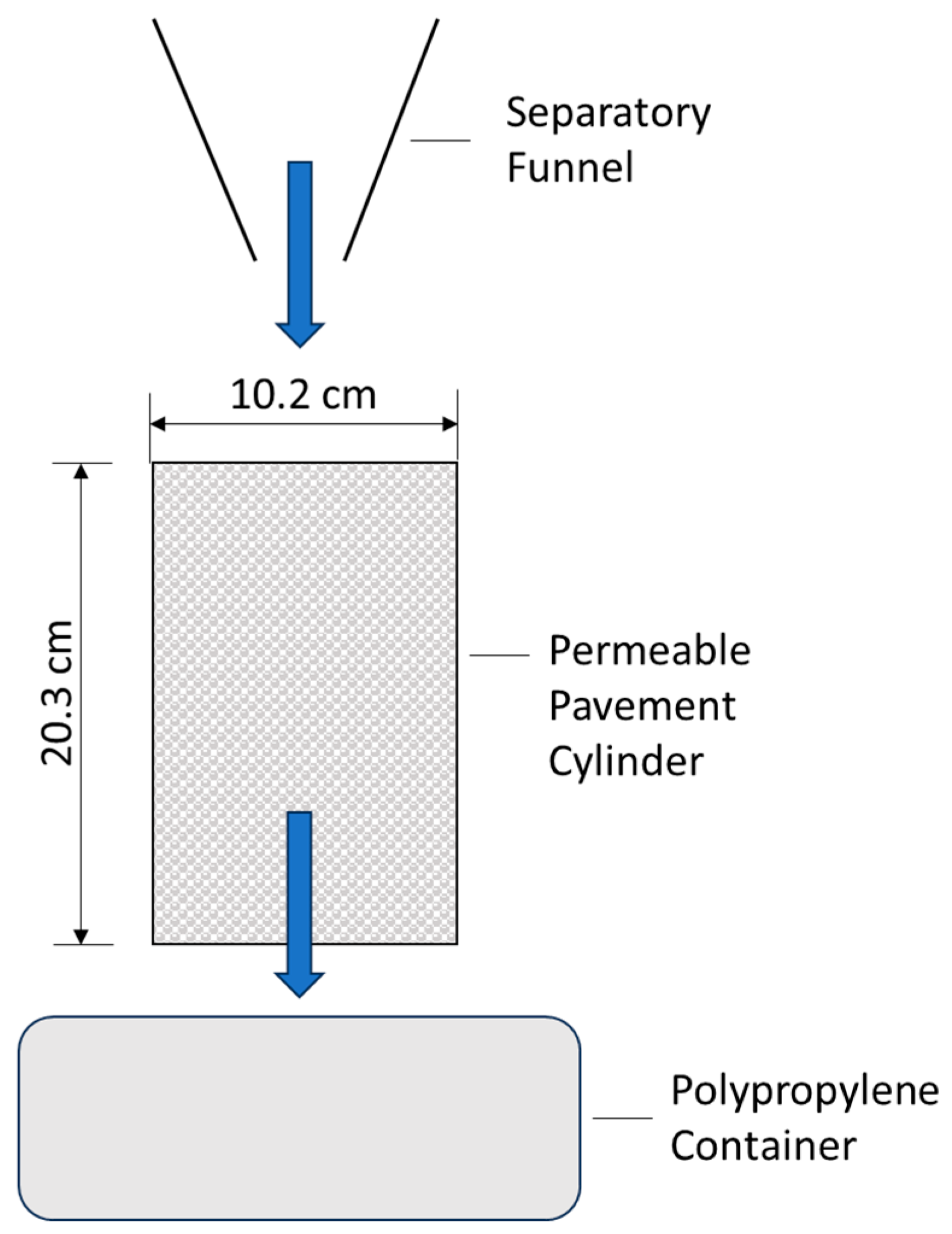
2.1. Test Specimens
2.2. Experiments
2.3. Statistical Analysis
3. Results and Discussion
3.1. Metals
3.2. Semi-Volatile Organics
3.3. Phosphorus
3.4. Comparison to Drinking Water Standards
4. Conclusions
- Pervious cement concrete and porous asphalt concrete can effectively remove most contaminants of concern for drinking water to below MCLs. Lead concentrations were slightly higher than the MCL, but further removal will likely occur in the soil layer beneath the pervious pavement;
- Zinc was exported from the permeable pavements, but concentrations were still well below the secondary MCL. Total phosphorus and phosphate were either exported or minimally removed, but this does not pose a threat to groundwater in terms of drinking water safety;
- Significant removal was observed for SVOCs, which indicates pervious cement concrete and porous asphalt concrete can effectively be used as a treatment method for organic contaminant removal;
- Permeable pavements minimize the risk of infiltrating stormwater polluting groundwater and provide a method of removing pollutants before reaching the groundwater table.
4.1. Study Limitations
4.2. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Weather Service. n.d. Monthly Climate Normals (1991–2020)—Portland International Airport, OR. NOAA Online Weather Data. Available online: https://www.weather.gov/wrh/Climate?wfo=pqr (accessed on 24 April 2023).
- Moretti, L.; Di Mascio, P.; Fusco, C. Porous Concrete for Pedestrian Pavements. Water 2019, 11, 2105. [Google Scholar] [CrossRef]
- Federal Highway Administration (FHWA). Rain and Flooding; U.S. Department of Transportation FHWA Road Weather Management Program: Washington, DC, USA, 2023. Available online: https://ops.fhwa.dot.gov/weather/weather_events/rain_flooding.htm#:~:text=Rain%20causes%20wet%20pavement%2C%20which,25%20percent%20on%20wet%20pavement (accessed on 10 April 2023).
- Environmental Protection Agency (EPA). Soak Up the Rain: What’s the Problem? Available online: https://www.epa.gov/soakuptherain/soak-rain-whats-problem#:~:text=And%20now%20when%20it%20rains,rivers%2C%20lakes%20and%20the%20ocean (accessed on 1 April 2023).
- Wethington, B. Green Infrastructure in the City of Portland, OR. City of Portland Bureau of Environmental Services Presentation, January 26, 2015. Available online: https://www.casfm.org/wp-content/uploads/2018/01/CASFM_Lunch_and_Learn_20150126.pdf (accessed on 3 July 2023).
- Jayasuriya, L.N.N.; Kadurupokune, N.; Othman, M.; Jesse, K. Contributing to the Sustainable Use of Stormwater: The Role of Pervious Pavements. Water Sci. Tech. 2007, 56, 69–75. [Google Scholar] [CrossRef]
- Brattebo, B.O.; Booth, D.B. Long-term Stormwater Quantity and Quality Performance of Permeable Pavement Systems. Water Res. 2003, 37, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.A.P.; Bradford, A.; Marsalek, J. Review of Environmental Performance of Permeable Pavement Systems: State of the Knowledge. Water Qual. Res. J. Canada 2013, 48, 203–222. [Google Scholar] [CrossRef]
- Selbig, W.R.; Buer, N. Hydraulic, Water-Quality, and Temperature Performance of Three Types of Permeable Pavement under High Sediment Loading Conditions; U.S. Geological Survey Scientific Investigations Report 2018-5037; U.S. Gelogical Service: Reston, VA, USA, 2018; 44p. [CrossRef]
- Zhang, W.; Li, Q.; Wang, J.; Meng, Y.; Zhou, Z. Aging Behavior of High-Viscosity Modified Asphalt Binder Based on Infrared Spectrum Test. Materials 2022, 15, 2778. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (EPA). Permeable Pavements. Stormwater Best Management Practice EPA-832-F-21-031W; Office of Water: Washington, DC, USA, 2021.
- Ndon, U.J.; Al-Manaseer, A. Permeable Pavement as a Sustainable Management Option for Highway Stormwater and Safe Use of Roadways; WP Report 12–13; Mineta Transportation Institute: San Jose, CA, USA, 2017. [Google Scholar]
- Holleran, I.; Wilson, D.J.; Holleran, G.; Walubita, L.F.; Byrony, J. Porous Asphalt—More Than Just Safety. In Proceedings of the IPENZ Transportation Group Conference, Auckland, NZ, USA, 7–9 March 2016. [Google Scholar]
- Environmental Protection Agency (EPA). Using Cool Pavements to Reduce Heat Islands. Available online: https://www.epa.gov/heatislands/using-cool-pavements-reduce-heat-islands#2 (accessed on 3 July 2023).
- Haddad, B.; Karaky, H.; Boutouil, M.; Boudart, B.; Sebaibi, N. Investigation Properties of Pervious and Water-Retaining Recycled Concrete to Mitigate Urban Heat Island Phenomena. Sustainability 2023, 15, 5384. [Google Scholar] [CrossRef]
- Zhu, H.; Wen, C.; Wang, Z.; Li, L. Study on the permeability of recycled aggregate pervious concrete with fibers. Materials 2020, 13, 321. [Google Scholar] [CrossRef]
- Vieira, G.L.; Schiavon, J.Z.; Borges, P.M.; da Silva, S.R.; de Oliveira Andrade, J.J. Influence of recycled aggregate replacement and fly ash content in performance of pervious concrete mixtures. J. Clean. Prod 2020, 271, 122665. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, D.; Wang, J.; Wu, D.; Liu, J. The Performance Evaluation of Asphalt Mortar and Asphalt Mixture Containing. Municipal Solid Waste Incineration Fly Ash. Materials 2022, 15, 1387. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, A.; Egodawatta, P.; McGree, J.; Goonetilleke, A. Assessment and Management of Human Health Risk from Toxic Metals and Polycyclic Aromatic Hydrocarbons in Urban Stormwater Arising from Anthropogenic Activities and Traffic Congestion. Sci. Total Environ. 2017, 579, 202–211. [Google Scholar] [CrossRef]
- Vaz, I.C.M.; Ghisi, E.; Thives, L.P. Stormwater Harvested from Permeable Pavements as a Means to Save Potable Water in Buildings. Water 2021, 13, 1896. [Google Scholar] [CrossRef]
- Iqbal, A.; Rahman, M.M.; Beecham, S. Spatial Analysis of the Water Harvesting Potential of Permeable Pavements in Australia. Sustainability 2022, 14, 16282. [Google Scholar] [CrossRef]
- Fletcher, T.; Duncan, H.; Poelsma, P.; Lloyd, S. Stormwater Flow and Quality, and the Effectiveness of Non-Proprietary Stormwater Treatment Measures: A Review and Gap Analysis; Cooperative Research Centre for Catchment Hydrology, Monash University: New South Wales, Australia, 2004. [Google Scholar]
- Booth, D.B.; Leavitt, J.; Peterson, K. The University of Washington Permeable Pavement Demonstration Project; Centre for Urban Water Resources Management, University of Washington: Seattle, WA, USA, 2003. [Google Scholar]
- Haselbach, L.; Poor, C.; Tilson, J. Dissolved Zinc and Copper Retention from Stormwater Runoff in Ordinary Portland Cement Pervious Concrete. Constr. Bldg. Mater. 2013, 53, 652–657. [Google Scholar] [CrossRef]
- Pilon, B.S.; Tyner, J.S.; Yoder, D.C.; Buchanan, J.R. The Effect of Pervious Concrete on Water Quality Parameters: A Case Study. Water 2019, 11, 263. [Google Scholar] [CrossRef]
- Kadurupokune, N.; Jayasuriya, N. Pollutant Load Removal Efficiency of Pervious Pavements: Is Clogging an Issue? Water Sci. Tech. 2009, 60, 1787–1794. [Google Scholar] [CrossRef]
- Weiss, P.T.; Kayhanian, M.; Gulliver, J.S.; Khazanovich, L. Permeable Pavement in Northern North American Urban Areas: Research Review and Knowledge Gaps. Int. J. Pavement Eng. 2017, 20, 143–162. [Google Scholar] [CrossRef]
- Legret, M.; Colandini, V. Effects of a porous pavement with reservoir structure on runoff water: Water quality and fate of heavy metals. Water Sci. Tech. 1999, 39, 111–117. [Google Scholar] [CrossRef]
- Dierkes, C.; Kuhlmann, L.; Kandasamy, J.; Angelis, G. Pollution Retention Capability and Maintenance of Permeable Pavements. In Proceedings of the 9th International Conference on Urban Drainage, Portland, OR, USA, 8–13 September 2002; p. 444. [Google Scholar]
- Charlesworth, S.M.; Beddow, J.; Nnadi, E.O. The Fate of Pollutants in Porous Asphalt Pavements, Laboratory Experiments to Investigate Their Potential to Impact Environmental Health. Int. J. Environ. Res. Public Health 2017, 14, 666. [Google Scholar] [CrossRef]
- Kandhal, P.S. Design, Construction, and Maintenance of Open-Graded Asphalt Friction Courses; Information Series 115; National Asphalt Pavement Association: Lanham, Maryland, 2002. [Google Scholar]
- City of Portland. Stormwater Management Manual; Bureau of Environmental Services: Portland, OR, USA, 2020.
- Oregon Department of Environmental Quality. Columbia Slough Sediment Project. Available online: https://www.oregon.gov/deq/hazards-and-cleanup/cleanupsites/pages/columbia-slough.aspx (accessed on 3 July 2023).
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Helsel, D.R.; Hirsch, R.M. Techniques of Water-Resources Investigations of the United States Geological Survey: Statistical Methods in Water Resources; United States Geological Survey: Reston, VA, USA, 2002.
- Sañudo-Fontaneda, L.A.; Charlesworth, S.M.; Castro-Fresno, D.; Andres-Valeri, V.C.A.; Rodriguez-Hernandez, J. Water Quality and Quantity Assessment of Pervious Pavements Performance in Experimental Car Park Areas. Water Sci. Tech. 2014, 69, 1526–1533. [Google Scholar] [CrossRef]
- Legret, M.; Colandini, V.; Le Marc, C. Effects of a porous pavement with reservoir structure on the quality of runoff water and soil. Sci Total Environ. 1996, 189–190, 335–340. [Google Scholar] [CrossRef]
- Washington Department of Ecology. Technology Assessment Protocol—Ecology (TAPE) Process Overview; Washington Department of Ecology: Olympia, WA, USA, 2018.
- Boving, T.B.; Stolt, M.H.; Augenstern, J.; Brosnan, B. Potential for localized groundwater contamination in a porous pavement parking lot setting in Rhode Island. Environ. Geol. 2008, 55, 571–582. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Quality Criteria for Water 1986; Office of Water Regulations and Standards: Washington, DC, USA, 1986; EPA 440/5-86/001.
- Litke, D.W. Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality; U.S. Geological Survey Water-Resources Investigations Report 99-4007; USGS: Denver, CO, USA, 1999.
- City of Portland. Decision Making Framework for Groundwater Protectiveness Demonstrations: Underground Injection Control System Evaluations and Response; Bureau of Environmental Services: Portland, OR, USA, 2008.
- Davis, A.; Shokouhian, M.; Sharma, H.; Minami, C.; Winogradoff, D. Water Quality Improvement through Bioretention: Lead, Copper, and Zinc removal. Water Environ. Res. 2003, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]




| Sieve Size | %Passing |
|---|---|
| 3/4″ | 100 |
| 1/2″ | 90–100 |
| 3/8″ | 40–70 |
| U.S. No. 4 | 0–15 |
| U.S. No. 8 | 0–5 |
| Contaminant |
|---|
| Acenaphthene |
| Acenaphthylene |
| Anthracene |
| Benzo(a)anthracene |
| Benzo(a)pyrene |
| Benzo(b)fluoranthene |
| Benzo(g,h,i)perylene |
| Benzo(k)fluoranthene |
| Chrysene |
| Dibenzo(a,h)anthracene |
| Fluoranthene |
| Fluorene |
| Indeno(1,2,3-cd)pyrene |
| Naphthalene |
| Pentachlorophenol |
| Phenanthrene |
| Pyrene |
| Butyl benzyl phthalate |
| Di-n-butyl phthalate |
| Diethyl phthalate |
| Dimethyl phthalate |
| Di-n-octyl phthalate |
| Bis(2-ethylhexyl) phthalate |
| Effluent Concentration (μg/L) | ||||||
|---|---|---|---|---|---|---|
| Pervious Concrete | Porous Asphalt | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Arsenic | 1.81 (0.02) | 1.03 (0.08) | 1.12 (0.10) | 1.59 (0.11) | 1.32 (0.41) | 1.09 (0.04) |
| Cadmium | 0.37 (0.02) | 0.30 (0.03) | 0.33 (0.04) | 0.39 (0.02) | 0.38 (0.02) | 0.41 (0.05) |
| Copper | 55.8 (5.83) | 33.4 (2.21) | 37.6 (1.97) | 47.3 (2.25) | 35.9 (1.89) | 35.8 (0.57) |
| Lead | 22.03 (0.83) | 14.7 (1.46) | 16.5 (0.44) | 19.7 (0.78) | 16.3 (0.71) | 15.8 (0.57) |
| Zinc | 732 (24.4) | 1277 (25.2) | 1377 (5.77) | 761 (19.5) | 1547 (64.3) | 1527 (49.3) |
| % Removal | ||||||
|---|---|---|---|---|---|---|
| Pervious Cement Concrete | Porous Asphalt Concrete | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Arsenic | 20.1 | 54.5 | 50.6 | 29.8 | 41.7 | 51.9 |
| Cadmium | 48.6 | 58.0 | 54.8 | 46.6 | 48.2 | 43.8 |
| Copper | 10.9 | 46.7 | 40.0 | 24.5 | 42.6 | 42.8 |
| Lead | 48.2 | 65.5 | 61.2 | 53.7 | 61.8 | 62.9 |
| Zinc | 13.0 | −51.8 | −63.7 | 9.6 | −83.9 | −81.5 |
| Effluent Concentrations (µg/L) | ||||||
|---|---|---|---|---|---|---|
| Pervious Concrete | Porous Asphalt | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Benzo(a)anthracene | ND | ND | ND | ND | ND | ND |
| Benzo(a)pyrene | ND | ND | ND | ND | ND | ND |
| Benzo(b)fluoranthene | ND | ND | ND | 0.06 (0.1) | 0.06 (0.1) | ND |
| Benzo(g,h,i)perylene | 0.20 (0.03) | ND | 0.06 (0.03) | 0.21 (0.02) | 0.18 (0.02) | ND |
| Chrysene | ND | ND | ND | ND | ND | ND |
| Fluoranthene | 0.29 (0.03) | 0.25 (0.02) | 0.28 (0.02) | 0.30 (0.01) | 0.30 (0.01) | 0.27 (0.01) |
| Indeno(1,2,3-cd) pyrene | ND | ND | ND | ND | ND | ND |
| Phenanthrene | 0.06 (0.1) | ND | ND | 0.17 (0.01) | 0.18 (0.01) | ND |
| Pyrene | 0.44 (0.05) | 0.37 (0.03) | 0.42 (0.03) | 0.46 (0.01) | 0.45 (0.01) | 0.42 (0.01) |
| Bis(2-ethylhexyl)phthalate | 5.27 (0.6) | 3.77 (0.25) | 4.5 (0.1) | 5.23 (0.85) | 4.77 (0.45) | 4.43 (0.21) |
| % Removal | ||||||
|---|---|---|---|---|---|---|
| Pervious Cement Concrete | Porous Asphalt Concrete | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Benzo(a)anthracene | 100 | 100 | 100 | 100 | 100 | 100 |
| Benzo(a)pyrene | 100 | 100 | 100 | 100 | 100 | 100 |
| Benzo(b)fluoranthene | 100 | 100 | 100 | 84.9 | 84.9 | 100 |
| Benzo(g,h,i)perylene | 60.7 | 100 | 88.7 | 58.0 | 63.3 | 100 |
| Chrysene | 100 | 100 | 100 | 100 | 100 | 100 |
| Fluoranthene | 63.7 | 68.4 | 65.0 | 62.4 | 62.4 | 65.4 |
| Indeno(1,2,3-cd) pyrene | 100 | 100 | 100 | 100 | 100 | 100 |
| Phenanthrene | 88.9 | 100 | 100 | 68.5 | 66.0 | 100 |
| Pyrene | 64.8 | 70.4 | 66.4 | 62.9 | 64.0 | 66.4 |
| Bis(2-ethylhexyl)phthalate | 59.5 | 71.0 | 65.4 | 59.7 | 63.3 | 65.9 |
| Effluent Concentrations (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Pervious Concrete | Porous Asphalt | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Total Phosphorus | 1.08 (0.34) | 0.68 (0.04) | 0.68 (0.01) | 2.8 (0.92) | 0.71 (0.02) | 0.63 (0.02) |
| Phosphate | 0.03 (0.05) | 0.11 (0.19) | 0.12 (0.11) | 0.19 (0.07) | 0.06 (0.08) | 0.09 (0.11) |
| % Removal | ||||||
|---|---|---|---|---|---|---|
| Pervious Cement Concrete | Porous Asphalt Concrete | |||||
| Contaminant | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 |
| Total Phosphorus | −13.7 | 1.0 | 12.1 | 2.8 | −2.4 | 18.6 |
| Phosphate | 73.3 | −22.2 | −71.4 | −90.0 | 33.3 | −33.3 |
| Average Concentration (mg/L) | |||
|---|---|---|---|
| Contaminant | Pervious Cement Concrete | Porous Asphalt Concrete | MCL (mg/L) |
| Arsenic | 0.00132 | 0.00133 | 0.010 |
| Cadmium | 0.00033 | 0.00039 | 0.005 |
| Copper | 0.0423 | 0.0397 | 1.3 * |
| Lead | 0.0177 | 0.0172 | 0.015 * |
| Zinc | 1.13 | 1.28 | 5 ** |
| Benzo(a)pyrene | ND | ND | 0.0002 |
| Pentachlorophenol | ND | ND | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poor, C.; Kaye, J.; Struck, R.; Gonzalez, R. Permeable Pavement in the Northwestern United States: Pollution Source or Treatment Option? Sustainability 2023, 15, 12926. https://doi.org/10.3390/su151712926
Poor C, Kaye J, Struck R, Gonzalez R. Permeable Pavement in the Northwestern United States: Pollution Source or Treatment Option? Sustainability. 2023; 15(17):12926. https://doi.org/10.3390/su151712926
Chicago/Turabian StylePoor, Cara, Jackson Kaye, Rodney Struck, and Ruben Gonzalez. 2023. "Permeable Pavement in the Northwestern United States: Pollution Source or Treatment Option?" Sustainability 15, no. 17: 12926. https://doi.org/10.3390/su151712926





